Abstract
A gene expression study of Haemophilus ducreyi identified the hypothetical lipoprotein HD0192, renamed here fibrinogen binder A (fgbA), as preferentially expressed in vivo. To test the role of fgbA in virulence, an isogenic fgbA mutant (35000HPfgbA) was constructed in H. ducreyi 35000HP, and six volunteers were experimentally infected with 35000HP and 35000HPfgbA. The overall pustule formation rate was 61.1% at parent sites and 22.2% at mutant sites (P = 0.019). Papules were significantly smaller at mutant sites than at parent sites (13.3 versus 37.9 mm2, P = 0.002) 24 h after inoculation. Thus, fgbA contributed significantly to virulence of H. ducreyi in humans. In vitro studies demonstrated that fgbA encodes a fibrinogen binding protein; no other fibrinogen binding proteins were identified in 35000HP. fgbA was conserved among clinical isolates of both class I and class II H. ducreyi strains, supporting the finding that fgbA is important for H. ducreyi infection.
Keywords: Haemophilus ducreyi, chancroid, human trials, fibrinogen, adhesins, virulence factors
INTRODUCTION
Haemophilus ducreyi is the causative agent of chancroid, a genital ulcer disease that contributes to the spread of human immunodeficiency virus (HIV) type 1 in endemic areas of the developing world [1]. H. ducreyi enters the body through microabrasions that occur during intercourse and primarily remains confined to the skin, where the organism resides extracellularly in a milieu of professional phagocytes [2, 3]. H. ducreyi colocalizes with fibrin, collagen, neutrophils, and macrophages at the papular, pustular, and ulcerative stages of disease in both a human infection model and naturally occurring chancroid [4, 5].
A limited number of H. ducreyi virulence factors have been identified. Consistent with the organism’s extracellular lifestyle and colocalization with collagen, full virulence of H. ducreyi in humans requires expression of the collagen-specific adhesin NcaA, the antiphagocytic proteins LspA1 or LspA2, and two outer membrane proteins, DsrA and DltA, which confer protection from serum-mediated killing [6–9]. Other virulence factors of H. ducreyi required for human infection include the hemoglobin receptor HgbA, the peptidoglycan-associated lipoprotein PAL, which is involved in outer membrane stability, an intact Flp locus, which encodes fimbria-like proteins and type IV-like secretory proteins, and a locus encoding enterobacterial common antigen-like biosynthetic genes [10–13].
With the goal of discovering new H. ducreyi virulence factors, we identified a panel of H. ducreyi genes whose corresponding mRNAs are preferentially expressed in experimental pustules [14]. The in vivo expressed genes included 133 ORFs encoding proteins of unknown function [14]. We performed in silico analyses of these in vivo-expressed hypothetical proteins, including subcellular localization algorithms and searches for conserved motifs, domains, and structural features [15–20]. From these analyses, we identified seven hypothetical outer membrane proteins (M. E. Bauer, unpublished data). In this study, we used the human model of H. ducreyi infection to examine the role of one such protein, the hypothetical lipoprotein HD0192, in virulence of H. ducreyi. We also explored a putative role for HD0192 in binding to fibrinogen; based on these studies, we named the protein FgbA (fibrinogen binder A).
METHODS
Bacterial strains and growth conditions
H. ducreyi strains (Table 1) were cultured at 33°C using media described previously [27]. Escherichia coli TOP10 (Invitrogen, Carlsbad, Calif.) was used for cloning and B21 DE3/pLysS (Invitrogen) for protein expression. A recombinant clone expressing the 36-kDa Fg-binding domain of Staphylococcus aureus ClfA in E. coli was kindly provided by M. Höök (Texas A&M Health Science Center) [28]. E. coli strains were cultured on Luria-Bertani agar or broth supplemented with kanamycin (50 µg/ml) or ampicillin (50 µg/ml).
Table 1.
H. ducreyi strains used in this study
| Strain Designation | Geographic Origin | Year of Isolation | Class | References |
|---|---|---|---|---|
| 35000HPa | Winnipeg, Canada | 1975 | Ib | [21, 22] |
| HD183 | Singapore | 1982 | Ic | [23] |
| HD188 | Kenya | 1982 | Ic | [23] |
| 82-029362 | California, USA | 1982 | Ic | [23] |
| 6644 | Boston, USA | 1989 | Ic | [23] |
| 85-023233 | New York City, USA | 1985 | Ic | [23] |
| CIP542 ATCC | Hanoi, Vietnam | 1954 | IIb | [22, 24] |
| HMC112 | not reported | 1984 | IIb | [24] |
| 33921 | Nairobi, Kenya | not reported | IId | [25] |
| DMC64 | Bangladesh | not reported | IIb | [24] |
Construction and complementation of an fgbA mutant
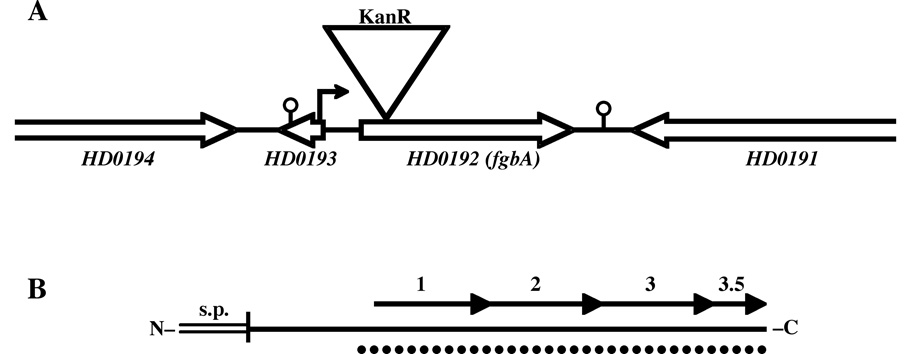
A map of the fgbA-containing locus in 35000HP is shown in Fig. 1A. The upstream and downstream ORFs, HD0193 and HD0191, were predicted to be transcribed from the opposite strand as fgbA, suggesting that fgbA was monocistronic (Fig. 1A). A 4.1 kb fragment containing HD0192 (fgbA) was PCR amplified from 35000HP genomic DNA (see Table 2 for primers). The amplicons were digested with BamHI and XbaI and cloned into pBluescriptIISK+ (Stratagene, La Jolla, Calif.). A kanamycin resistance (KanR) cassette from pUC18K [29] was inserted into the cloned fgbA ORF; the mutated fgbA was subcloned into pRSM2072, which expresses lacZ and acts as a suicide vector in H. ducreyi [30], and introduced into H. ducreyi 35000HP by electroporation. KanR transformants were propagated in medium containing 5-bromo-4-chloro-3-indoyl-b-D-galactopyranoside (X-gal), as described [30], to identify colonies that had undergone allele exchange. One such colony was designated 35000HPfgbA. The fgbA mutation in 35000HPfgbA was confirmed by sequencing, southern blotting, and PCR analyses (data not shown). 35000HP and 35000HPfgbA had similar outer membrane protein (OMP) and lipooligosaccharide profiles, assessed as described [11], and demonstrated similar growth rates in broth (data not shown).
