Abstract
Transplantation of neural precursor cells has been proposed as a possible approach for replacing missing or damaged central nervous system myelin. Neonatal and adult myelin-deficient shiverer (shi) mice, bearing a mutation of the myelin basic protein (MBP) gene, have been used extensively as hosts for testing cell engraftment, migration, and myelination, but relatively little progress has been made in reversing shi motor deficits. Here we describe a prenatal cell replacement strategy, showing that embryonic stem cells injected into shi blastocyst embryos can generate chimeric mice with strong and widespread immunoreactive MBP expression throughout the brain and a behavioral (motor) phenotype that appears essentially rescued.
Keywords: Stem cells, Myelin basic protein, X-gal histochemistry, Blastocyst injection, Chimera, Oligodendrocytes, Shiverer mice, White matter
1. Introduction
The prospect that repair of the damaged central nervous system (CNS) might be achieved by exogenous cell replacement has generated much recent excitement. Therapeutic efficacy will surely depend on the identity of both the transplanted cell type and the local host environment, and animal models are proving valuable for assessing the possible influences of these factors.
One powerful model has been the mutant mouse shiverer (shi), bearing a large deletion in the myelin basic protein (MBP) gene [1]. Homozygous shi mice fail to produce the four “classic” isoforms of MBP, and their CNS shows extensive dysmyelination [2]. Shi mice exhibit severe tremor beginning at about postnatal day 10, later develop hindlimb ataxia, and survive for only a few months. Both the morphological and motor phenotype of shi mice can be genetically rescued by expression of the wild-type MBP transgene [3].
Shi mice are a useful transplant host because any myelin produced by engrafted wide-type donor cells will be MBP-positive and can be unequivocally identified by light microscopic immunocytochemistry. Neonatal and adult shi mice have been transplanted with oligodendrocytes; oligodendrocyte progenitors; neural “stem” cells, either freshly-dissected, immortalized, or growth-factor expanded in vitro; non-neural precursor cells; and embryonic stem (ES) cell-derived progenitors by injection into CNS parenchyma or ventricles [for reviews, references, and some recent examples, see 4–9]. The cells engraft, migrate, and myelinate, but – with the exception of two reports describing diminished tremor [10] and somewhat improved rotarod performance [11] – fail to rescue the mutant’s motor deficits. Most recently, Windrem et al. [12] have reported some success by first generating immunodeficient shi mice, using sorted fetal human glial progenitor cells, and implanting the cells into five cerebral and cerebellar sites in pups within a day of birth. There was prolonged survival in 23% of the transplanted mice, with two-thirds of these survivors apparently rescued.
To our knowledge, a prenatal cell replacement strategy has been reported only once, in which aggregation chimeras were created using wild-type and shi embryos at the 8-cell stage; although few in number, the resulting animals showed patches of MBP-positive and -negative axons intermixed within white matter [13]. Here we show that ES cells injected into shi blastocyst embryos can generate chimeric mice with strong and widespread immunoreactive MBP expression throughout the brain and a motor phenotype that appears essentially rescued.
2. Materials and Methods
2.1. Animals and blastocyst injections
Shi mice are maintained in our colony on a B6C3F1 hybrid-based stock, greater than 99.9% congenic at other loci. Twice per year, homozygous shi males are outcrossed to B6C3F1 wild-type females to produce unaffected heterozygous shi breeders for the next generation. For the current study, homozygous shi offspring were identified by their pronounced tremor and ataxia. Blastocysts from homozygous intercrosses were flushed from the uteri of shi females 3.5 days postcoitum. Each shi blastocyst was injected with 10 to 12 ES cells (Omnibank no. OST32815) bearing a retroviral promoter trap insertion that functionally inactivates one allele of the Ini1 gene; expression of a reporter β-galactosidase (β-gal)-neomycin gene fusion cassette within the retroviral insertion is regulated by the Ini1 promoter [14]. Ten ES cell-injected blastocysts were surgically implanted into the uterine horn of a pseudo-pregnant Swiss Webster dam, and pups were delivered about 17 days later. All animal procedures were approved by the University of Massachusetts Institutional Animal Care and Use Committee.
Homozygous shi mice genetically rescued with the wild-type MBP transgene (shi/shi; MBP/MBP) were initially a gift of Carol Readhead [3], and they have been bred onto the same hybrid background on which the shi mice are maintained. Ini1-heterozygous mice were created and maintained in our colony as previously described [14].
