Abstract
Cardiac function is regulated by interactions among intrinsic and extrinsic autonomic neurons, and the mechanisms responsible for organizing these circuits are poorly understood. Parasympathetic neurons elsewhere synthesize the neurotrophin NGF, which may promote postganglionic axonal associations where parasympathetic axons inhibit sympathetic transmitter release. Previous studies have shown that parasympathetic NGF content and neurochemical phenotype are regulated by sympathetic innervation. In this study we assessed contributions of sympathetic input on cardiac ganglion neuronal phenotype and NGF expression. Because cardiac ganglia are reported to contain putative noradrenergic neurons, we eliminated sympathetic input both surgically (extrinsic) and chemically (extrinsic plus intrinsic). In controls, most cardiac ganglion neurons expressed vesicular acetylcholine transporter, frequently colocalized with vesicular monoamine transporter, but lacked catecholamine histofluorescence. Most cardiac ganglion neurons expressed NGF transcripts, and 40% contained mature and 47% proNGF immunoreactivity. Guanethidine treatment for 7 days decreased numbers of neurons expressing vesicular acetylcholine transporter, NGF transcripts and NGF immunoreactivity, but did not affect proNGF or vesicular monoamine transporter immunoreactivity. Stellate ganglionectomy had comparable effects on neurochemical phenotype and mature NGF immunoreactivity, but proNGF expression was additionally reduced.
These findings show that individual cardiac ganglion neurons display markers of both cholinergic and noradrenergic transmission. Sympathetic noradrenergic innervation maintains levels of cholinergic but not noradrenergic marker protein. Sympathetic innervation also promotes cardiac ganglion neuronal NGF synthesis. Because chemical blockade of all noradrenergic transmission is no more effective than extrinsic sympathectomy, local intrinsic noradrenergic transmission is not a factor in regulating ganglion neuron phenotype.
Keywords: Nerve Growth Factor, ProNGF, Stellate Ganglionectomy, Guanethidine, Parasympathetic Nervous System
1. INTRODUCTION
The heart is richly supplied by sympathetic nerves, particularly in cardiac pacemaker regions. Parasympathetic tone normally predominates over sympathetic heart rate-promoting activity. Interactions between these autonomic nerves may occur within the parasympathetic cardiac ganglia, but also at the level of the axon terminals which innervate pacemaker regions and form inhibitory axo-axonal connections (Wetzel et al., 1985; Loiacono et al., 1986). Appropriate interactions among these nerve populations are crucial for normal cardiac function (Randall et al., 2003). Indeed, during the progression to heart failure, uncoupling of autonomic nerves (Dunlap et al., 2003; Nihei et al., 2005) may diminish parasympathetic inhibition of sympathetic activity contributing to unfettered sympathetic overdrive.
The factors responsible for establishing and maintaining connections among cardiac nerves have not been delineated. However, one possibility is that neural interactions serve to promote and reinforce the complex cardiac circuitry. Indeed, we have shown previously that sympathetic innervation can modulate both the expression of the prototypal neurotrophin nerve growth factor (NGF) and transmitter peptides in parasympathetic ganglion neurons projecting to cranial targets (Hasan et al., 2000a). NGF is known to modulate sympathetic innervation through effects on neuronal survival, innervation density, transmitter release, and synaptic plasticity (Chun et al., 1977; Korsching et al., 1988; Rush et al., 1997; Kohn et al., 1999; Lockhart et al., 2000; Reichardt, 2006). Thus, NGF synthesis by parasympathetic cardiac ganglion neurons may play a role in maintaining axo-axonal or axo-somatic connections between cardiac parasympathetic and sympathetic neurons. Similarly, the expression of some parasympathetic traits such as vasoactive intestinal polypeptide and nitric oxide synthase expression are diminished in the absence of functional sympathetic innervation (Fan et al., 1993; Warn et al., 1997; Smith et al., 2002). Thus, sympathetic regulation of parasympathetic neurochemical phenotype and/or neurotrophin synthesis could play a role in dysregulation of cardiac autonomic function in some pathophysiological conditions.
While parasympathetic neurons projecting to cranial targets are believed to represent populations of cholinergic and nitrergic cells, a number of immunohistochemical studies have suggested that cardiac ganglion neurons are heterogeneous, including both cholinergic neurons and intrinsic adrenergic neurons (Baluk et al., 1990; Forsgren et al., 1990; Horackova et al., 1999; Singh et al., 1999; Slavikova et al., 2003; Weihe et al., 2005). Presumptive adrenergic neurons have been variously described as being either dual cholinergic/adrenergic or purely adrenergic large (20–40 μm diameter) neurons with features similar to autonomic neurons elsewhere, or small (8–10 μm) adrenergic cells with characteristics similar to small intensely fluorescent (SIF) interneurons (Baluk et al., 1990; Forsgren et al., 1990; Horackova et al., 1999; Slavikova et al., 2003). Therefore, it is possible that cardiac ganglion neuronal phenotype and NGF expression may be regulated by both extrinsic sympathetic nerves deriving from the paravertebral chain as well as intrinsic noradrenergic neurons.
In the present study, we examined the effect of interrupting noradrenergic innervation to cardiac ganglion neurons with respect to markers of cholinergic and noradrenergic function, and NGF expression. We selectively eliminated extrinsic noradrenergic innervation by performing surgical sympathectomy and compared this to the effect of chronic blockade of all noradrenergic transmission (intrinisic and extrinsic) by the sympatholytic agent guanethidine. Our findings show that noradrenergic transmission provided by extrinsic neurons is essential in maintaining cardiac parasympathetic neuronal phenotype and NGF expression.
2. METHODS
2.1. Guanethidine treatment
Seven adult Sprague-Dawley rats (60–70 days postnatal, ~225g, Harlan Breeding Laboratories, Indianapolis, IN) were injected intrascapularly 2xdaily with guanethidine (25mg/kg, Sigma) for 5 days. Control rats (n=5) were injected with sterile saline. Guanethidine-treated rats exhibited ptosis and diarrhea within 24 h, which persisted throughout treatment (Smith, 1985; Smith et al., 1987). Presence of ptosis has been correlated with adrenergic neuronal blockade in the heart (Chang et al., 1965; Maxwell, 1982). The base of the heart including atria and major cardiac vessels, which includes the cardiac ganglia, was removed, snap-frozen and stored at −80 °C.
2.2. Surgical Sympathectomy
Surgical sympathectomy was accomplished by removing the inferior and middle cervical (stellate) ganglion complex, which comprise the primary cardiac-projecting sympathetic innervation (Pardini et al., 1989). Five adult male Sprague-Dawley rats (60–70 days postnatal, ~225g, Harlan) were anesthetized (60 mg/kg ketamine, 8 mg/kg xylazine, and 0.4mg/kg atropine, intraperitoneal injection), intubated, respired mechanically and a unilateral thoracotomy (2nd rib) carried out. The subclavian artery was identified and followed inferiorly where it branches into the costocervical artery and internal thoracic artery; the stellate ganglion lies in a cleft between the latter vessels (Hansson et al., 1998; Tseng et al., 2001). The superior and inferior branches were cut and the ganglia were removed bilaterally. Sham surgery (n=4) involved exposing the ganglion then closing the chest wall with sutures. Buprenorphine (0.1 mg/kg sc/im) was administered as post-operative analgesia. Rats exhibited ptosis for the duration of the study, consistent with the disruption of the cranially directed preganglionic axons traveling through the stellate ganglionic complex. Two weeks after surgery, the base of the heart including the cardiac ganglia was snap-frozen and stored at −80 °C.