Fig. 1. Maps of the HD0192 (fgbA)-containing genetic locus (A) and the Fg-binding protein FgbA (B).
(A) Scale map of the fgbA locus in 35000HP. Open arrows indicate direction of transcription of ORFs; single lines indicate intergenic sequences. Triangle denotes site of insertional mutagenesis with KanR cassette in 35000HPfgbA. Small arrow indicates putative promoter upstream of the fgbA ORF; stalked circles indicate predicted Rho-independent transcriptional terminators. (B) Scale map of the protein product of fgbA. The horizontal solid line represents the 1° structure of the mature protein, the double line indicates the signal 2 peptide, and the vertical line between them denotes the site of signal peptide cleavage and lipidation. Arrows represent the 29-amino acid direct repeats. Dotted line indicates the predicted alpha-helical coiled-coil structure.
Table 2.
Oligonucleotides Used in This Study
| Uses | Forward Primera | Forward Primera |
|---|---|---|
| recombinant expression of fgbA (HD0192) |
CCTCAAGTTGAAGAAATGAAACA AACGG |
CGAATTCGTATTTGGTAATAA ATGACCGC |
| recombinant expression of HD0581 |
TGTAATAAACCTGATCCTGCTACA GA |
AGTCATTTGAAAGTCCTATGTC AG |
| recombinant expression of HD1218 |
ACCATCTCTGCACACGCAACCATA | GGGACCATTCCTAATATGCAA ATCC |
| mutagenesis of fgbA | catatcggatccCCGCCAACGTTTAAGC CCATCATT |
catatctctagaTCGGGCGACTTTAG CGCAATATCA |
|
trans–complementation with fgbA; survey of fgbA loci in H. ducreyi strains |
taagaatgcggccgcTACGCTGCGCCACA GACTACTAAA |
taagaatgcggccgcTGACCGCGATA AGCGGTCTTT |
| class I-specific primers dsrA 14 and dsrA 24b |
GACAGCATTCAGTGAATAATGGC | AATGAAGTCCGCACCTTTAAC GGC |
| class II-specific primers dsrA 42 and dsrA 43c |
TGCCTTGCTCTTAATGACG | TAAAAGCACATAAACAAGCG |
For complementation, the fgbA ORF and 197 bp of upstream flanking sequence was PCR-amplified (Table 2) and cloned into shuttle vector pLSKS [31]. The resulting construct was introduced into 35000HPfgbA by electroporation and selected on plates containing kanamycin and streptomycin.
Human Inoculation Protocol
Six healthy volunteers (5 men, 1 woman; 5 whites, 1 black; age range: 21–54 years, mean ± standard deviation, 32 ± 14) were recruited for the study. Subjects gave informed consent for participation and for HIV serology, in accordance with the human experimentation guidelines of the U.S. Department of Health and Human Services and the Institutional Review Board of Indiana University-Purdue University of Indianapolis. All aspects of the mutant-parent trial and determining colony phenotypes were performed exactly as described previously [7, 10, 13]. Statistical analysis compared papule and pustule formation rates using a logistical regression model with generalized estimating equations to adjust for within subject correlation, as described [12]. Papule size was compared between strains using a mixed effects model with a random subject effect.
Recombinant fusion protein construction and expression
Genes encoding HD0192, HD0581, and HD1218 were PCR-amplified (Table 2), starting immediately downstream of their lipidation sites, cloned into pCR-XL-TOPO (Invitrogen), and subcloned into pRSET B (Invitrogen) to express the ORFs fused in frame with an N-terminal 6xHis tag. The constructs were expressed in B21 DE3/pLysS, following the manufacturer’s directions. Recombinant proteins were purified with the QIAexpressionist System, following the manufacturer’s instructions (Qiagen Inc, Valencia, Calif).
Fibrinogen binding ligand blot assay
Purified, human Fg (Enzyme Research Laboratories, Inc., South Bend, Ind.) was labeled with digoxigenin (dig) using the DIG protein labeling kit from Roche Applied Sciences (Indianapolis, Ind.), following the manufacturer’s instructions. Bacterial proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to polyvinylidene fluoride (PVDF) membranes, blocked with 2% nonfat milk in phosphate-buffered saline pH 7.4 (PBS), and probed with a 1:1000 dilution of dig-Fg (10 µg/ml) in 2% milk-PBS overnight. Dig-Fg was detected using digoxigenin detection reagents (Roche), following the manufacturer’s protocols.
Bacterial protein preparations
Whole cell lysates and OMPs of 35000HP were prepared as described previously [27]. Triton X-114 phase partitioning was performed on mid-logarithmic cultures of 35000HP as described [32]. Following separation, both phases were precipitated with acetone to collect proteins. Aqueous and detergent phase protein samples were suspended in identical volumes of Laemmli sample buffer for comparison by standard SDS-PAGE or in rehydration buffer containing 9 M urea, 4% Igepal CA-630, 1% dithiothreitol, and 2% carrier ampholytes (pH 4–7) [33] for two-dimensional gel electrophoresis (2DE).