2.2. Phenotype assessment
Pups were assessed at least every other week from 3 to 4 weeks of age until 9 weeks at time of sacrifice by one of us (S.B.-G.) experienced in the handling and observation of developing shi and normal mice. Animals were scored on the presence of tremor and ataxia, with 0 as “absent,” 1 as “barely detectable,” 2 as “mild-moderate,” and 3 as “severe.” For reporting purposes, we categorized the motor phenotype of each mouse at sacrifice as: “rescued” if it was scored 0 – tremor and 0 – ataxia (abbreviated 0,0), “essentially rescued” (0,1) or (1,0), “not rescued” (3,3), and “intermediate” (all other combinations).
2.3. Animals sacrifice and brain assessment
Mice were deeply anesthetized with ketamine (25 mg/kg IP) and xylazine (5 mg/kg IP) and perfused with ice-cold heparinized phosphate-buffered saline (PBS), followed by ice-cold 4% buffered paraformaldehyde fixative. Brains were removed, post-fixed for 4 h, and 50 μm thick coronal sections were cut on a vibratome, or saturated with 20% sucrose and cut on a freezing microtome, and stored at −20°C in cryoprotectant (30% sucrose, 30% ethylene glycol, 0.25 mM polyvinylpyrrolidone in PBS).
For X-gal histochemistry to detect β-gal activity, free-floating sections were rinsed in PBS and incubated in a solution of 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, 2 mM magnesium chloride, 0.01% deoxycholic acid, 0.02% Nonidet P-40, and 1 mg/ml 5-bromo-4-chloro-3-indolyl-beta-D-galactopyranoside (X-gal; Gold Biotechnology, St. Louis, MO) overnight at 37°C. After rinsing in PBS and water, the sections were counterstained with Nuclear Fast Red (Vector Laboratories, Burlingame, CA), rinsed in water, mounted onto slides, dried, and coverslipped in Permount. For MBP immunofluorescence, free-floating sections were rinsed in Tris-buffered saline (TBS), blocked in 10% normal goat serum and 0.4% Triton X-100 in TBS for 30 min to 1 h, followed by incubation with anti-MBP antibody (mouse; 1:1000; Covance, Berkeley, CA) diluted in 2% normal goat serum and 0.4% Triton X-100 in TBS overnight at 4°C. After washing, sections were incubated with Alexa Fluor secondary antibody (1:200; Invitrogen/Molecular Probes, Eugene, OR) for 2 h at room temperature in the dark, washed, and cover-slipped in Prolong anti-fade reagent (Invitrogen/Molecular Probes).
For cellular double labeling, free-floating sections were first treated with 1% hydrogen peroxide for 20 min, blocked with 10% normal goat serum and 0.4% Triton X-100 in TBS for 1 h, and incubated with antibodies directed against either Olig-1 (rabbit; 1:200; Chemicon International, Temecula, CA), glial fibrillary acidic protein (GFAP) (rabbit; 1:1000; MP Biomedicals, Aurora, OH), or NeuN (mouse; 1:1000; Chemicon International) diluted in 2% normal goat serum and 0.4% Triton X-100 in TBS overnight at 4°C. After rinsing in TBS, sections were treated with the appropriate biotinylated secondary antibodies (1:500; Vector Laboratories) for 1 h and the avidin-biotin method, with diaminobenzidine (Sigma-Aldrich, Milwaukee, WI) as the chromogen. After rinsing in TBS, sections were then subjected to X-gal histochemistry, as described above. For dual histochemical X-gal and immunofluorescent MBP labeling, sections were treated first for X-gal (omitting the counterstaining step) and second for MBP.
