Abstract
Background and purpose
Restless Legs Syndrome (RLS) is a common sensorimotor disorder often associated with significant chronic sleep loss. Previous studies looking at the effects of sleep loss on daytime function in RLS individuals, using subjective reporting techniques have yielded mixed results. In this study we used more objective measures of alertness and compared RLS subjects who are off treatments and chronically sleep restricted to chronic sleep-restricted controls.
Subjects and Methods
The final sample consisted of 20 RLS subjects (10 male and 10 female) and 13 sleep-restricted controls (seven male and six female). Thirteen controls underwent a 14-day chronic partial sleep-restriction protocol in order to closely match the degree of chronic sleep loss reportedly experienced by untreated RLS patients. On the final day of the protocol each subject performed a morning and evening Suggested Immobilization Test (SIT) which served as a modified Maintenance of Wakefulness Test (MWT). RLS and control groups were compared for differences in alertness as measured objectively by the sleep latency on the morning and evening SITs.
Results
The RLS subjects had a longer sleep latency on the morning and evening SIT than controls (t = 3.80, p = .001, U = 31.0, p < .001, respectively). Even after controlling for the potential arousal impact associated with increased leg activity, RLS individuals still demonstrated a higher degree of objective alertness (p = 0.023, p = 0.006, Fisher's exact test).
Conclusions
RLS subjects, despite having, if anything, greater sleep loss, displayed greater sustained alertness than sleep-restricted controls. Thus, the heightened degree of alertness demonstrated by RLS patients may be in contrast to the perceived impairment in mood, vigor, and vigilance commonly reported in previous studies.
Keywords: Restless legs syndrome, Sleep deprivation, Chronic sleep restriction, Polysomnogram, Sleep Latency, Suggested Immobilization Test (SIT)
Introduction
Restless Legs Syndrome is a common sensorimotor sleep disorder that affects approximately 7-10% of the general population (1). Individuals with moderately severe Restless Legs Syndrome (RLS) commonly exist in a chronic sleep restricted state, often obtaining an average of three to five hours of sleep nightly (2). Only a limited number of studies have looked at alertness in the moderately severe RLS population specifically, and the results have been mixed. One large epidemiological study found significantly higher complaints of increased daytime fatigue and decreased vigor on the SF-36 for a population judged to have clinically significant RLS symptoms compared to normal controls (1). Another population-based study of RLS patients seeking medical treatment for their symptoms showed similar findings (3). However, other studies using different subjective sleep questionnaires evaluating RLS patients seeking treatment at a medical clinic found no significant differences in the degree of sleepiness between the RLS and controls (4)(5). All of these studies used only questionnaires to evaluate the impact of RLS on daytime function. In addition to daytime sleepiness, RLS subjects frequently endorse other neuropsychiatric complaints including depression, anxiety, and cognitive impairment (6)(7). One model of the causal relation for RLS co-morbidities indicated that the sleep loss and non severity of RLS accounted for these subjectively reported co-morbidities of RLS (8). In order to examine the impact that chronic sleep has on RLS individuals' daytime function, we compared RLS subjects' alertness on a SIT performance to the performance of similarly sleep restricted normal controls.
The SIT was originally developed to evaluate RLS severity by assessing leg sensations and periodic leg movements while awake (PLMW)(9). Since the SIT test requires maintained alertness, it monitors the sleep-wake state based on EEG and also records the EMG for leg activity. Thus, the SIT test can serve as a modified Maintenance of Wakefulness Test (MWT) (10)(11). Like the MWT, the SIT is conducted under soporific conditions and the subject is directed to stay awake with specific instruction that they refrain from voluntarily moving their legs during the test. In order to simulate the conditions of the MWT, the customary repeated sensory report on a visual analog scale was not used. Thus, the patients were not disturbed during the test except they were to stay awake and remain immobile. In this study RLS subjects are compared to sleep-restricted control subjects on their ability to maintain alertness based on the sleep latency (as used in the MWT) from the Suggested Immobilization Test (SIT). Based on our clinical observations and on those reported by others (5)(4), we hypothesized that RLS subjects with reduced sleep times despite being off medications will display longer sleep latencies on the SIT compared to controls who have had a similar degree of reduced sleep times.