2DE and tandem mass spectrometry
2DE was performed by the Indiana Center for Applied Protein Sciences as described previously [33, 34]. Briefly, detergent phase proteins from 35000HP were separated by isoelectric focusing with a pH 4–7 linear gradient, followed by molecular size through 4–20% gradient gels (Bio-Rad). Samples were analyzed in parallel on two gels; one gel was stained with Coomassie [34], and the other was transferred to PVDF and probed with dig-Fg by ligand blot. Coomassie-stained spots corresponding to positive signals in the ligand blot were excised and subjected to tandem mass spectrometry (MS-MS) for peptide sequence analysis. The results indicated multiple protein IDs in each spot; proteins with at least two distinct matching peptides were considered for further analysis (Table 3).
Table 3.
H. ducreyi proteins identified by MS-MS of Fg-binding spots
| Protein Annotation | Accession no. |
No. matching peptides |
predicted MW, kDa |
predicted pI |
predicted signal peptidea |
|---|---|---|---|---|---|
| hypothetical protein HD0192 (FgbA) | AAP95185 | 4 | 16.8b | 5.03b | residues 1–18 |
| lipoprotein HlpB | AAP95513 | 2 | 17.8c | 5.33c | residues 1–19 |
| NifU-like protein | AAP95949 | 5 | 13.6 | 4.66 | none |
| hypothetical protein HD1218 | AAP96060 | 2 | 12.6d | 4.72d | residues 1–21 |
| universal stress protein UspA | AAP96234 | 2 | 15.7 | 4.5 | none |
| 10 kDa chaperonin GroES | AAP96537 | 3 | 10.3 | 4.88 | none |
| 50S ribosomal protein L7/L12 | AAP96610 | 4 | 12.2 | 4.60 | none |
Signal peptide prediction based on the SignalP algorithm [15].
Predicted mature FgbA protein, excluding signal peptide, is 14.9 kDa with pI = 4.85.
Predicted mature HlpB protein, excluding signal peptide, is 15.9 kDa with pI = 4.99.
Predicted mature HD1218 protein, excluding signal peptide, is 10.4 kDa with pI = 4.39.
Conservation of fgbA sequence in H. ducreyi strains
H. ducreyi strains and class designations are listed in Table 1. For strains whose phenotypic class had not been previously reported, we PCR-amplified the dsrA locus from genomic DNA using class-specific primer pairs described by White et al. [Table 2 and ref. 24]. All strains tested contained a dsrA locus that amplified with class I-specific but not class II-specific primers (data not shown) and were therefore considered class I strains (Table 1).
fgbA-containing sequences, including 145 bp 5’ and 130 bp 3’ to the fgbA ORF, were PCR-amplified from genomic DNA of all listed H. ducreyi strains (Table 2). The amplicons were sequenced, and the resulting sequences aligned by the ClustalW algorithm in the Lasergene software package (DNASTAR, Madison, WI).
RESULTS
fgbA contributed to virulence in the human model of infection
fgbA transcripts are expressed during human infection [14]. To determine whether FgbA played a role in disease, fgbA was inactivated in H. ducreyi 35000HP by insertion of a kanamycin resistance cassette (Fig. 1A). The resulting mutant, 35000HPfgbA, was directly compared with 35000HP for virulence in the human model of H. ducreyi infection. Six healthy adults volunteered for the study (Table 4). In the first iteration, each of two subjects was infected with a fixed estimated delivered dose (EDD) (88 CFU) of 35000HP at three sites on one arm and varying EDDs (59, 118, and 256 CFU) of 35000HPfgbA at three sites on the other arm. Pustules formed at 3 of 6 parent sites and 1 of 6 mutant sites (Table 4). In the second iteration, 3 volunteers were inoculated with 98 CFU of 35000HP on one arm and 43, 85, and 169 CFU of 35000HPfgbA on the other arm. Pustules formed at 5 of 9 parent sites and 3 of 9 mutant sites (Table 4). In the third iteration, the volunteer was inoculated with 50 CFU of 35000HP at 3 sites and 78 CFU of 35000HPfgbA at 3 sites; pustules formed at all 3 parent sites and at 0 of 3 mutant sites.
Table 4.
Response to Inoculation of live H. ducreyi Strains
| Volunteer no.a |
Genderb | Days of Observation |
Strain | No. of Initial Papules |
No. of Pustules |
Final Outcomes of Sites: |
||
|---|---|---|---|---|---|---|---|---|
| Papule | Pustule | Resolved | ||||||
| 297 | F | 14 | 35000HP | 3 | 1 | 0 | 1 | 2 |
| 35000HPfgbA | 3 | 1 | 0 | 1 | 2 | |||
| 300 | M | 8 | 35000HP | 2 | 2 | 0 | 2 | 0 |
| 35000HPfgbA | 2 | 0 | 0 | 0 | 2 | |||
| 301 | M | 7 | 35000HP | 3 | 2 | 1 | 2 | 0 |
| 35000HPfgbA | 2 | 0 | 0 | 0 | 2 | |||
| 302 | M | 7 | 35000HP | 3 | 3 | 0 | 3 | 0 |
| 35000HPfgbA | 3 | 3 | 0 | 3 | 0 | |||
| 303 | M | 6 | 35000HP | 3 | 0 | 0 | 0 | 3 |
| 35000HPfgbA | 3 | 0 | 0 | 0 | 3 | |||
| 304 | M | 6 | 35000HP | 3 | 3 | 0 | 3 | 0 |
| 35000HPfgbA | 2 | 0 | 0 | 0 | 3 | |||
Volunteers 297 and 300 were inoculated in iteration one. Volunteers 301, 302 and 303 were inoculated in iteration two. Volunteer 304 was inoculated in iteration three.
F, female; M, male.
Overall, the pustule formation rate was 61.1% (95% CI, 32.7%–89.6%) at 18 parent sites and 22.2% (95% CI, 0.1%–51.7%) at 18 mutant sites (P = 0.019). Papules were significantly smaller at mutant sites (mean, 13.3 mm2) than at parent sites (mean, 37.9 mm2) 24 h after inoculation (P = 0.002). These results suggested that expression of FgbA facilitated the ability of H. ducreyi to initiate disease and progress to pustule formation in humans.