2.4. Genotype assessment
Genomic DNA was isolated from brain sections using a Genomic DNA Isolation Kit (Lamda Biotech, St. Louis, MO) according to manufacturer’s instructions. The presence of wild-type (WT) or shi alleles in the brain samples was detected using two sets of specific primers in a polymerase chain reaction (PCR), as previously described [15]. One set of oligonucleotide primers amplified a 169-base-pair (bp) fragment from WT mouse DNA: 5′-AGCTCTGGTCTTTCTTGCAG-3′ and 5′-CCCCGTGGTAGGAATATTACATAAC-3′. The second set of primers amplified a 380-bp fragment unique to shi DNA: 5′-CAGGGGATGGGGAGTCAGAAGTGAG-3′ and 5′-ATGTATGTGTGTGTGTGCTTATCTAGTGTA-3′. PCR was performed using 0.4 μM of both primer sets, 400 μM dNTP, 2.0 mM MgCl2, 2.5U AmpliTaq DNA polymerase (Applied Biosystems, Foster City, CA) and approximately 100 ng template DNA in a 1X PCR buffer (50 mM KCl, 10 mM Tris-HCl pH 8.3, 0.001% gelatin). Amplifications were performed in a thermocycler using 41 cycles of 95°C for 30 s, 60°C for 30 s, 72°C for 1 min, followed by a final extension at 72°C for 10 min. Amplification products were visualized under ultraviolet light after gel electrophoresis on a 2% agarose gel and staining with GelGreen Nucleic Acid Gel Stain (Biotium, Hayward, CA).
3. Results
Seventy homozygous shi blastocysts were injected with ES cells in 6 independent sessions, generating 48 pups, of which 2 died before any behavioral analysis could be performed. Of the 46 viable mice, 21 exhibited motor deficits indistinguishable from those of homozygous shi mice (“not rescued”), and their cut brain sections revealed no evidence of immunoreactive MBP (at a minimum, 7 coronal sections mapped at atlas [16] levels 0.2 mm, −0.2 mm, −2.4 mm, −3.3 mm, −5.2 mm, −6.4 mm, and −7.5 mm from bregma).
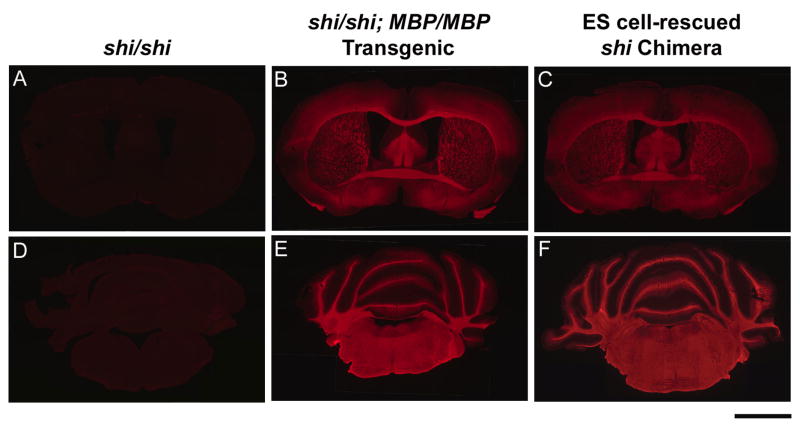
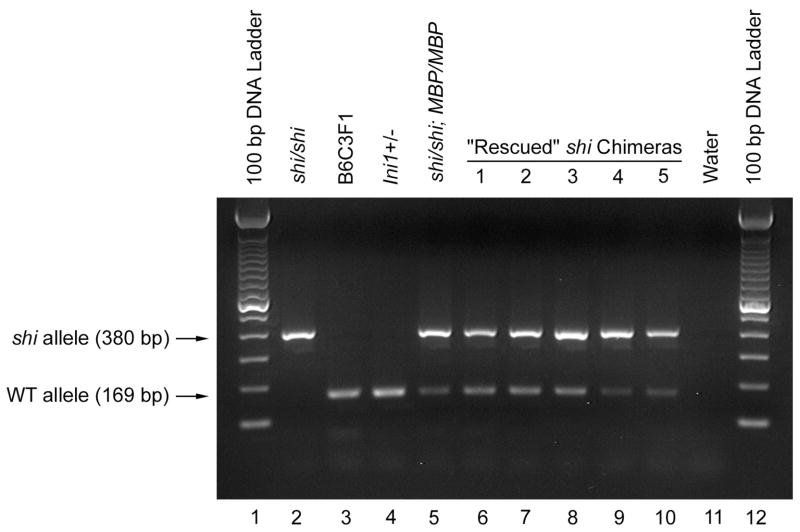
The brains of the remaining 25 mice were immunopositive for MBP to varying degrees. In 10 mice (40%), the motor phenotype was categorized as “rescued” or “essentially rescued,” and MBP staining was strong and widespread throughout all these brains (Fig. 1). Each of the “essentially rescued” mice (n = 5) was scored as (1,0), and MBP labeling in forebrain areas of these mice was widespread but tended to be more variable than that of the fully “rescued” mice (hindbrain areas appeared equally dense in the two groups). Although the behavioral phenotype and the pattern of MBP immunoreactivity of the fully “rescued” mice were indistinguishable from those of WT mice, PCR analysis of brain samples demonstrated that the “rescued” mice were truly chimeric for both shi and WT alleles (Fig. 2).