2.3. Glyoxcylic Acid Histofluorescence
To confirm noradrenaline depletion and to examine the prevalence of intrinsic catecholaminergic neurons within the cardiac ganglion, we carried out glyoxylic acid histofluorescence on control and guanethidine-treated rats. Twenty-four hours before tissue harvest, rats were injected intraperitoneally with the monoamine oxidase inhibitor pargyline (100mg/kg, Sigma-RBI), which increases catecholamine levels allowing optimum visualization of catecholaminergic cells and their projections. Rats were anesthetized (60 mg/kg ketamine, 8 mg/kg xylazine, and 0.4mg/kg atropine, intraperitoneal injection) and perfused with 50ml heparin-saline then 1L/kg of Krebs-Ringers solution with 2% glyoxylic acid (pH 7.4, Sigma) (Smith et al., 1996). The base of the heart including atria and major cardiac vessels were removed, washed in perfusion solution (2×10min, 4°C), snap-frozen and stored at −80°C. Slides were cryosectioned at 10μm, dipped in sucrose–phosphate–glyoxylic acid solution (pH 7.4) for 60sec, air dried for 10min, and heated at 80°C for 5 min. Slides were dehydrated, cleared and mounted. Histofluorescence was examined with a Leitz Diaplan microscope with 100 W mercury epifluorescence and ultraviolet filters. Superior cervical ganglia (SCG) were also examined to confirm somal catecholamine depletion.
2.4. In Situ Hybridization
NGF transcripts were detected in cardiac ganglia from control and guanethidine-treated rats using a digoxigenin-labeled antisense probe to rat pre-pro-NGF cDNA (Hasan et al., 2000b). Sense probes, RNase A treatment, or probe omission were carried out as negative controls, with salivary gland as a positive control. Control and experimental sections were processed together to ensure that variations in processing did not contribute to differences in staining intensity.
2.5. Indirect Single and Double Immunostaining
Cardiac ganglion sections from control, surgically sympathectomized and guanethidine-treated rats were incubated with primary antibody overnight at 4°C, followed by 90 min incubation with cy3-conjugated secondary antibodies. For double staining sections were incubated with 2 compatible primary antibodies, followed by secondary antisera from a common species conjugated to cy2 or cy3 fluorophores (1:200, Jackson). Combinations of primary and secondary antibodies were screened to exclude possible antibody cross-reactivity. Antibodies used were directed against the cholinergic marker vesicular acetylcholine transporter (VAChT, goat IgG, 1:200, Chemicon), the adrenergic marker vesicular monoamine transporter-2 (VMAT, rabbit IgG, 1:200, Chemicon), mature NGF (NGFβ, rabbit IgG, 1:100, sc-549, Santa Cruz) and proNGF (rabbit IgG, 1:100, AB5583, Chemicon). Specificity of the mature NGF and proNGF antibodies was determined by probing immunoblots loaded with 500 ng human recombinant NGF (Sigma); the mature NGFβ antibody primarily detected the 13.5 and 16 kDa mature forms of NGF whereas the proNGF antibody detected bands at 35 and 53 kDa. Antibody omission, pre-adsorption to the relevant antigen, and substitution of naïve immunoglobulin for the primary antibody served as negative controls.
2.6. Quantitation of Stained Neurons
Sets of 10μm sections perpendicular to the basal-apical axis of the atria were collected as a stepped series onto 5 slides. Sections were collected beginning ~2 mm superior from the point of entry of the right superior vena cava into the atrium and continued until at least two cardiac ganglia had been identified under differential interference microscopy and completely sectioned. Cardiac ganglia were typically clustered within atrial tissue or in epicardial fat pads at the base of the right vena cava. Within every 2nd section in one stepped series through the entire ganglion, all neurons displaying hybridization product or immunofluorescence and a nucleus whose uppermost membrane boundary was contained within the section were counted and divided by the total number of neuronal nuclei in that section (Hasan et al., 2005). Total neuron counts per ganglion within sampled sections ranged from 50–150 neurons. Percentages of stained neurons were averaged to obtain a mean value (± S.E.M.) per ganglion. All experimental manipulations were approved by the Institutional Animal Care and Use Committee of the University of Kansas Medical Center and conformed to local and national statutes.
2.7. Statistical analysis
All data are expressed as means ±SEM. Comparisons were performed by Student’s t-test, with significance set at p≤0.05.
3. RESULTS
3.1. Guanethidine treatment
3.1.1. Control Tissue
Cardiac ganglion neuronal phenotypes
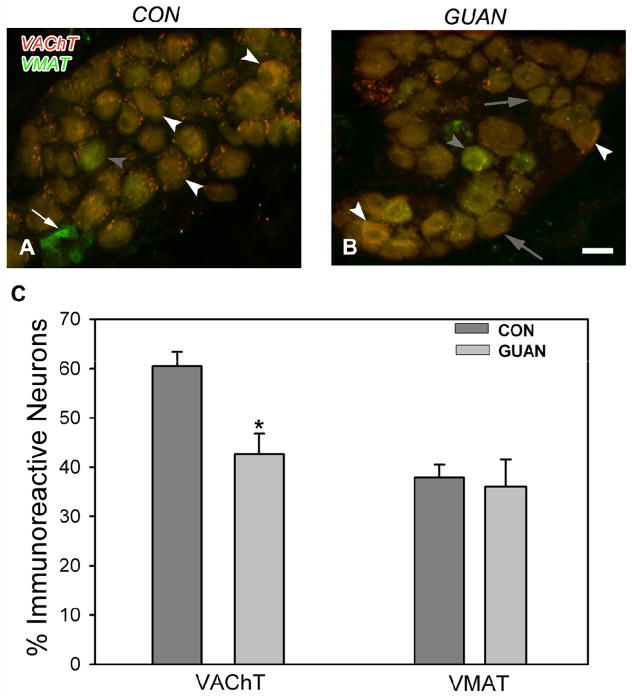
The cardiac ganglion contains large neurons with diameters of 20–40 μm and small neurons 8–10 μm in diameter. VAChT-ir was prominent within the cytoplasm of 60% of large cardiac ganglion neurons (Figure 1A, C) but not small cells. Axons and presynaptic varicosities around large neurons also expressed VAChT-ir (Figure 1A). Thirty-eight percent of the large cardiac ganglion neurons expressed VMAT immunoreactivity (Figure 1A, C). Double staining studies showed that 89% of those neurons expressing VMAT-ir contained VAChT-ir. VMAT-ir was also consistently observed within all small neurons (Figure 1A).
Figure 1.
Vesicular acetylcholine transporter (VAChT) and vesicular monoamine transporter (VMAT) immunoreactivity (ir) in control (A) and guanethidine-treated (B) tissue. A: Double-stained photomicrograph of VAChT (cy3, red) and VMAT (cy2, green) –ir shows mostly yellow co-localized (white arrowheads) large neurons, as well as occasional VAChT negative, VMAT-ir (filled arrowhead) neurons. Small neurons (arrow) stain strongly for VMAT (cy3, green)-ir. B: Following guanethidine treatment, a photomicrograph of VAChT (cy3, red) and VMAT (cy2, green) –ir shows many large neurons negative for VAChT-ir (filled arrows), although co-localized neurons (white arrowheads) are still evident. Large neurons strongly VMAT-ir are also observed (filled arrowhead). C: Quantitative representation of the percentage of large cardiac ganglion neurons immunoreactive for VAChT and VMAT. Scale bar in B is 40 μm.