For the parent and mutant broth cultures used to prepare the inocula, all 107 parent and 107 mutant colonies tested were phenotypically correct for kanamycin resistance. Ten of 15 sites (67.7%) inoculated with the parent yielded positive surface cultures, while 2 of 15 mutant sites (13%) yielded a positive surface culture. All colonies obtained from surface cultures (n = 406 and n = 43) and biopsy specimens (n = 72 and n = 36) from parent sites and mutant sites, respectively, had the expected antibiotic susceptibility.
Recombinant FgbA bound to human fibrinogen
To discern possible functions for FgbA, we performed in silico analysis of the deduced protein sequence (Fig. 1B). The N-terminus contained a signal II peptide and lipidation site consistent with a lipoprotein (Fig. 1B).The C-terminus contained 3.5 direct, tandem repeats of a 29-amino acid motif with a consensus sequence of N-EMKDAAKAKLEDMKESAAEAKESLAEKAN-C. The repetitive region was predicted to form an alpha-helical coiled-coil structure (Fig. 1B). Coiled coil motifs are found in a wide variety of proteins with structural or regulatory functions and frequently involve homomeric or heteromeric protein-protein interactions [18]. Several bacterial proteins with coiled coils interact with host proteins [35–37]. The only known host protein that H. ducreyi colocalizes with, and for which no adhesin has yet been identified, is fibrin. Many bacteria interact with fibrin via Fg-binding proteins with coiled coil motifs. Thus, we hypothesized that FgbA may bind Fg.
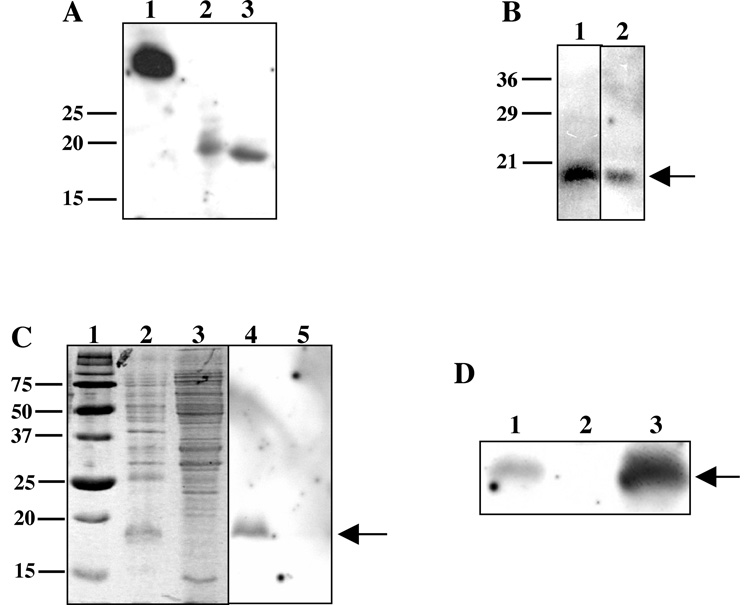
To determine whether FgbA could bind Fg, the fgbA ORF was expressed, without its signal peptide, as a recombinant N-terminal 6xHis tagged fusion protein (designated r-FgbA) and probed with dig-Fg in a ligand blot assay. The Fg-binding domain of ClfA from Staphylococcus aureus served as a positive control [28]. As shown in Fig. 2A, r-FgbA demonstrated Fg binding activity, both within an E. coli whole cell lysate (Fig. 2A lane 2) and after purification over a nickel column (Fig. 2A lane 3). These data suggested that FgbA was able to bind human Fg.
Fig. 2. FgbA conferred fibrinogen binding in a ligand blot assay.
(A) Lane 1, lysate of E. coli strain expressing recombinant Fg-binding portion of S. aureus ClfA; lane 2, lysate of E. coli strain expressing recombinant HD0192; lane 3, recombinant HD0192 after purification over a Ni column. (B) Whole cell lysate (lane 1) and OMP preparation (lane 2) of 35000HP probed with dig-Fg and developed to detect dig. Note the Fg-binding bands at 18 kDa (arrow). (C) Fg-binding activity was enriched in a hydrophobic protein preparation. Triton X-114 detergent (lanes 2, 4) or aqueous (lanes 3, 5) phase proteins from whole cell lysates of 35000HP were stained with Coomassie (lanes 2, 3) or probed with dig-Fg in the ligand blot (lanes 4, 5). Lane 1, molecular size markers. Note the 18 kDa bands (arrow) present in detergent phase samples. (D) FgbA conferred Fg-binding activity in H. ducreyi. Whole cell lysates of parent strain 35000HP (lane 1), mutant 350000HPfgbA (lane 2), and complemented mutant (lane 3) were probed with dig-Fg in the ligand blot assay. Note the FgbA band present in the parent, absent in the fgbA mutant, and restored in the complemented mutant.
Identification of Fg-binding protein(s) in H. ducreyi
Because many pathogens express redundant adhesins for important ligands, we examined 35000HP for additional Fg-binding proteins using the ligand blot assay. We first probed whole cell lysates and OMP preparations of H. ducreyi 35000HP with dig-Fg. As shown in Fig. 2B, dig-Fg bound to a single, approximately18 kDa band present in whole cell lysates and in OMPs.
To partially purify the Fg-binding protein, H. ducreyi 35000HP cells were subjected to Triton X-114 phase partitioning (Fig. 2C). Fg binding activity partitioned to the detergent phase; no activity was observed in the aqueous phase (Fig. 2C). Thus, the Fg binding activity was likely due to a protein with hydrophobic domains or components.
To identify the Fg-binding band, detergent phase proteins were separated by 2DE and subjected to ligand blot analysis. Two Fg binding spots were detected by ligand blot; each migrated to the same molecular size and slightly different pIs, in the range of pI 4–5.5 (data not shown), suggesting one protein with heterogeneous post-translational modification. Coomassie-stained spots corresponding to the Fg binding spots were excised and subjected to MS-MS analysis. Peptides were identified that corresponded to seven proteins, each with a predicted molecular weight between 10 and 20 kDa and acidic pI (Table 3). One of the proteins identified was FgbA (Table 3). Two additional identified proteins, lipoprotein HlpB and hypothetical protein HD1218, had no known function; the remaining four proteins were orthologous to bacterial proteins with assigned functions (Table 3). Three of the proteins, including FgbA, lipoprotein HlpB, and hypothetical protein HD1218, contained predicted signal peptides indicating an extracytoplasmic location [15, 16]; the remaining four proteins were predicted to be cytoplasmic (Table 3).