Fig. 1.
Coronal brain sections at two rostrocaudal levels labeled for immunoreactive MBP from homozygous shi mice (shi/shi) (A,D), a homozygous shi mouse genetically rescued with the wild-type MBP transgene (shi/shi; MBP/MBP) (B,E), and chimeras derived from ES cell-injected shi blastocysts (ES cell-rescued shi Chimera) (C,F). Scale bar represents 2 mm.
Fig. 2.
Agarose gel electrophoretic analysis of PCR amplification for the shi and WT alleles, displayed as 380- and 169-bp amplification products, respectively, from brain genomic DNA of shi/shi (Lane 2), B6C3F1 (Lane 3), Ini1+/− (Lane 4), shi/shi; MBP/MBP (Lane 5), and the 5 “rescued” shi chimeric (1, 2, 3, 4 and 5; Lanes 6 – 10) mice. The size of the amplification products was determined by reference to the 100-bp DNA ladder (Lanes 1 and 12). For negative control, water was used in place of genomic DNA as template (Lane 11).
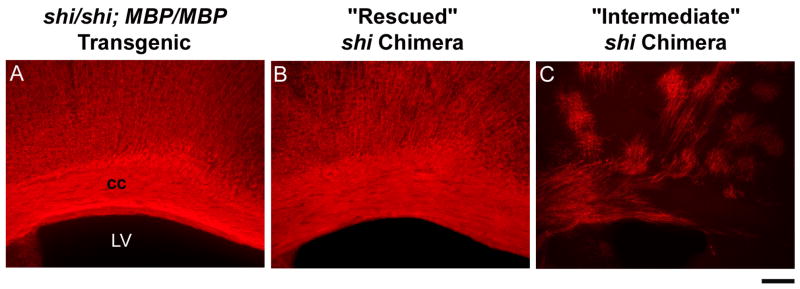
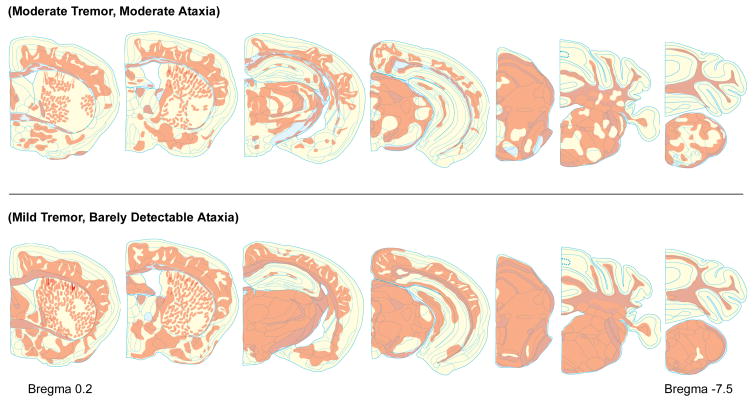
The 15 “intermediate” mice displayed regional gaps in the appearance of brain MBP, and the brains from animals with a “severe” (3) score for either tremor or ataxia (n = 6) had an abnormal, patchy pattern of MBP expression (Fig. 3). Visual inspection of rostrocaudal maps of MBP immunoreactivity suggested that less severe “intermediate” phenotypes correlated with more widespread distribution of MBP (Fig. 4); we could not implicate staining in any one specific region or nucleus as critical for relieving tremor or ataxia.
Fig. 3.
Coronal brain sections labeled for immunoreactive MBP from a homozygous shi mouse genetically rescued with the wild-type MBP transgene (shi/shi; MBP/MBP) (A), a “rescued” chimeric mouse (“Rescued” shi Chimera) (B) and a chimeric mouse with severe motor deficits (“Intermediate” shi Chimera) (C) derived from ES cell-injected shi blastocysts. cc, corpus callosum; LV, lateral ventricle. Scale bar represents 0.2 mm.
Fig. 4.