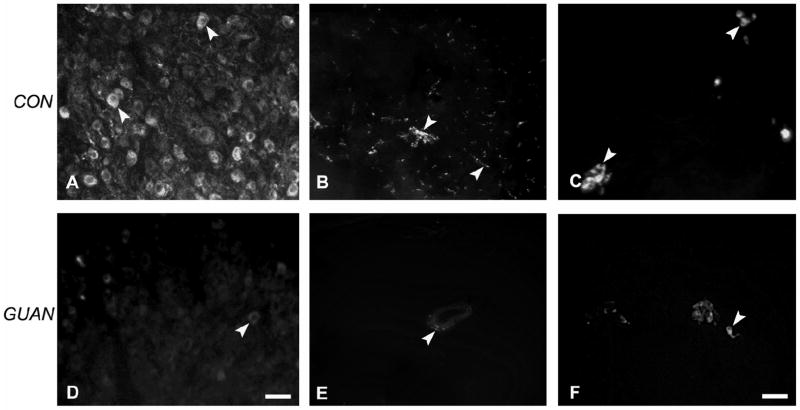
SCG neurons were examined for catecholamine histofluorescence to confirm the efficacy of the glyoxylic acid method. The majority of SCG neurons from control rats displayed intense histofluorescence (Figure 2A). In the heart, histofluorescence was prominent within atrial axons (Figure 2B), but large cardiac ganglion neurons lacked catecholamine histofluorescence altogether (Figure 2C). Small cardiac ganglion neurons were intensely fluorescent (Figure 2C).
Figure 2.
Catecholamine histofluorescence in control (A–C) and guanethidine-treated (D–F) tissue. A: Most neurons of the superior cervical ganglion (SCG) display histofluorescence (arrowheads). B: Axons in the atria, both nerve bundles and free (arrowheads) are strongly stained for catecholamines. C: Cardiac ganglion neurons do not express catecholamine histofluorescence, however small neurons (arrowheads) are strongly fluorescent. D: Occasional SCG neurons from guanethidine-treated rats display weak histofluorescence (arrowhead). E: Only rarely are weakly-stained histofluorescent axons (arrowhead) observed in atrial tissue, principally around blood vessels. F: Small neurons (arrowhead) with axonal processes express reduced histofluorescence in cardiac ganglia. Scale bar in D is 50μm for panels A, B, D, E; scale bar in F is 20μm for panels C,F.
NGF transcript and protein expression
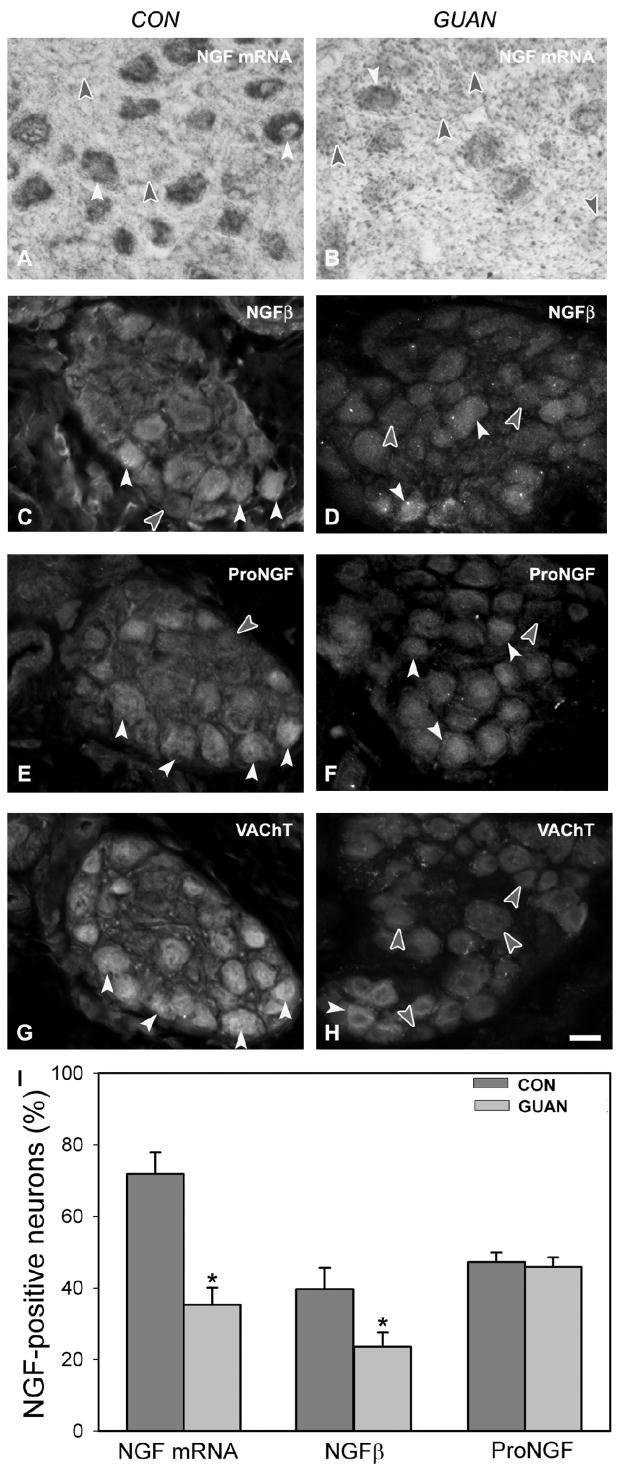
Transcripts for NGF were detected as a cytoplasmic reaction product within 72% of large cardiac ganglion neurons (Figure 3A, I). Mature NGF immunoreactivity (ir) was present in 40% of large cardiac ganglion neurons (Figure 3C,I), and those neurons were predominantly (93%) VAChT-ir (Figure 3G). ProNGF-ir was strong within 47% of large cardiac ganglion neurons (Figure 3E, I) and was localized 82% of the time within VAChT-ir neurons (Figure 3G).
Figure 3.
Nerve growth factor (NGF) transcript and protein expression in cardiac ganglia from control (CON; A, C, E, G) and guanethidine-treated (GUAN; B, D, F, H) rats. A: NGF transcripts are expressed strongly in most large cardiac ganglion neurons (white arrowheads); non-transcript expressing neurons (filled arrowheads) are also observed. B: After guanethidine treatment, non-NGF mRNA-expressing neurons (filled arrowheads) are more prominent than positive neurons (white arrowhead). C: Mature NGFβ immunoreactivity (ir) is present within a subpopulation of control cardiac ganglion neurons (white arrowheads); many neurons (filled arrowhead) do not express mature NGFβ-ir. D: After guanethidine treatment, mature NGFβ-negative neurons (filled arrowheads) are more frequent than those mature NGFβ-ir (white arrowheads). E: ProNGF-ir (white arrowheads) is prominent in cardiac ganglion neurons; a similar proportion does not stain for ProNGF (filled arrowheads). F: Following guanethidine treatment, ProNGF-ir neurons (white arrowheads), or unstained (filled arrowheads), are observed in similar numbers to controls. G, H: Vesicular acetylcholine transporter (VAChT)-ir is observed in neurons (white arrowheads) from adjacent sections to those processed for mature NGFβ (C, D) and proNGF (E, F). VAChT-negative neurons are indicated by filled arrowheads. I: Quantitative representation of the percentage of large cardiac ganglion neurons immunoreactive for mature NGFβ and proNGF. Scale bar in H is 40μm for panels A-H.
3.1.2. Guanethidine treatment
Neurochemical phenotype
Guanethidine treatment resulted in a 29% decrease in numbers of large VAChT-ir neurons (Figure 1B, p=0.002, Figure 1C) whereas VMAT-ir was unaltered (Figure 1B, C).