To determine whether HlpB or HD1218 was able to bind Fg, the genes corresponding to both proteins were recombinantly expressed as N-terminal 6xHis tagged fusion proteins and subjected to ligand blot analysis. Neither recombinant HlpB nor recombinant HD1218 bound to dig-Fg in the ligand blot assay (data not shown). Thus, FgbA was the only H. ducreyi OMP identified by ligand blot to bind human Fg. The predicted lipidation of FgbA was consistent with the hydrophobic nature and heterogeneous pI observed for the Fg-binding activity in H. ducreyi.
A fgbA mutant did not bind Fg
To confirm that fgbA conferred H. ducreyi binding to Fg by ligand blot, we compared 35000HP and 3500HPfgbA for Fg binding. 35000HPfgbA did not express the 18 kDa Fg binding protein; however, trans-complementation with the fgbA ORF restored Fg binding activity to the mutant (Fig. 2D). The complemented mutant showed a much stronger Fg-binding band than did the parent strain. Although FgbA levels in 35000HP and the complemented mutant have not been quantitatively compared, the increased activity is likely from overexpression of fgbA on the multi-copy shuttle vector. The mutagenesis and complementation data demonstrate that FgbA encodes the H. ducreyi Fg binding protein detected by ligand blot.
fgbA was conserved among H. ducreyi strains
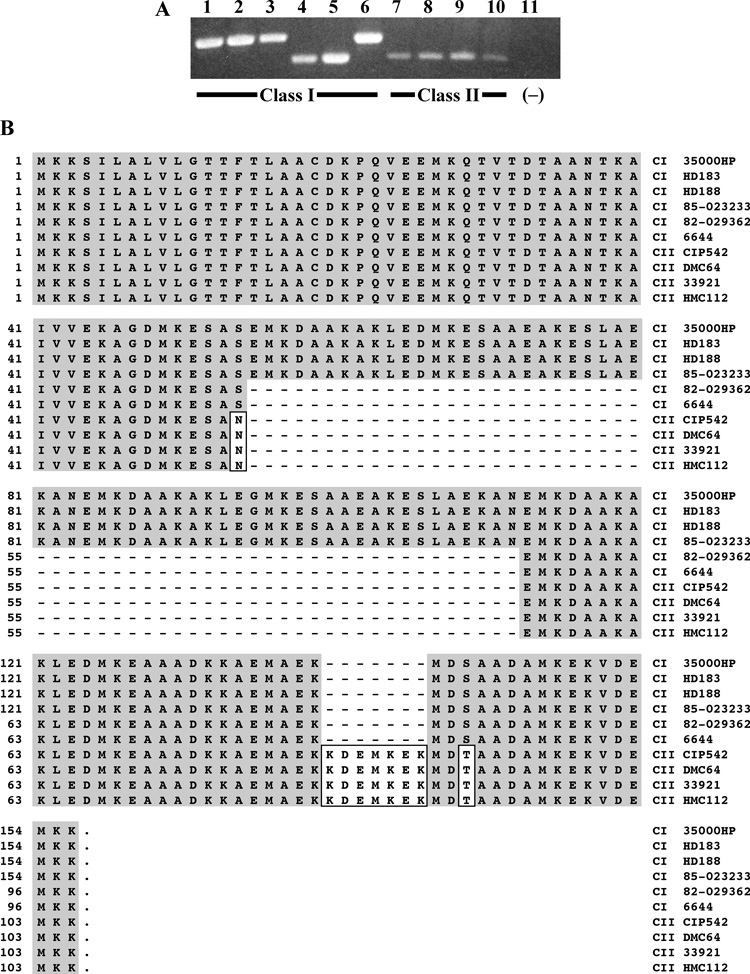
Two phenotypic classes of H. ducreyi strains have been identified, based on differences in OMPs and LOS migration patterns [24]. To examine conservation of fgbA among strains of both classes, we PCR-amplified the fgbA locus from 10 clinical H. ducreyi isolates, including six class I and four class II strains (Table 1). fgbA-containing amplicons were obtained from all strains; however, amplicons from six strains migrated faster than that of 35000HP, indicating size variations in the fgbA loci (Fig. 3A).Sequencing showed that the faster migrating amplicons contained deletions within the repetitive motif of fgbA; however, no strains harbored frameshift mutations or premature stop codons (Fig. 3B).
Fig. 3. fgbA varied in size but was conserved among class I and class II clinical isolates of H. ducreyi.
(A) PCR amplicons of the fgbA locus from genomic DNA of clinical isolates listed in Table 1. Lanes 1–6, class I strains 35000HP, HD183, HD188, 82-029362, 6644, and 85-023233; lanes 7–10, class II strains CIP542 ATCC, DMC64, 33921, and HMC112. Lane 11, negative control (no template added). (B) Alignment of deduced FgbA sequences among H. ducreyi clinical isolates. Strain names are listed on the right-hand side. C I, class I strain; C II, class II strain. Shaded residues match those of 35000HP, boxed residues differ from those of 35000HP, and dashes indicate missing residues.
Class-specific changes were noted among the sequenced fgbA loci. Compared with fgbA of 35000HP, three class I strains had identical fgbA ORFs, and two strains contained a 58-amino acid deletion but were otherwise identical (Fig. 3B). This deletion removed the first two copies of the 29-amino acid repeat in 35000HP FgbA (Fig. 1B). No class I loci had changes in the sequenced regions outside the ORF (data not shown).
The four class II isolates tested were identical to each other and all contained the same 58-amino acid deletion observed in two of the class I strains (Fig. 3B). However, compared with the class I fgbA ORFs, the class II ORFs harbored two conservative amino acid substitutions and a 7-amino acid insertion (Fig. 3B). Upstream of the ORF, the class II strains harbored a 1-bp deletion (data not shown). Although this deletion did not affect the predicted promoter elements of fgbA, it did produce a frameshift mutation in the 5’ gene, HD0193. The class II strains also harbored a T–C base change 98 bp downstream of the 3’ end of the fgbA ORF that was outside the predicted Rho-independent terminator and HD0191 (Fig. 1A). Overall, the fgbA ORF was conserved among class I and class II strains of H. ducreyi but with differences in the length of the repetitive motif.