Representative rostrocaudal maps of MBP immunoreactivity from two “intermediate” shi chimeras with behavioral phenotypes that were relatively more (top) or less (bottom) severe. Shown are coronal brain sections at atlas [16] levels 0.2 mm, −0.2 mm, −2.4 mm, −3.3 mm, −5.2 mm, −6.4 mm, and −7.5 mm from bregma.
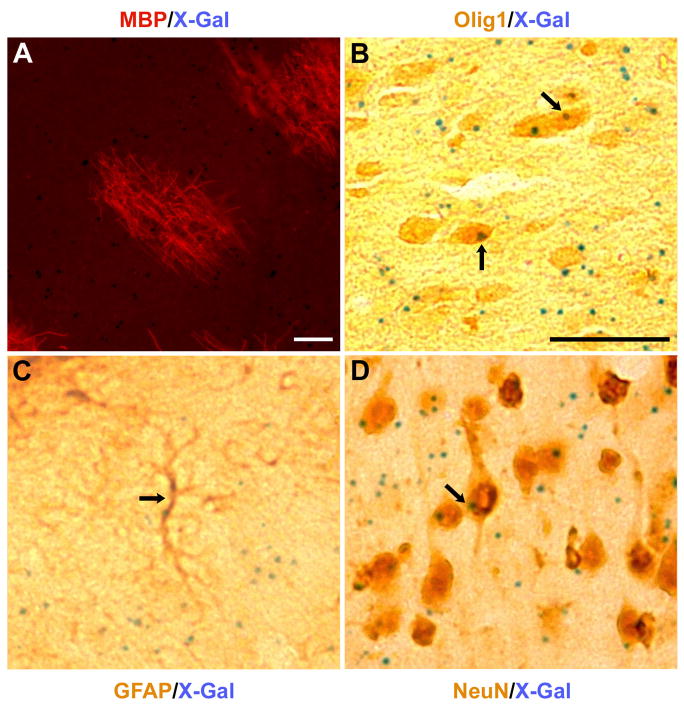
Further quantitative analyses were not attempted, as we found variable expression of the ES cell β-gal reporter after cellular differentiation. In selected cases, however, the punctate histochemical reaction product of the reporter could be co-localized with immunoreactive Olig1, GFAP, or NeuN, and it was clear that donor-derived cells formed not only myelinating oligodendrocytes but putative astrocytes and neurons as well (Fig. 5).
Fig. 5.
Dual immuno- and histochemical labeling of single sections, showing (A) an immunoreactive MBP patch along with punctate cellular X-gal reaction products from an “intermediate” chimeric mouse (MBP, red; X-gal, blue), and (B–D) co-localization of immunoreactive Olig1, GFAP, or NeuN (brown) with X-gal (blue, arrows) in presumptive oligodendrocytes (B), astroglia (C), and neurons (D) from a “rescued” chimeric mouse. Scale bars represent 50 μm.
4. Discussion
To our knowledge, no previous cell grafting technique has restored brain MBP expression in immunocompetent shi mice as extensively as our ES cell injections in shi blastocysts, resulting in a distribution that can be ostensibly normal and comparable to that observed in animals genetically rescued with the wild-type MBP transgene. Behavioral rescue of our chimeric shi mice depended on this widespread MBP expression; in no case did partial or patchy regional expression lead to a “rescued” or “essentially rescued” mouse. Because we used shi mice with a large MBP deletion as the host, we expect that ES cell restoration of MBP expression and motor function was by differentiation into the oligodendrocyte lineage. In contrast to our data, there have been examples in which ES cells restore defective gene expression in a non-cell autonomous manner [17,18], by secreting circulating factors rather than by differentiating and replacing specific cell lineages.
Although we did not measure brain MBP levels, we suspect that the critical factor for correcting shi motor deficits is the broad distribution of MBP (and, presumably, associated normal myelination) rather than MBP amount. Improved rotarod performance has been reported in heterozygous mice with one wild-type MBP transgene and expression levels of MBP mRNA and MBP protein of about 12.5% of normal; and animals essentially rescued by two copies of the transgene express MBP levels 20 – 25% of normal, with a similar percentage of normally-myelinated axons [19,20]. Another critical factor for successful shi rescue may be the initiation of treatment at an early stage of development, before dysmyelination leads to axonal abnormalities. Postnatal shi brains exhibit abnormal regions around the nodes of Ranvier, with paranodal loops ending in a disorderly jumble, and axoglial junctions form in inappropriate locations [21] with an anomolous distribution of related axonal ion channels [22,23]. Additional axonal pathology involving the cytoskeleton has been reported [24].