Within the SCG, histofluorescence was present within very few neurons (Figure 2D) confirming catecholamine depletion. Similarly, axons in atrial myocardium displaying histofluorescence were rarely encountered (Figure 2E). In cardiac ganglia, catecholamine histofluorescence was absent in large cardiac ganglion neurons as in controls, while small cardiac ganglion neurons retained their histofluorescent staining (Figure 2F).
NGF transcript and protein expression
Guanethidine treatment decreased numbers of NGF mRNA-expressing large neurons by 51% (Figure 3B, I). The percentage of neurons containing mature NGF-ir was decreased by 41% by guanethidine (p=0.038, Figure 3D, I). ProNGF protein expression was comparable to control (Figure 3F, I).
3.2. Surgical Sympathectomy
Neurochemical phenotype
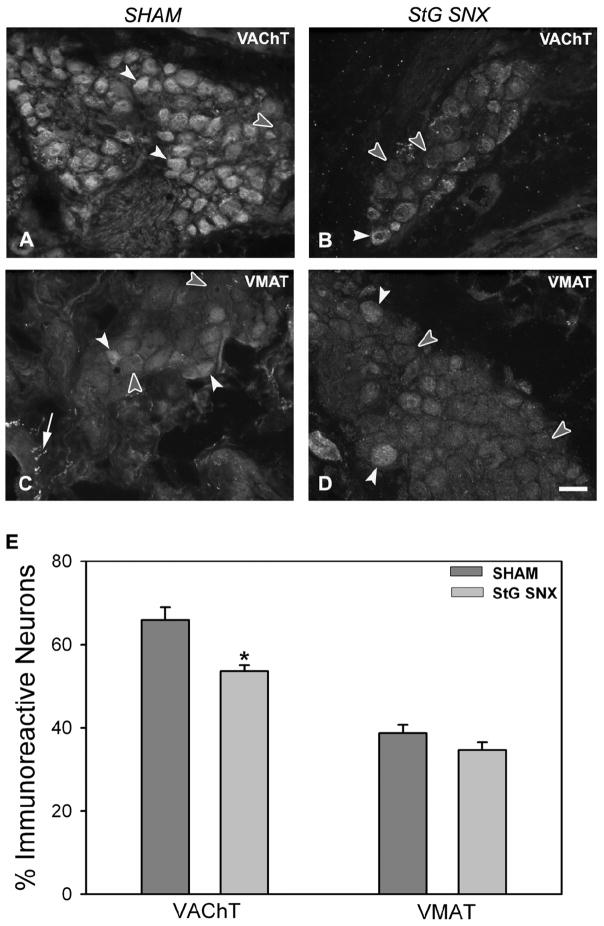
Numbers of large neurons displaying VAChT-ir decreased from 66% to 53% (p<0.001; Figure 4A, B, E) after surgical sympathectomy. VMAT-ir was present in 38% of large neurons in sham-operated rats and did not change significantly after stellate ganglionectomy (35%; Figure 4C, D, E). Numbers of VMAT-ir axons were markedly reduced in atrial tissue (cf. Figure 4C and D) consistent with degeneration of extrinsic sympathetic innervation.
Figure 4.
Vesicular acetylcholine transporter (VAChT) and vesicular monoamine transporter (VMAT) immunoreactivity (ir) in cardiac ganglia from sham-operated (A, C) and stellate ganglionectomized (B, D) rats. A: Photomicrograph of VAChT staining shows the majority of large neurons (white arrowheads) are strongly immunoreactive in cardiac ganglia from sham-operated rats, however unstained neurons are also present (filled arrowhead). B: Following stellate sympathectomy, more neurons are negative for VAChT-ir (filled arrowheads) as compared to shams. C: Some cardiac ganglion neurons from sham-operated rats display immunoreactivity for VMAT (white arrowheads), however the majority are not stained for VMAT (filled arrowheads). Presumptive VMAT-ir sympathetic axons (arrow) are observed around the ganglia primarily in perivascular locations. D: Following sympathectomy, staining for VMAT in cardiac ganglion neurons is similar to that seen in tissue from sham-operated rats, however VMAT axonal staining is rarely observed around cardiac ganglion indicating cardiac sympathectomy. E: Quantitative representation of the percentage of large cardiac ganglion neurons immunoreactive for VAChT and VMAT. Scale bar in D is 40 μm.
NGF expression
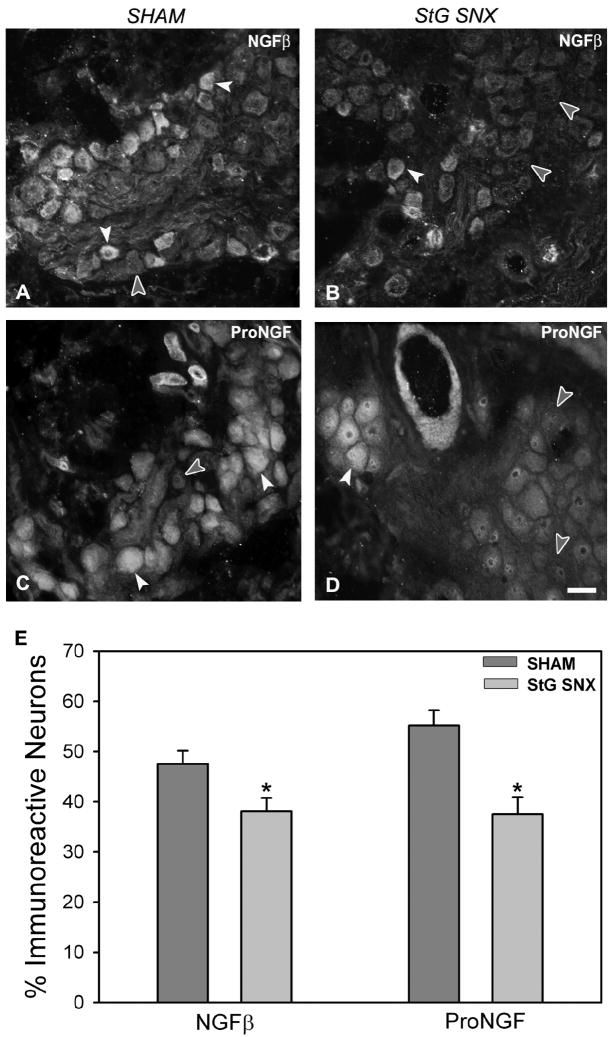
Immunoreactivity for mature NGF was present in 48% (Figure 5A) of large cardiac ganglion neurons from sham-operated rats and decreased to 38% (p=0.03; Figure 5B, E) two weeks after stellate ganglionectomy. In contrast to findings observed after chemical sympathectomy, proNGF immunoreactivity was decreased from 55% (Figure 5C) to 38% (p=0.007; Figure 5D, E) after surgical sympathectomy.
Figure 5.
NGF protein expression in cardiac ganglia from sham-operated (A, C) and stellate ganglionectomized (B, D) rats. A: Mature NGFβ immunoreactivity (ir) is present within a subpopulation of large control cardiac ganglion neurons (white arrowheads); many neurons (filled arrowhead) do not express mature NGFβ-ir. B: After sympathectomy, although mature NGFβ-ir (white arrowhead) neurons are observed, the frequency of mature NGFβ-negative (filled arrowheads) neurons is increased. C: ProNGF-ir (white arrowheads) is prominent in cardiac ganglion neurons; a similar proportion does not stain for ProNGF (filled arrowhead). D: Following sympathectomy, neurons unstained for ProNGF (filled arrowheads) are increased within cardiac ganglia. E: Quantitative representation of the percentage of large cardiac ganglion neurons immunoreactive for mature NGFβ and proNGF. Scale bar in D is 40μm.