Methodological Approach
RLS data were collected from subjects as part of a large study. All RLS medications were stopped at least 14 days prior to the current reported study. Appropriate controls were recruited separately to match RLS subjects for age and gender. All subjects consented to this Institutional Review Board (IRB) approved study prior to participation. Both control and RLS subjects were initially screened with several questionnaires including medical questionnaires, sleep questionnaires (Pittsburgh Sleep Quality Inventory) (12), RLS diagnostic questionnaires, leg activity meters and when applicable, a home apnea monitor.
All subjects (control & RLS) with a BMI of >27.5 were given an ambulatory apnea monitor and were excluded if they had significant apnea (≥ 15/hour), and leg activity monitors were worn by the controls to exclude sleep disruptions secondary to PLMD. The control subjects at baseline had to report an average sleep duration of seven hours between the hours of 9pm and 9am in order to exclude short sleepers and individuals with Circadian rhythm disturbance and chronic insufficient sleep. All with a PSQI score greater than five were also excluded. The PSQI is a validated screen of “good” and “poor” sleepers based on a global estimate across one month that includes items related to daytime sleepiness and function and has been utilized to screen for sleepiness related to primary sleep disorders including circadian Rhythm Disorders and insomnia (13, 14).
Face-to-face interviews included completion of the RLS diagnostic interview and review of the medical history and consent form. A “definite not RLS” (control) was determined during screening by telephone interviews using the Johns Hopkins Telephone diagnostic interview. All subjects enrolled in the study had a second interview at the admission to the General Clinical Research Center (GCRC) by a separate investigator to verify the diagnosis.
Sleep-Restriction Protocol: Sleep Restriction in the RLS subjects was by report while at home and by sleep logs while in the GCRC. All RLS patients were on medication prior to the study. Medication was stopped 14 days prior to admission to the GCRC. At the inpatient intake evaluation, the subjects were interviewed by one of the co-authors who is a sleep specialist. All RLS subjects reported minimal or no sleep for the first 48 hours after stopping their treatment medications. No subject had more than four hours sleep by day seven after medication withdrawal, which corresponds to reports in the literature of untreated RLS patients obtaining approximately three to five hours of sleep nightly (15). On admission to GCRC, all of the RLS subjects reported less than six hours of sleep nightly. Therefore, it was estimated that the RLS subjects obtained slightly less than five hours per night on average over the course of 14 days. Sleep Restricted Control subjects (SRCs) restricted their sleep to a maximum of six hours per night at home, with no daytime naps, for 12 days. We could not restrict the sleep further because of IRB concerns regarding safety. SRCs were contacted daily and provided with transportation if they reported pathological sleepiness on the Epworth Sleepiness scale (≥ 11 out of 24). Sleep-wake duration was monitored using daily sleep-wake logs, body-position and wrist-activity monitors in order to monitor compliance. Control subjects were not allowed to continue with the admission to the GCRC if their activity and body position meters did not show they had complied with the sleep-restriction. Thus, on day 13, upon admission to the GCRC, the sleep-restricted control (SRC) was disqualified from the study if there was >20% non-compliance (from the maximum daily six hour sleep time) on average over the 12 day period shown on any one of the three monitoring tools. During the 2½ -day inpatient stay, the SRCs underwent a protocol similar to that experienced by the RLS subjects, which included four (two morning and two evening) suggested immobilization tests (SIT) and two polysomnograms. Sleep latency on the SIT was considered to be the first epoch of any stage of sleep. During the inpatient stay, the SRC's sleep was restricted to five hours nightly, matching that observed for the RLS patients.
SITs were performed twice daily on two consecutive days: each morning starting between 5:00 and 6:30 am and each evening starting between 10:00 and 10:30 pm. To potentially avoid a first-night effect as has been reported with PSG, only the second set of morning and evening SITs were used to assess alertness.
Statistics
The differences between control and RLS subjects on sleep latencies were evaluated using t and Mann-Whitney U tests.