Although the shi white matter disease has no clinical counterpart, other mouse mutants, e.g., jimpy, twitcher, and rumpshaker, are believed to represent the murine equivalents of human inherited disorders of myelination, sp., Pelizaeus-Merzbacher disease, Krabbe (globoid cell) leukodystrophy, and spastic paraplegia type 2, respectively. As more pluripotent cell types become available, injections into blastocyst embryos may be a useful approach for testing the cells’ functional activity in such animal models. Ultimately, prenatal cell therapies might offer promise for correcting disorders of defective myelination before secondary, irreversible axonal damage can occur.
Acknowledgments
This work was supported by National Institute of Neurological Disorders and Stroke Grant NS43879 to L.D.R. and National Aeronautics and Space Administration Grant NAG9-1356 to W.J.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roach A, Takahashi N, Pravtcheva D, Ruddle F, Hood L. Chromosomal mapping of mouse myelin basic protein gene and structure and transcription of the partially deleted gene in shiverer mutant mice. Cell. 1985;42:149–155. doi: 10.1016/s0092-8674(85)80110-0. [DOI] [PubMed] [Google Scholar]
- 2.Chernoff GF. Shiverer: an autosomal recessive mutant mouse with myelin deficiency. J Hered. 1981;72:128. doi: 10.1093/oxfordjournals.jhered.a109442. [DOI] [PubMed] [Google Scholar]
- 3.Readhead C, Popko B, Takahashi N, Shine HD, Saavedra RA, Sidman RL, Hood L. Expression of a myelin basic protein gene in transgenic shiverer mice: correction of the dysmyelinating phenotype. Cell. 1987;48:703–712. doi: 10.1016/0092-8674(87)90248-0. [DOI] [PubMed] [Google Scholar]
- 4.Mitome M, Low HP, van den Pol A, Nunnari JJ, Wolf MK, Billings-Gagliardi S, Schwartz WJ. Towards the reconstruction of central nervous system white matter using neural precursor cells. Brain. 2001;124:2147–2161. doi: 10.1093/brain/124.11.2147. [DOI] [PubMed] [Google Scholar]
- 5.Duncan ID. Oligodendrocytes and stem cell transplantation: their potential in the treatment of leukoencephalopathies. J Inherit Metab Dis. 2005;28:357–368. doi: 10.1007/s10545-005-7058-z. [DOI] [PubMed] [Google Scholar]
- 6.Keyoung HM, Goldman SA. Glial progenitor-based repair of demyelinating neurological diseases. Neurosurg Clin N Am. 2007;18:93–104. doi: 10.1016/j.nec.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Radtke C, Spies M, Sasaki M, Vogt PM, Kocsis JD. Demyelinating diseases and potential repair strategies. Int J Dev Neurosci. 2007;25:149–153. doi: 10.1016/j.ijdevneu.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eftekharpour E, Karimi-Abdolrezaee S, Wang J, Beheiry HE, Morshead C, Fehlings MG. Myelination of congenitally dysmyelinated spinal cord axons by adult neural precursor cells results in formation of nodes of Ranvier and improved axonal conduction. J Neurosci. 2007;27:3416–3428. doi: 10.1523/JNEUROSCI.0273-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Izrael M, Zhang P, Kaufman R, Shinder V, Ella R, Amit M, Itskovitz-Eldor J, Chebath J, Revel M. Human oligodendrocytes derived from embryonic stem cells: Effect of noggin on phenotypic differentiation in vitro and on myelination in vivo. Mol Cell Neurosci. 2007;34:310–323. doi: 10.1016/j.mcn.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Yandava BD, Billinghurst LL, Snyder EY. “Global” cell replacement is feasible via neural stem cell transplantation: evidence from the dysmyelinated shiverer mouse brain. Proc Natl Acad Sci USA. 1999;96:7029–7034. doi: 10.1073/pnas.96.12.7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuhn PL, Petroulakis E, Zazanis GA, McKinnon RD. Motor function analysis of myelin mutant mice using a rotarod. Int J Dev Neurosci. 1995;13:715–722. doi: 10.1016/0736-5748(96)81215-9. [DOI] [PubMed] [Google Scholar]