4. DISCUSSION
4.1. Organization of the rodent cardiac ganglion
The cardiac ganglion of the normal adult rat heart is well characterized as a classic parasympathetic ganglion composed of predominantly large autonomic neurons expressing cholinergic phenotypes (Hassall et al., 1986; Mawe et al., 1996; Kummer et al., 1998; Pauza et al., 2002). Stimulation of preganglionic vagal axons to the cardiac ganglion elicits 2 primary cholinergic effects; a slowing of heart rate via hyperpolarization of cardiac muscles, and prejunctional inhibition of excitatory sympathetic neurotransmission (Loffelholz et al., 1985). Accordingly, the predominant effects of cardiac ganglia on cardiac function are mediated by direct cholinergic hyperpolarization of sino-atrial and atrioventricular pacemaker conductive cells and indirect muscarinic inhibition of excitatory sympathetic axons (Loffelholz et al., 1985; Wetzel et al., 1985; Levy, 1990).
However, the cardiac ganglion is considerably more complicated than simply a cholinergic relay station. In addition to autonomic neurons with cholinergic properties, at least two cell types display adrenergic traits. Small neurons are abundant within the ganglion. These cells express the catecholaminergic synthetic enzymes TH and to a lesser degree DBH (Forsgren et al., 1990; Moravec et al., 1990), as well as the VMAT transporter protein, but not cholinergic markers such as VAChT. Glyoxylic acid treatment reveals intense histofluorescence indicating accumulation and storage of catecholamines. While the precise roles of these cells in cardiac autonomic neurotransmission are unclear, they are believed to represent small intensely fluorescent (SIF) cells which frequently populate autonomic ganglia (Baluk et al., 1990; Forsgren et al., 1990; Cheng et al., 1997; Horackova et al., 1999; Slavikova et al., 2003) and may be involved in local modulation of vascular tone and large cholinergic neuron function (Jacobowitz, 1967).
A second source of noradrenergic traits reside within the larger cardiac ganglion neurons themselves. Previous studies have shown that cardiac ganglion neurons express enzymes normally associated with catecholamine synthesis including DBH and TH (Singh et al., 1999; Slavikova et al., 2003), as well as monoamine transporter proteins as shown in the present study and previously in primate cardiac ganglia (Weihe et al., 2005). These catecholaminergic traits appear to be co-expressed to a large degree within individual neurons, as 89% of neurons with VMAT-ir also express VAChT. However, unlike SIF-like cells and sympathetic axons, large cardiac ganglion neurons with catecholaminergic proteins do not display glyoxylic-induced histofluorescence, consistent with previous reports that these cells do not contain detectable monoaminergic stores under normal conditions (Hassall et al., 1986; Baluk et al., 1990; Seabrook et al., 1990). There are several explanations as to why this may be the case. Both sympathetic and parasympathetic neurons derive from common precursor cells (Smith, 2008), and some catecholamine biosynthetic pathway proteins may be retained after differentiation while others are absent. Alternatively, biosynthetic enzymes may undergo pre- or post-translational modifications that diminish their biological activity, thus rendering catecholamine levels below those detectable by glyoxylic acid histochemistry.
In contrast to parasympathetic neurons, neonatal sympathetic neurons in culture can express a dual transmitter phenotype allowing both adrenergic and cholinergic modes of transmission depending on environmental signals (Furshpan et al., 1976; Furshpan et al., 1986; Matsumoto et al., 1987; Yang et al., 2002). This plasticity in neurotransmission is evidenced in vivo by neonatal sympathetic neurons projecting to the periosteum and sweat glands that undergo a phenotypic switch in transmission, from adrenergic to cholinergic, that is induced by target-derived factors (Habecker et al., 1994; Asmus et al., 2001). Whether conditions exist that may similarly modulate parasympathetic phenotype towards a full adrenergic profile remain to be determined, however our results indicate that sympathetic nerves promote expression of some adrenergic traits within cardiac parasympathetic neurons.
Several lines of evidence suggest that the neurochemical phenotypes of cardiac ganglion neurons may not be stable and can be influenced by several factors. In particular, studies of cranial parasympathetic ganglia have shown that noradrenergic sympathetic nerves play a significant role in modulating the expression of both cholinergic and adrenergic properties in the pterygopalatine ganglion (Mione et al., 1991; Warn et al., 1997). Hence, when sympathetic innervation to this cranial sympathetic parasympathetic ganglion is interrupted, neuronal expression of some cholinergic markers including VIP, nitric oxide synthase and NADPH diaphorase are reduced, implying that optimal expression of parasympathetic transmitter properties requires intact sympathetic innervation. Similarly, expression of catecholaminergic traits such as DBH and TH are concurrently upregulated in these parasympathetic neurons after sympathectomy (Mione et al., 1991; Warn et al., 1997). If similar mechanisms are operative in the heart, then this could have major implications regarding cardiac function under conditions such as congestive heart failure and Parkinson’s disease, both of which are characterized by sympathetic nerve abnormalities and possible disturbances in vagal control of heart rate (Azevedo et al., 1999; Goldstein et al., 2002; Goldstein, 2004; Sroka, 2004).
Unlike the cranial parasympathetic ganglion where noradrenergic innervation in the adult is derived strictly from the ipsilateral superior cervical sympathetic ganglion (Smith et al., 1987), catecholaminergic innervation to the cardiac ganglia derives potentially from both extrinsic and intrinsic sources, and these may potentially mediate different actions. Accordingly, in the present study we employed surgical sympathectomy which eliminates extrinsic noradrenergic innervation while leaving any intrinsic innervation intact, and guanethidine which is an effective sympatholytic (Chang et al., 1965) and also appears to impair catecholaminergic properties in atrial SIF cells (Kniazeva et al., 1982), thus providing a potential means for distinguishing between extrinsically and intrinsically mediated catecholaminergic actions on cardiac ganglion neuronal properties.
4.2. Effects of sympatholysis on cardiac ganglion neurochemical phenotype
To examine the effect of extrinsic sympathetic innervation, we excised sympathetic nerves projecting from the paravertebral ganglia to the cardiac ganglia. This reduced the numbers of cardiac ganglion neurons with VAChT-ir, consistent with the reductions in other parasympathetic markers noted previously in cranial parasympathetic ganglia post-sympathectomy (Fan et al., 1993; Hasan et al., 2000a). Interestingly, upregulation of catecholaminergic proteins in cranial parasympathetic neurons previously noted were not detected in surgically sympathectomized cardiac ganglia. This could be due either to an intrinsic difference in the response to sympathectomy, or possibly a result of the influence of intrinsic catecholaminergic systems, which may provide sufficient adrenergic input to suppress the full extent of phenotypic alterations. However, sympatholysis with guanethidine neither initiated alterations in VMAT phenotype nor augmented the suppression of VAChT expression seen after surgical sympathectomy. It therefore appears that, in contrast to extrinsic sympathetic innervation, intrinsic catecholaminergic traits do not play a significant role in determining the neurochemical phenotype of cardiac ganglion neurons. Although we have not investigated the functional consequence of attenuated parasympathetic phenotype, previous studies (Ferrari et al., 1996; Paton et al., 2005) indicate the ability of the parasympathetic system to increase cardiac output after sympathectomy through positive compensatory effects on heart rate.