Results
Thirteen SRCs (six women, seven men) and 20 RLS subjects (10 women, 10 men) completed the study. The SRCs did not differ from RLS subjects in age (Mean ± SD: 58.1 ± 8.4 and 63.1 ± 10.9, respectively; t = 1.39, p = 0.17). RLS and SRCs were matched for gender (χ2 = 0.47, p = 0.83) and total sleep time during the second PSG (Mean ± SD, 290.7 ± 87.6 and Mean ± SD, 281.3 ± 16.5, respectively; t = 0.38, p = 0.71). As expected, based on the nature of the syndrome, the RLS subjects had a significantly higher number of leg movements compared to controls (Mean ± SD, 37.5 ± 60.0 and Mean ± SD, 33.8 ± 62.2, respectively; t = 120, p = 0.91) on the evening SIT, with no significant difference for the two groups on the morning SIT (Table 1). The analyses revealed statistically significant differences between both morning and evening sleep latencies (Table 1). Specifically, RLS subjects had a longer sleep latency on the morning SIT than did the SRCs (Mean ± SD: 57.3 ± 8.1 and 33.7 ± 25.4, respectively; t = 3.80, p = .001). RLS subjects also had a longer sleep latency on the evening SIT than did SRCs (Mean ± SD: 53.9 ± 15.0 and 21.0 ± 22.7, respectively; U = 31.0, p < .001).
Table 1.
Means and Standard Deviations for the sleep latency during the SIT.
| Tests | Sleep-restricted Control | RLS | t or U | |
|---|---|---|---|---|
| M (SD) | M (SD) | t | p | |
| Sleep Latency Morning | 33.7 (25.4) | 57.3 (8.1) | 3.80** | 0.001 |
| U | p | |||
| Sleep Latency Night | 21.0 (22.7) | 53.9 (15.0) | 31.0** | 0.000 |
| PLMW during Morning SIT | 33.8 (62.2) | 37.5 (60.0) | 120 | 0.91 |
| PLMW during Evening SIT | 15.5 (22.5) | 97.4 (86.3) | 45.5** | 0.001 |
p < .05;
p < .01
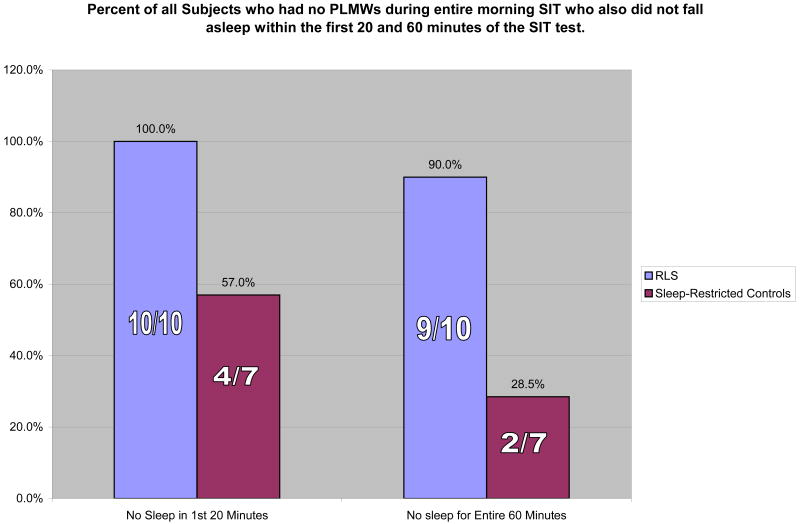
Due to the circadian pattern of RLS, most subjects experience a symptom-free period in the morning. In order to control for the potential arousal impact associated with increased leg activity, an additional analysis was conducted looking at only those RLS and SRCs who had no PLMWs during the entire morning SIT (Figure 1). Of 10 RLS participants and seven SRCs without PLMWs on the am SIT, three of the controls fell asleep within the first 20 minutes and another two fell asleep within 60 minutes. Of the RLS subjects without PLMWs, none fell asleep in the first 20 minutes and only one fell asleep within 60 minutes (p = 0.023 and p = 0.006, respectively, Fisher's' exact test).