DISCUSSION
In this study, we identified a novel Fg-binding protein, FgbA, that contributes significantly to the disease process of H. ducreyi in human volunteers. We originally identified fgbA among H. ducreyi transcripts expressed during human infection [14] and, because of its predicted outer membrane location, we hypothesized that FgbA is involved in virulence. Using the human model of H. ducreyi infection, we demonstrated that an isogenic fgbA mutant, although able to cause pustules, did so at a significantly lower rate than that of 35000HP; thus, 35000HPfgbA was partially attenuated [9, 13]. Biosafety considerations preclude testing a trans-complemented mutant in human subjects because of potential horizontal transfer of resistance plasmids to skin flora. With this caveat, the human challenge experiments demonstrate that FgbA is important for the organism’s virulence.
Our in vitro assays sought to identify a function for this novel virulence factor. The predicted coiled coil structure suggested that FgbA may interact with host proteins. H. ducreyi colocalizes with collagen and fibrin in vivo, and a collagen-specific adhesin has already been identified in H. ducreyi [4–6]. We therefore tested the ability of FgbA to bind Fg, the soluble precursor of fibrin. r-FgbA bound to Fg, and FgbA was subsequently identified as the major H. ducreyi protein binding Fg in the ligand blot assay. Although FgbA bears no sequence homology with other known Fg binding proteins, several other extracellular pathogens, including Staphylococcal and Streptococcal species, express Fg binding proteins with coiled coils and repetitive motifs [38–40]. Another notable feature of FgbA is the number of charged residues, which represent 45% of the mature peptide and confer a net charge of -7. Charged residues frequently indicate sites of protein-protein interaction; however, the large number of charges in FgbA is unusual. The role of these charged residues in interactions with Fg is being investigated.
The predicted outer membrane location of FgbA was based on sorting rules for E. coli lipoproteins, since lipoprotein sorting has not been well studied in H. ducreyi [17, 19, 20]. Consistent with an outer membrane location, Fg-binding activity was observed in sarkosyl-insoluble protein preparations, which are enriched for OMPs (Fig. 2B); however, this is not proof of outer membrane location. Further, even if anchored to the outer membrane by its lipid portion, the protein portion of FgbA could face either the periplasm or the external milieu. Thus, additional work is needed to define the location and possible surface exposure of FgbA.
The fgbA-containing locus is conserved among class I and class II strains of H. ducreyi. Although the strains differed in the lengths of their ORFs, all strains maintained an intact fgbA ORF. This conservation among clinical isolates supports the conclusion that fgbA is important for H. ducreyi. The major difference in the deduced FgbA sequences was the length of the repetitive coiled coil region. Despite the smaller size, the strains with deletions relative to 35000HP were still predicted to form coiled coils. Whether these deletions, or the class II-specific amino acid changes, affect the capacity to bind Fg is under investigation.
A BLASTP search with FgbA or its 29-amino acid repetitive motif showed homology with hypothetical proteins in several Pasteurellaceae, including Actinobacillus pleuropneumoniae Aple 020001366, H. somnus HS1338, Pasteurella multocida PM0442, and NTHI1667 from nontypeable H. influenzae 86-028NP. All four homologs are predicted lipoproteins of 15–22 kDa with pI of 4.6–4.9 containing a disproportionate number of charged amino acids and a net negative charge. The C-terminal portions of these homologs encoded 3–5 direct, tandem repeats of 22–29 amino acids Thus, FgbA may represent a family of lipidated virulence factors in several pathogenic genera of the Pasteurellaceae.
What role might FgbA play for H. ducreyi? Although not a component of normal skin, Fg transudates from serum into H. ducreyi-infected lesions and is cleaved to form a matrix of fibrin strands. Fg and fibrin are plentiful in H. ducreyi-infected lesions throughout the disease process [4, 5]. Thus, FgbA may act as an adhesin to anchor the bacterium to the fibrin matrix. Although the ligand blot assay used in this study does not define FgbA as an adhesin, the assay demonstrated that FgbA can bind Fg.
An alternative role of Fg binding proteins in pathogenesis is to protect extracellular bacteria from phagocytosis, as exemplified by S. pyogenes M protein [39, 41]. Another possible role of Fg binding proteins is to occlude opsonization with antibodies or complement, although this function is controversial [42–45]. Because H. ducreyi associates with Fg in vivo and resists phagocytosis, we hypothesize that FgbA may be involved in adherence or antiphagocytic activity for H. ducreyi. Studies to detail the mechanism(s) by which FgbA contributes to virulence in vivo are underway.
Acknowledgments
Financial Support: This work was supported by Public Health Service grants R01 AI27863 (to S.M.S.), U19 AI31494 (to S.M.S.), K08 AI74657 (to D.M.J.), and T32 AI07637 (to D.M.J.) from the National Institute of Allergy and Infectious Disease within the National Institutes of Health, by a Research Support Funds Grant (M.E.B.) and a Biomedical Research Grant (M.E.B.) from Indiana University-Purdue University at Indianapolis, and by the Indiana Genomics Initiative of Indiana University (M.E.B.), which is supported in part by Lilly Endowment, Inc. The human challenge trials were also supported by NIH grant M01 RR00750 to the General Clinic Research Center at Indiana University.
We thank X. Frank Yang, Barbara Van Der Pol, and Kristy L. B. Mount for critical review of the manuscript and Sheila Ellinger for recruiting the volunteers who participated in the trial.
Footnotes
Potential conflicts of interest: no conflicts.
Presented in part: 108th General Meeting of the American Society for Microbiology, Boston, MA, June, 2008 (abstract B023).