4.3. Sympathetic nerves modulate NGF expression within cardiac ganglia
The diminution of parasympathetic neurochemical phenotype with reduced sympathetic input represents a potential mechanism whereby direct parasympathetic input to cardiac cells may be diminished, thus contributing to autonomic dysfunction in pathological states such as heart failure. However, another key deficit is the loss of parasympathetic axonal inhibition of excitatory sympathetic axons. Associations between nerves and targets typically involve production of neurotrophic proteins that serve to initiate and stabilize synaptic contacts. Perhaps the most potent neurotrophic protein for sympathetic neurons is mature NGF acting on the trkA receptor. We have shown previously that cranial parasympathetic neurons synthesize NGF, and that normal expression levels also require intact sympathetic neurotransmission. Because attenuated prejunctional parasympathetic inhibition of sympathetic transmitter release is a major contributor to cardiac autonomic dysfunction in congestive heart failure (Azevedo et al., 1999; Dunlap et al., 2003), we further investigated whether sympathetic disruption affected NGF expression in cardiac ganglion neurons.
Our findings demonstrate that NGF transcripts and the proNGF and mature NGF isoforms are present within cardiac ganglion neurons. The presence of NGF mRNA and proteins is consistent with synthesis and post-translational processing of this neurotrophin by cardiac ganglion parasympathetic neurons, and occurs in roughly the same proportions as those observed previously in cranial parasympathetic pterygopalatine ganglion neurons. Moreover, NGF protein appears to be transported anterogradely in parasympathetic axons (Hasan et al., 2000a). Thus, NGF synthesis by parasympathetic neurons appears to occur with relative frequency in normal adult parasympathetic neurons.
Because the prejunctional coupling of cardiac autonomic axons is important for inhibiting sympathetic cardiac transmitter release and this is impaired in congestive heart failure, we examined whether extrinsic and intrinsic catecholaminergic systems might also affect NGF expression in cardiac ganglion neurons. To demonstrate that adrenergic influences do affect cardiac ganglion NGF synthesis, we confirmed that guanethidine sympatholysis markedly reduced numbers of cardiac ganglion neurons with NGF transcripts, as noted previously in the pterygopalatine ganglion (Hasan et al., 2000a). We next assessed the effects of extrinsic sympathectomy on the 2 major NGF isoforms, mature NGF (NGFβ) and its precursor protein proNGF. The relative abundance of these 2 isoforms may be of critical significance, given that mature NGF binds with high affinity to the trkA receptor thereby promoting axon growth and neuronal survival whereas proNGF binds preferentially to the pan-neurotrophin receptor p75 and can induce cell death and axon degeneration (Lee et al., 2001; Lu et al., 2005; Reichardt, 2006).
Following surgical sympathectomy, there was a clear reduction in the number of cardiac ganglion neurons expressing mature NGF, consistent with the hypothesis that adrenergic input to cardiac ganglion neurons is necessary for NGF production. While the mechanism by which sympathetic innervation modulates parasympathetic NGF synthesis is unclear, cardiac ganglion neurons express beta-adrenergic receptors and respond to beta-adrenergic agonists (Horackova et al., 1993; Huang et al., 1993), and studies in a number of tissues show that adrenoceptor activation can increase NGF (Dal Toso et al., 1988; Carswell et al., 1992; Hayes et al., 1995; Semkova et al., 1996; Culmsee et al., 1999; Samina Riaz et al., 2000; Colangelo et al., 2004). Hence, a reduction in cardiac ganglion neuronal adrenoceptor activation following sympathetic denervation provides a plausible explanation for the reduced NGF expression. It is also noteworthy that guanethidine treatment was no more efficacious in reducing numbers of neurons expressing NGF than was surgical sympathectomy, again suggesting that the major noradrenergic system regulating cardiac ganglion neuronal phenotype is the extrinsic sympathetic innervation.
However, an important distinction between sympathectomy and sympatholysis is in the response to proNGF expression. While surgical sympathectomy reduced both mature and proNGF-ir, chemical sympatholysis selectively reduced only the mature form. Because maintained levels of proNGF in the face of diminished mature NGF might be expected to further contribute to retraction of prejunctional sympathetic associations, it is interesting to speculate that some intrinsic adrenergic factor is acting beneficially to suppress proNGF expression after surgical sympathectomy, but that this is impaired by guanethidine treatment.
5. CONCLUSION
Our findings provide support for the idea that both neurochemical and trophic support mechanisms in the rodent cardiac ganglion are dependent upon noradrenergic input. While both extrinsic (sympathetic) and intrinsic catecholaminergic systems could potentially both contribute to cardiac ganglion properties, the extrinsic innervation appears to be the predominating influence, with the exception of regulating proNGF expression.
Disturbances to the catecholaminergic input to the cardiac ganglion could have serious consequences on 2 fronts. First, diminutions in cholinergic phenotype could reduce the ability of the parasympathetic system to directly suppress cardiac cell excitability. Second, alterations in production of neurotrophic proteins could facilitate uncoupling between axo-axonal synapses inhibiting noradrenaline release. Because sympathetic function is impaired in congestive heart failure, both through diminution in numbers of intact nerves and through down-regulation of beta-adrenoceptor mechanisms (Himura et al., 1993; Ungerer et al., 1994; Ungerer et al., 1998; Ogita et al., 2001; Brodde, 2007), further study is necessary to determine the extent to which altered cardiac ganglion properties contribute to post-ischemic parasympathetic dysfunction.
Acknowledgments
Supported by NIH HL079652 (PGS, WH) with core support by RR016475 and HD02528. The authors would like to thank Ms. Gwenaelle Wernli for immunoblot analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asmus SE, Tian H, Landis SC. Induction of cholinergic function in cultured sympathetic neurons by periosteal cells: cellular mechanisms. Dev Biol. 2001;235:1–11. doi: 10.1006/dbio.2001.0282. [DOI] [PubMed] [Google Scholar]
- Azevedo ER, Parker JD. Parasympathetic control of cardiac sympathetic activity: normal ventricular function versus congestive heart failure. Circulation. 1999;100:274–279. doi: 10.1161/01.cir.100.3.274. [DOI] [PubMed] [Google Scholar]
- Baluk P, Gabella G. Some parasympathetic neurons in the guinea-pig heart express aspects of the catecholaminergic phenotype in vivo. Cell Tissue Res. 1990;261:275–285. doi: 10.1007/BF00318669. [DOI] [PubMed] [Google Scholar]