Figure 1. Percent of all Subjects who had no PLMWs during entire morning SIT who also did not fall asleep within the first 20 and 60 minutes of the SIT test.
Discussion
To our knowledge, this is the first study to objectively compare the alertness of untreated sleep-deprived RLS subjects to a similarly sleep-deprived control group. This ability of RLS subjects to maintain alertness as demonstrated under these testing conditions does not appear to be an artifact of the potential arousal impact of leg kicks. Thus, sleep deprived RLS individuals are better able to maintain wakefulness independent of movements producing arousals. In order to simulate an MWT-like condition, we did not conduct VASs every 10 minutes during the SIT. For this reason, the contribution that sensory symptoms may have played toward the maintenance of alertness was not assessed and therefore represents one of the primary drawbacks for this pilot study. In the future, the two (RLS and control) groups should undergo the same amount of sleep restriction and be placed under the same conditions, particularly since the impact of sleep deprivation due to chronic sleep fragmentation appears to be more detrimental than an undisturbed period of restricted sleep (16). For this study we were limited by IRB restrictions of only six hours of sleep as an outpatient for control subjects, but under the inpatient condition we were able to match both RLS and controls to five hours per night. Under our current research design, the RLS subjects endured, if anything, greater sleep loss than controls, which makes the RLS subjects' performance on the SIT test even more striking.
Our findings support the Saletu and Bassetti reports that RLS subjects often do not report significant daytime sleepiness despite chronic sleep loss. Perhaps the mixed results seen in the literature may result from semantics (4)(5). Although Bassetti reported primarily normal scores on the Epworth sleepiness scale for his 55 patients (4), these same patients also reported a significantly high degree of daytime fatigue, which corresponds with the lack of vigor reported in the Allen and Abetz studies (1)(3). In addition, all of the previous studies surveyed subjects whose RLS may have been treated or untreated so the impact of medications on daytime function may have also been a factor. Thus, our assessment of RLS subjects withdrawn from all medication provides a unique opportunity to evaluate the daytime function of RLS subjects under conditions free of drug treatment.
Our findings are consistent with the view that the RLS disease state itself appears to enhance alertness in the face of sleep loss, and thus the RLS patient has less sleepiness than would be expected for the degree of sleep loss. This is in contrast to findings reported in Parkinson's disease, another neurological disorder like RLS that is associated with hypodopaminergic function. Parkinson's disease is strongly associated with increased daytime sleepiness independent of drug treatments (17)(18). So, despite the common beneficial response to dopaminergic treatments seen in these two conditions, PD and RLS appear to demonstrate markedly different effects on sleep-wake mechanisms. This may reflect differences in the nature of any underlying pathologies and, in particular, may indicate differences in status of the dopaminergic arousal system in the two disorders. In this respect RLS provides an interesting and unique model for exploring some of the neurobiology contributing to alertness in the face of sleep deprivation. Future studies examining the relationship of disease onset, severity, and symptom features are warranted to explore this concept further.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Allen RP, Walters A, Montplaisi J, Hening W, Myers A, Bell TJ, et al. Restless legs syndrome prevalence and impact: REST general population study. Arch Intern Med. 2005;165(11):1286–92. doi: 10.1001/archinte.165.11.1286. [DOI] [PubMed] [Google Scholar]