References
- 1.Steen R. On eradicating chancroid. Bull. WHO. 2001;79:818–826. [PMC free article] [PubMed] [Google Scholar]
- 2.Spinola SM, Bauer ME, Munson RS., Jr Immunopathogenesis of Haemophilus ducreyi infection (chancroid) Infect. Immun. 2002;70:1667–1676. doi: 10.1128/IAI.70.4.1667-1676.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauer ME, Spinola SM. Localization of Haemophilus ducreyi at the pustular stage of disease in the human model of infection. Infect. Immun. 2000;68:2309–2314. doi: 10.1128/iai.68.4.2309-2314.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauer ME, Goheen MP, Townsend CA, Spinola SM. Haemophilus ducreyi associates with phagocytes, collagen, and fibrin and remains extracellular throughout infection of human volunteers. Infect. Immun. 2001;69:2549–2557. doi: 10.1128/IAI.69.4.2549-2557.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bauer ME, Townsend CA, Ronald AR, Spinola SM. Localization of Haemophilus ducreyi in naturally acquired chancroidal ulcers. Microb. Infect. 2006;8:2465–2468. doi: 10.1016/j.micinf.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Fulcher RA, Cole LE, Janowicz DM, et al. Expression of Haemophilus ducreyi collagen binding outer membrane protein NcaA is required for virulence in swine and human challenge models of chancroid. Infect. Immun. 2006;74:2651–2658. doi: 10.1128/IAI.74.5.2651-2658.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janowicz DM, Fortney KR, Katz BP, et al. Expression of the LspA1 and LspA2 proteins by Haemophilus ducreyi is required for virulence in human volunteers. Infect. Immun. 2004;72:4528–4533. doi: 10.1128/IAI.72.8.4528-4533.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bong CTH, Throm RE, Fortney KR, et al. A DsrA-deficient mutant of Haemophilus ducreyi is impaired in its ability to infect human volunteers. Infect. Immun. 2001;69:1488–1491. doi: 10.1128/IAI.69.3.1488-1491.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janowicz DM, Leduc I, Fortney KR, Katz BP, Elkins C, Spinola SM. A DltA mutant of Haemophilus ducreyi is partially attenuated in its ability to cause pustules in human volunteers. Infect. Immun. 2006;74:1394–1397. doi: 10.1128/IAI.74.2.1394-1397.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Tawfiq JA, Fortney KR, Katz BP, Elkins C, Spinola SM. An isogenic hemoglobin receptor-deficient mutant of Haemophilus ducreyi is attenuated in the human model of experimental infection. J. Infect. Dis. 2000;181:1049–1054. doi: 10.1086/315309. [DOI] [PubMed] [Google Scholar]
- 11.Fortney KR, Young RS, Bauer ME, et al. Expression of peptidoglycan-associated lipoprotein is required for virulence in the human model of Haemophilus ducreyi infection. Infect. Immun. 2000;68:6441–6448. doi: 10.1128/iai.68.11.6441-6448.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spinola SM, Fortney KR, Katz BP, et al. Haemophilus ducreyi requires an intact flp gene cluster for virulence in humans. Infect. Immun. 2003;71:7178–7182. doi: 10.1128/IAI.71.12.7178-7182.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banks KE, Fortney KR, Baker B, et al. The enterobacterial common antigen-like gene cluster of Haemophilus ducreyi contributes to virulence in humans. J. Infect. Dis. 2008;197:1531–1536. doi: 10.1086/588001. [DOI] [PubMed] [Google Scholar]
- 14.Bauer ME, Fortney KR, Harrison A, Janowicz DM, Munson RS, Jr, Spinola SM. Identification of Haemophilus ducreyi genes expressed during human infection. Microbiology. 2008;154:1152–1160. doi: 10.1099/mic.0.2007/013953-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP. 3.0. J. Mol. Biol. 2004;340:783–795. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 16.Gardy JL, Laird MR, Chen F, et al. PSORTb v.2.0: Expanded prediction of bacterial protein subcellular localization and insights gained from comparative proteome analysis. Bioinformatics. 2005;21:617–623. doi: 10.1093/bioinformatics/bti057. [DOI] [PubMed] [Google Scholar]
- 17.Gennity JM, Inouye M. The protein sequence responsible for lipoprotein membrane localization in Escherichia coli exhibits remarkable specificity. J. Biol. Chem. 1991;266:16458–16464. [PubMed] [Google Scholar]
- 18.Letunic I, Copley RR, Pils B, Pinkert S, Schultz J, Bork P. SMART 5: domains in the context of genomes and networks. Nucleic Acids Res. 2006;34:D257–D260. doi: 10.1093/nar/gkj079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terada M, Kuroda T, Matsuyama S, Tokuda H. Lipoprotein sorting signals evaluated as the LolA-dependent release of lipoproteins from the cytoplasmic membrane of Escherichia coli. J. Biol. Chem. 2001;276:47690–47694. doi: 10.1074/jbc.M109307200. [DOI] [PubMed] [Google Scholar]
- 20.Yamaguchi K, Yu F, Inouye M. A single amino acid determinant of the membrane localization of lipoproteins in E. coli. Cell. 1988;53:423–432. doi: 10.1016/0092-8674(88)90162-6. [DOI] [PubMed] [Google Scholar]
- 21.Al-Tawfiq JA, Thornton AC, Katz BP, et al. Standardization of the experimental model of Haemophilus ducreyi infection in human subjects. J. Infect. Dis. 1998;178:1684–1687. doi: 10.1086/314483. [DOI] [PubMed] [Google Scholar]