- Brodde OE. Beta-adrenoceptor blocker treatment and the cardiac beta-adrenoceptor-G-protein(s)-adenylyl cyclase system in chronic heart failure. Naunyn Schmiedebergs Arch Pharmacol. 2007;374:361–372. doi: 10.1007/s00210-006-0125-7. [DOI] [PubMed] [Google Scholar]
- Carswell S, Hoffman EK, Clopton-Hartpence K, Wilcox HM, Lewis ME. Induction of NGF by isoproterenol, 4-methylcatechol and serum occurs by three distinct mechanisms. Brain Res Mol Brain Res. 1992;15:145–150. doi: 10.1016/0169-328x(92)90162-5. [DOI] [PubMed] [Google Scholar]
- Chang CC, Costa E, Brodie BB. Interaction of Guanethidine with Adrenergic Neurons. J Pharmacol Exp Ther. 1965;147:303–312. [PubMed] [Google Scholar]
- Cheng Z, Powley TL, Schwaber JS, Doyle FJ., 3rd Vagal afferent innervation of the atria of the rat heart reconstructed with confocal microscopy. J Comp Neurol. 1997;381:1–17. doi: 10.1002/(sici)1096-9861(19970428)381:1<1::aid-cne1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Chun LL, Patterson PH. Role of nerve growth factor in the development of rat sympathetic neurons in vitro. I. Survival, growth, and differentiation of catecholamine production. J Cell Biol. 1977;75:694–704. doi: 10.1083/jcb.75.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colangelo AM, Mallei A, Johnson PF, Mocchetti I. Synergistic effect of dexamethasone and beta-adrenergic receptor agonists on the nerve growth factor gene transcription. Brain Res Mol Brain Res. 2004;124:97–104. doi: 10.1016/j.molbrainres.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Culmsee C, Semkova I, Krieglstein J. NGF mediates the neuroprotective effect of the beta2-adrenoceptor agonist clenbuterol in vitro and in vivo: evidence from an NGF-antisense study. Neurochem Int. 1999;35:47–57. doi: 10.1016/s0197-0186(99)00032-7. [DOI] [PubMed] [Google Scholar]
- Dal Toso R, De Bernardi MA, Brooker G, Costa E, Mocchetti I. Beta adrenergic and prostaglandin receptor activation increases nerve growth factor mRNA content in C6-2B rat astrocytoma cells. J Pharmacol Exp Ther. 1988;246:1190–1193. [PubMed] [Google Scholar]
- Dunlap ME, Bibevski S, Rosenberry TL, Ernsberger P. Mechanisms of altered vagal control in heart failure: influence of muscarinic receptors and acetylcholinesterase activity. Am J Physiol Heart Circ Physiol. 2003;285:H1632–1640. doi: 10.1152/ajpheart.01051.2002. [DOI] [PubMed] [Google Scholar]
- Fan Q, Smith PG. Decreased vasoactive intestinal polypeptide-immunoreactivity of parasympathetic neurons and target innervation following long-term sympathectomy. Regul Pept. 1993;48:337–343. doi: 10.1016/0167-0115(93)90162-2. [DOI] [PubMed] [Google Scholar]
- Ferrari AU, Franzelli C, Daffonchio A, Perlini S, Dirienzo M. Sympathovagal interplay in the control of overall blood pressure variability in unanesthetized rats. Am J Physiol. 1996;270:H2143–2148. doi: 10.1152/ajpheart.1996.270.6.H2143. [DOI] [PubMed] [Google Scholar]
- Forsgren S, Moravec M, Moravec J. Catecholamine-synthesizing enzymes and neuropeptides in rat heart epicardial ganglia; an immunohistochemical study. Histochem J. 1990;22:667–676. doi: 10.1007/BF01047451. [DOI] [PubMed] [Google Scholar]
- Furshpan EJ, Landis SC, Matsumoto SG, Potter DD. Synaptic functions in rat sympathetic neurons in microcultures. I. Secretion of norepinephrine and acetylcholine. J Neurosci. 1986;6:1061–1079. doi: 10.1523/JNEUROSCI.06-04-01061.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furshpan EJ, MacLeish PR, O’Lague PH, Potter DD. Chemical transmission between rat sympathetic neurons and cardiac myocytes developing in microcultures: evidence for cholinergic, adrenergic, and dual-function neurons. Proc Natl Acad Sci USA. 1976;73:4225–4229. doi: 10.1073/pnas.73.11.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS. Functional neuroimaging of sympathetic innervation of the heart. Ann N Y Acad Sci. 2004;1018:231–243. doi: 10.1196/annals.1296.028. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, Robertson D, Esler M, Straus SE, Eisenhofer G. Dysautonomias: clinical disorders of the autonomic nervous system. Ann Intern Med. 2002;137:753–763. doi: 10.7326/0003-4819-137-9-200211050-00011. [DOI] [PubMed] [Google Scholar]
- Habecker BA, Landis SC. Noradrenergic regulation of cholinergic differentiation. Science. 1994;264:1602–1604. doi: 10.1126/science.8202714. [DOI] [PubMed] [Google Scholar]
- Hansson M, Kjorell U, Forsgren S. Increased immunoexpression of atrial natriuretic peptide in the heart conduction system of the rat after cardiac sympathectomy. J Molec Cell Cardiol. 1998;30:2047–2057. doi: 10.1006/jmcc.1998.0767. [DOI] [PubMed] [Google Scholar]
- Hasan W, Smith HJ, Ting AY, Smith PG. Estrogen alters trkA and p75 neurotrophin receptor expression within sympathetic neurons. J Neurobiol. 2005;65:192–204. doi: 10.1002/neu.20183. [DOI] [PubMed] [Google Scholar]
- Hasan W, Smith PG. Nerve growth factor expression in parasympathetic neurons: regulation by sympathetic innervation. Eur J Neurosci. 2000a;12:4391–4397. doi: 10.1046/j.0953-816x.2000.01353.x. [DOI] [PubMed] [Google Scholar]
- Hasan W, Zhang R, Liu M, Warn JD, Smith PG. Coordinate expression of NGF and alpha-smooth muscle actin mRNA and protein in cutaneous wound tissue of developing and adult rats. Cell Tissue Res. 2000b;300:97–109. doi: 10.1007/s004410000175. [DOI] [PubMed] [Google Scholar]
- Hassall CJ, Burnstock G. Intrinsic neurones and associated cells of the guinea-pig heart in culture. Brain Res. 1986;364:102–113. doi: 10.1016/0006-8993(86)90991-1. [DOI] [PubMed] [Google Scholar]
- Hayes VY, Isackson PJ, Fabrazzo M, Follesa P, Mocchetti I. Induction of nerve growth factor and basic fibroblast growth factor mRNA following clenbuterol: contrasting anatomical and cellular localization. Exp Neurol. 1995;132:33–41. doi: 10.1016/0014-4886(95)90056-x. [DOI] [PubMed] [Google Scholar]
- Himura Y, Felten SY, Kashiki M, Lewandowski TJ, Delehanty JM, Liang CS. Cardiac noradrenergic nerve terminal abnormalities in dogs with experimental congestive heart failure. Circulation. 1993;88:1299–309. doi: 10.1161/01.cir.88.3.1299. [DOI] [PubMed] [Google Scholar]
- Horackova M, Armour JA, Byczko Z. Distribution of intrinsic cardiac neurons in whole-mount guinea pig atria identified by multiple neurochemical coding. A confocal microscope study. Cell Tissue Res. 1999;297:409–421. doi: 10.1007/s004410051368. [DOI] [PubMed] [Google Scholar]
- Horackova M, Huang MH, Armour JA, Hopkins DA, Mapplebeck C. Cocultures of adult ventricular myocytes with stellate ganglia or intrinsic cardiac neurones from guinea pigs: spontaneous activity and pharmacological properties. Cardiovasc Res. 1993;27:1101–1108. doi: 10.1093/cvr/27.6.1101. [DOI] [PubMed] [Google Scholar]
- Huang MH, Sylven C, Pelleg A, Smith FM, Armour JA. Modulation of in situ canine intrinsic cardiac neuronal activity by locally applied adenosine, ATP, or analogues. Am J Physiol. 1993;265:R914–922. doi: 10.1152/ajpregu.1993.265.4.R914. [DOI] [PubMed] [Google Scholar]
- Jacobowitz D. Histochemical studies of the relationship of chromaffin cells and adrenergic nerve fibers to the cardiac ganglia of several species. J Pharmacol Exp Ther. 1967;158:227–240. [PubMed] [Google Scholar]