- 2.Allen RP, Earley CJ. Restless legs syndrome: a review of clinical and pathophysiologic features. J Clin Neurophysiol. 2001;18(2):128–47. doi: 10.1097/00004691-200103000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Abetz L, Allen R, Follet A, Washburn T, Early C, Kirsch J, et al. Evaluating the quality of life of patients with restless legs syndrome. Clinical Therapeutics. 2004;26(6):925–35. doi: 10.1016/s0149-2918(04)90136-1. 2004/6. [DOI] [PubMed] [Google Scholar]
- 4.Bassetti CL, Mauerhofer D, Gugger M, Mathis J, Hess CW. Restless legs syndrome: a clinical study of 55 patients. Eur Neurol. 2001;45(2):67–74. doi: 10.1159/000052098. [DOI] [PubMed] [Google Scholar]
- 5.Saletu M, Anderer P, Saletu B, Lindeck-Pozza L, Hauer C, Saletu-Zyhlarz G. EEG mapping in patients with restless legs syndrome as compared with normal controls. Psychiatry Res. 2002 Aug 20;115(12):49–61. doi: 10.1016/s0925-4927(02)00023-9. [DOI] [PubMed] [Google Scholar]
- 6.Hornyak M, Kopasz M, Berger M, Riemann D, Voderholzer U. Impact of sleep-related complaints on depressive symptoms in patients with restless legs syndrome. J Clin Psychiatry. 2005 Sep;66(9):1139–45. doi: 10.4088/jcp.v66n0909. [DOI] [PubMed] [Google Scholar]
- 7.Ulfberg J, Nystrom B, Carter N, Edling C. Prevalence of restless legs syndrome among men aged 18 to 64 years: an association with somatic disease and neuropsychiatric symptoms. Mov Disord. 2001 Nov;16(6):1159–63. doi: 10.1002/mds.1209. [DOI] [PubMed] [Google Scholar]
- 8.Atkinson MJ, Allen RP, DuChane J, Murray C, Kushida C, Roth T. Validation of the Restless Legs Syndrome Quality of Life Instrument (RLS-QLI): findings of a consortium of national experts and the RLS Foundation. Qual Life Res. 2004 Apr;13(3):679–93. doi: 10.1023/B:QURE.0000021322.22011.d0. [DOI] [PubMed] [Google Scholar]
- 9.Michaud M, Paquet J, Lavigne G, Desautels A, Montplaisir J. Sleep laboratory diagnosis of restless legs syndrome. Eur Neurol. 2002;48(2):108–13. doi: 10.1159/000062996. [DOI] [PubMed] [Google Scholar]
- 10.Sangal RB, Thomas L, Mitler MM. Maintenance of wakefulness test and multiple sleep latency test. Measurement of different abilities in patients with sleep disorders [see comments] Chest. 1992 Apr;101(4):898–902. doi: 10.1378/chest.101.4.898. [DOI] [PubMed] [Google Scholar]
- 11.Doghramji K, Mitler MM, Sangal RB, Shapiro C, Taylor S, Walsleben J, et al. A normative study of the maintenance of wakefulness test (MWT) Electroencephalogr Clin Neurophysiol. 1997 Nov;103(5):554–62. doi: 10.1016/s0013-4694(97)00010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989 May;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 13.Buysse DJ. Diagnosis and assessment of sleep and circadian rhythm disorders. J Psychiatr Pract. 2005 Mar;11(2):102–15. doi: 10.1097/00131746-200503000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Fictenberg NL, Putnam SH, Mann NR, Zafonte RD, Millard AE. Insomnia screening in postacute traumatic brain injury: utility and validity of the Pittsburgh Sleep Quality Index. Am J Phys Med Rehabil. 2001 May;80(5):339–45. doi: 10.1097/00002060-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Borreguero D, Larrosa O, de la Llave Y, Granizo JJ, Allen R. Correlation between rating scales and sleep laboratory measurements in restless legs syndrome. Sleep Med. 2004 Nov;5(6):561–5. doi: 10.1016/j.sleep.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2005 Mar;25(1):117–29. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- 17.Dhawan V, Dhoat S, Williams AJ, Dimarco A, Pal S, Forbes A, et al. The range and nature of sleep dysfunction in untreated Parkinson's disease (PD). A comparative controlled clinical study using the Parkinson's disease sleep scale and selective polysomnography. J Neurol Sci. 2006 Oct 25;248(12):158–62. doi: 10.1016/j.jns.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Ondo WG, Dat Vuong K, Khan H, Atassi F, Kwak C, Jankovic J. Daytime sleepiness and other sleep disorders in Parkinson's disease. Neurology. 2001 Oct 23;57(8):1392–6. doi: 10.1212/wnl.57.8.1392. [DOI] [PubMed] [Google Scholar]