- 12.Windrem MS, Schanz SJ, Guo M, Tian G-F, Washco V, Stanwood N, Rasband M, Roy NS, Nedergaard M, Havton LA, Wang S, Goldman SA. Neonatal chimerization with human glial progenitor cells can both remyelinate and rescue the otherwise lethally hypomyelinated shiverer mouse. Cell Stem Cell. 2008;2:553–565. doi: 10.1016/j.stem.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inoue Y, Mikoshiba K, Yokoyama M, Inoue K, Terashima T, Nomura T, Tsukada Y. Alteration of the primary pattern of central myelin in a chimaeric environment – study of shiverer ↔ wild-type chimaeras. Dev Brain Res. 1986;26:239–247. doi: 10.1016/0165-3806(86)90288-9. [DOI] [PubMed] [Google Scholar]
- 14.Guidi CJ, Sands AT, Zambrowicz BP, Turner TK, Demers DA, Webster W, Smith TW, Imbalzano AN, Jones SN. Disruption of Ini1 leads to peri-implantation lethality and tumorigenesis in mice. Mol Cell Biol. 2001;21:3598–3603. doi: 10.1128/MCB.21.10.3598-3603.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomez CM, Muggleton-Harris AL, Whittingham DG, Hood LE, Readhead C. Rapid preimplantation detection of mutant (shiverer) and normal alleles of the mouse myelin basic protein gene allowing selective implantation and birth of live young. Proc Natl Acad Sci USA. 1990;87:4481–4484. doi: 10.1073/pnas.87.12.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hof PR, Young WG, Bloom FE, Belichenko PV, Celio MR. Comparative Cytoarchitectonic Atlas of the C57BL/6 and 129/Sv Mouse Brains. Elsevier Science BV; Amsterdam: 2000. [Google Scholar]
- 17.Fraidenraich D, Stillwell E, Romero E, Wilkes D, Manova K, Basson CT, Benezra R. Rescue of cardiac defects in Id knockout embryos by injection of embryonic stem cells. Science. 2004;306:247–252. doi: 10.1126/science.1102612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vermot J, Messaddeq N, Niederreither K, Dierich A, Dolle P. Rescue of morphogenetic defects and of retinoic acid signaling in retinaldehyde dehydrogenase 2 (Raldh2) mouse mutants by chimerism with wild-type cells. Differentiation. 2006;74:661–668. doi: 10.1111/j.1432-0436.2006.00094.x. [DOI] [PubMed] [Google Scholar]
- 19.Popko B, Puckett C, Lai E, Shine HD, Readhead C, Takahashi N, Hunt SW, III, Sidman RL, Hood L. Myelin deficient mice: expression of myelin basic protein and generation of mice with varying levels of myelin. Cell. 1987;48:713–721. doi: 10.1016/0092-8674(87)90249-2. [DOI] [PubMed] [Google Scholar]
- 20.Shine HD, Readhead C, Popko B, Hood L, Sidman RL. Morphometric analysis of normal, mutant, and transgenic CNS: correlation of myelin basic protein expression to myelinogenesis. J Neurochem. 1992;58:342–349. doi: 10.1111/j.1471-4159.1992.tb09316.x. [DOI] [PubMed] [Google Scholar]
- 21.Rosenbluth J. Axoglial junctions in the mouse mutant shiverer. Brain Res. 1981;208:283–297. doi: 10.1016/0006-8993(81)90558-8. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Allen ML, Grigg JJ, Noebels JL, Tempel BL. Hypomyelination alters K+ channel expression in mouse mutants shiverer and trembler. Neuron. 1995;15:1337–1347. doi: 10.1016/0896-6273(95)90012-8. [DOI] [PubMed] [Google Scholar]
- 23.Rasband MN, Peles E, Trimmer JS, Levinson SR, Lux SE, Shrager P. Dependence of nodal sodium channel clustering on paranodal axoglial contact in the developing CNS. J Neurosci. 1999;19:7516–7528. doi: 10.1523/JNEUROSCI.19-17-07516.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brady ST, Witt AS, Kirkpatrick LL, de Waegh SM, Readhead C, Tu P-H, Lee VM-Y. Formation of compact myelin is required for maturation of the axonal cytoskeleton. J Neurosci. 1999;19:7278–7288. doi: 10.1523/JNEUROSCI.19-17-07278.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]