- 22.Hammond GW, Lian CJ, Wilt JC, Ronald AR. Antimicrobial susceptibility of Haemophilus ducreyi. Antimicrob. Agents Chemother. 1978;13:608–612. doi: 10.1128/aac.13.4.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spinola SM, Griffiths GE, Bogdan JA, Menegus MA. Characterization of an 18,000 molecular-weight outer membrane protein of Haemophilus ducreyi that contains a conserved surface-exposed epitope. Infect. Immun. 1992;60:385–391. doi: 10.1128/iai.60.2.385-391.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White CD, Leduc I, Olsen B, Jeter C, Harris C, Elkins C. Haemophilus ducreyi outer membrane determinants, including DsrA, define two clonal populations. Infect. Immun. 2005;73:2387–2399. doi: 10.1128/IAI.73.4.2387-2399.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deneer HG, Slaney L, Maclean IW, Albritton WL. Mobilization of nonconjugative antibiotic resistance plasmids in Haemophilus ducreyi. J. Bacteriol. 1982;149:726–732. doi: 10.1128/jb.149.2.726-732.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Post DM, Gibson BW. Proposed second class of Haemophilus ducreyi strains show altered protein and lipooligosaccharide profiles. Proteomics. 2007;7:3131–3142. doi: 10.1002/pmic.200600830. [DOI] [PubMed] [Google Scholar]
- 27.Hiltke TJ, Bauer ME, Klesney-Tait J, Hansen EJ, Munson RS, Jr, Spinola SM. Effect of normal and immune sera on Haemophilus ducreyi 35000HP and its isogenic MOMP and LOS mutants. Microb. Pathog. 1999;26:93–102. doi: 10.1006/mpat.1998.0250. [DOI] [PubMed] [Google Scholar]
- 28.McDevitt D, Nanavaty T, House-Pompeo K, et al. Characterization of the interaction between the Staphylococcus aureus clumping factor (ClfA) and fibrinogen. Eur. J. Biochem. 1997;247:416–424. doi: 10.1111/j.1432-1033.1997.00416.x. [DOI] [PubMed] [Google Scholar]
- 29.Menard R, Sansonetti PJ, Parsot C. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J. Bacteriol. 1993;175:5899–5906. doi: 10.1128/jb.175.18.5899-5906.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bozue JA, Tarantino L, Munson RS., Jr Facile construction of mutations in Haemophilus ducreyi using lacz as a counter-selectable marker. FEMS Microbiology Letters. 1998;164:269–273. doi: 10.1111/j.1574-6968.1998.tb13097.x. [DOI] [PubMed] [Google Scholar]
- 31.Wood GE, Dutro SM, Totten PA. Target cell range of Haemophilus ducreyi hemolysin and its involvement in invasion of human epithelial cells. Infect. Immun. 1999;67:3740–3749. doi: 10.1128/iai.67.8.3740-3749.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crother TR, Champion CI, Wu X-Y, Blanco DR, Miller JN, Lovett MA. Antigenic composition of Borrelia burgdorferi during infection of SCID mice. Infect. Immun. 2003;71:3419–3428. doi: 10.1128/IAI.71.6.3419-3428.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Decker ED, Zhang Y, Cocklin RR, Witzmann FA, Wang M. Proteomic analysis of differential protein expression induced by ultraviolet light radiation in HeLa cells. Proteomics. 2003;3:2019–2027. doi: 10.1002/pmic.200300473. [DOI] [PubMed] [Google Scholar]
- 34.Brown DPG, Gökmen-Polar Y, Jiang L, et al. A comparative proteomic study to characterize the vinblastine resistance in human ovarian cancer cells. Proteomics Clin. Appl. 2007;1:18–31. doi: 10.1002/prca.200600171. [DOI] [PubMed] [Google Scholar]
- 35.Delahay RM, Frankel G. Coiled-coil proteins associated with type III secretion systems: a versatile domain revisited. Mol. Microbiol. 2002;45:905–916. doi: 10.1046/j.1365-2958.2002.03083.x. [DOI] [PubMed] [Google Scholar]
- 36.Hovis KM, Freedman JC, Zhang H, Forbes JL, Marconi RT. Identification of an antiparallel coiled-coil/loop domain required for ligand binding by the Borrelia hermsii FgbA protein: additional evidence for the role of FhbA in the host-pathogen interaction. Infect. Immun. 2008;76:2113–2122. doi: 10.1128/IAI.01266-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McNamara C, Zinkernagel AS, Macheboeuf P, Cunningham MW, Nizet V, Ghosh P. Coiled-coil irregularities and instabilities in group A Streptococcus M1 are required for virulence. Science. 2008;319:1405–1408. doi: 10.1126/science.1154470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meehan M, Muldowney DA, Watkins NJ, Owen P. Localization and characterization of the ligand-binding domain of the fibrinogen-binding protein (FgBP) of Streptococcus equi subsp. equi. Microbiology. 2000;146:1187–1194. doi: 10.1099/00221287-146-5-1187. [DOI] [PubMed] [Google Scholar]
- 39.Ringdahl U, Svensson HG, Kotarsky H, Gustafsson M, Weineisen M, Sjobring U. A role for the fibrinogen-binding regions of streptococcal M proteins in phagocytosis resistance. Mol. Microbiol. 2000;37:1318–1326. doi: 10.1046/j.1365-2958.2000.02062.x. [DOI] [PubMed] [Google Scholar]
- 40.Schubert A, Zakikhany K, Schreiner M, et al. A fibrinogen receptor from group B Streptococcus interacts with fibrinogen by repetitive units with novel ligand binding sites. Mol. Microbiol. 2002;46:557–569. doi: 10.1046/j.1365-2958.2002.03177.x. [DOI] [PubMed] [Google Scholar]
- 41.Celli J, Finlay BB. Bacterial avoidance of phagocytosis. Trends in Microbiol. 2002;10:232–237. doi: 10.1016/s0966-842x(02)02343-0. [DOI] [PubMed] [Google Scholar]
- 42.Whitnack E, Beachey EH. Antiopsonic activity of fibrinogen bound to M protein on the surface of group A streptococci. J. Clin. Invest. 1982;69:1042–1045. doi: 10.1172/JCI110508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whitnack E, Beachey EH. Inhibition of complement-mediated opsonization and phagocytosis of Streptococcus pyogenes by D fragments of fibrinogen and fibrin bound to cell surface M protein. J. Exp. Med. 1985;162:1983–1997. doi: 10.1084/jem.162.6.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boschwitz JS, Timoney JF. Characterization of the antiphagocytic activity of equine fibrinogen for Streptococcus equi subsp. equi. Microb. Pathog. 1994;17:121–129. doi: 10.1006/mpat.1994.1058. [DOI] [PubMed] [Google Scholar]
- 45.Campo RE, Schultz DR, Bisno AL. M proteins of group G streptococci: mechanisms of resistance to phagocytosis. J. Infect. Dis. 1995;171:601–606. doi: 10.1093/infdis/171.3.601. [DOI] [PubMed] [Google Scholar]