- Kniazeva LA, Iarygin VN, Pylaev AS. [Cytofluorimetric study of the small, intensely fluorescent cells of the rat atria in pharmacological sympathectomy] Biulleten’ eksperimental’noi biologii i meditsiny. 1982;94:90–92. [PubMed] [Google Scholar]
- Kohn J, Aloyz RS, Toma JG, Haak-Frendscho M, Miller FD. Functionally antagonistic interactions between the TrkA and p75 neurotrophin receptors regulate sympathetic neuron growth and target innervation. J Neurosci. 1999;19:5393–5408. doi: 10.1523/JNEUROSCI.19-13-05393.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsching S, Thoenen H. Developmental changes of nerve growth factor levels in sympathetic ganglia and their target organs. Dev Biol. 1988;126:40–46. doi: 10.1016/0012-1606(88)90236-9. [DOI] [PubMed] [Google Scholar]
- Kummer W, Fink L, Dvorakova M, Haberberger R, Bohle RM. Rat cardiac neurons express the non-coding R-exon (exon 1) of the cholinergic gene locus. Neuroreport. 1998;9:2209–2212. doi: 10.1097/00001756-199807130-00011. [DOI] [PubMed] [Google Scholar]
- Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- Levy MN. Autonomic interactions in cardiac control. Ann N Y Acad Sci. 1990;601:209–221. doi: 10.1111/j.1749-6632.1990.tb37302.x. [DOI] [PubMed] [Google Scholar]
- Lockhart ST, Mead JN, Pisano JM, Slonimsky JD, Birren SJ. Nerve growth factor collaborates with myocyte-derived factors to promote development of presynaptic sites in cultured sympathetic neurons. J Neurobiol. 2000;42:460–476. [PubMed] [Google Scholar]
- Loffelholz K, Pappano AJ. The parasympathetic neuroeffector junction of the heart. Pharmacol Rev. 1985;37:1–24. [PubMed] [Google Scholar]
- Loiacono RE, Story DF. Effect of alpha-adrenoceptor agonists and antagonists on cholinergic transmission in guinea-pig isolated atria. Naunyn Schmiedebergs Arch Pharmacol. 1986;334:40–47. doi: 10.1007/BF00498738. [DOI] [PubMed] [Google Scholar]
- Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat Rev Neurosci. 2005;6:603–614. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- Matsumoto SG, Sah D, Potter DD, Furshpan EJ. Synaptic functions in rat sympathetic neurons in microcultures. IV. Nonadrenergic excitation of cardiac myocytes and the variety of multiple-transmitter states. J Neurosci. 1987;7:380–390. doi: 10.1523/JNEUROSCI.07-02-00380.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawe GM, Talmage EK, Lee KP, Parsons RL. Expression of choline acetyltransferase immunoreactivity in guinea pig cardiac ganglia. Cell Tissue Res. 1996;285:281–286. doi: 10.1007/s004410050645. [DOI] [PubMed] [Google Scholar]
- Maxwell RA. Guanethidine after twenty years: a pharmacologist’s perspective. Br J Clin Pharmacol. 1982;13:35–44. doi: 10.1111/j.1365-2125.1982.tb01334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mione MC, Sancesario G, D’Angelo V, Bernardi G. Increase of dopamine beta-hydroxylase immunoreactivity in non-noradrenergic nerves of rat cerebral arteries following long-term sympathectomy. Neurosci Lett. 1991;123:167–171. doi: 10.1016/0304-3940(91)90922-g. [DOI] [PubMed] [Google Scholar]
- Moravec M, Moravec J, Forsgren S. Catecholaminergic and peptidergic nerve components of intramural ganglia in the rat heart. An immunohistochemical study. Cell Tissue Res. 1990;262:315–327. doi: 10.1007/BF00309887. [DOI] [PubMed] [Google Scholar]
- Nihei M, Lee JK, Honjo H, Yasui K, Uzzaman M, Kamiya K, Opthof T, Kodama I. Decreased vagal control over heart rate in rats with right-sided congestive heart failure: downregulation of neuronal nitric oxide synthase. Circ J. 2005;69:493–499. doi: 10.1253/circj.69.493. [DOI] [PubMed] [Google Scholar]
- Ogita H, Shimonagata T, Fukunami M, Kumagai K, Yamada T, Asano Y, Hirata A, Asai M, Kusuoka H, Hori M, Hoki N. Prognostic significance of cardiac (123)I metaiodobenzylguanidine imaging for mortality and morbidity in patients with chronic heart failure: a prospective study. Heart (British Cardiac Society) 2001;86:656–660. doi: 10.1136/heart.86.6.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardini BJ, Lund DD, Schmid PG. Organization of the sympathetic postganglionic innervation of the rat heart. J Auton Nerv Syst. 1989;28:193–201. doi: 10.1016/0165-1838(89)90146-x. [DOI] [PubMed] [Google Scholar]
- Paton JF, Boscan P, Pickering AE, Nalivaiko E. The yin and yang of cardiac autonomic control: vago-sympathetic interactions revisited. Brain Res Brain Res Rev. 2005;49:555–565. doi: 10.1016/j.brainresrev.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Pauza DH, Pauziene N, Pakeltyte G, Stropus R. Comparative quantitative study of the intrinsic cardiac ganglia and neurons in the rat, guinea pig, dog and human as revealed by histochemical staining for acetylcholinesterase. Ann Anat. 2002;184:125–136. doi: 10.1016/S0940-9602(02)80005-X. [DOI] [PubMed] [Google Scholar]
- Randall DC, Brown DR, Mcardiac ganglionuirt AS, Thompson GW, Armour JA, Ardell JL. Interactions within the intrinsic cardiac nervous system contribute to chronotropic regulation. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1066–1075. doi: 10.1152/ajpregu.00167.2003. [DOI] [PubMed] [Google Scholar]
- Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush RA, Chie E, Liu D, Tafreshi A, Zettler C, Zhou XF. Neurotrophic factors are required by mature sympathetic neurons for survival, transmission and connectivity. Clin Exp Pharm Physiol. 1997;24:549–555. doi: 10.1111/j.1440-1681.1997.tb02089.x. [DOI] [PubMed] [Google Scholar]
- Samina Riaz S, Tomlinson DR. Pharmacological modulation of nerve growth factor synthesis: a mechanistic comparison of vitamin D receptor and beta(2)-adrenoceptor agonists. Brain Res Mol Brain Res. 2000;85:179–188. doi: 10.1016/s0169-328x(00)00254-0. [DOI] [PubMed] [Google Scholar]
- Seabrook GR, Fieber LA, Adams DJ. Neurotransmission in neonatal rat cardiac ganglion in situ. Am J Physiol. 1990;259:H997–1005. doi: 10.1152/ajpheart.1990.259.4.H997. [DOI] [PubMed] [Google Scholar]
- Semkova I, Schilling M, Henrich-Noack P, Rami A, Krieglstein J. Clenbuterol protects mouse cerebral cortex and rat hippocampus from ischemic damage and attenuates glutamate neurotoxicity in cultured hippocampal neurons by induction of NGF. Brain Res. 1996;717:44–54. doi: 10.1016/0006-8993(95)01567-1. [DOI] [PubMed] [Google Scholar]
- Singh S, Johnson PI, Javed A, Gray TS, Lonchyna VA, Wurster RD. Monoamine- and histamine-synthesizing enzymes and neurotransmitters within neurons of adult human cardiac ganglia. Circulation. 1999;99:411–419. doi: 10.1161/01.cir.99.3.411. [DOI] [PubMed] [Google Scholar]
- Slavikova J, Kuncova J, Reischig J, Dvorakova M. Catecholaminergic neurons in the rat intrinsic cardiac nervous system. Neurochem Res. 2003;28:593–598. doi: 10.1023/a:1022837810357. [DOI] [PubMed] [Google Scholar]
- Smith PG. Role of the sympathetic nervous system in functional maturation of Muller’s smooth muscle in the rat. J Pharmacol Exp Ther. 1985;235:330–334. [PubMed] [Google Scholar]
- Smith PG. Autonomic Neuroplasticity: Development. In: Squire LAT, Bloom F, Gage F, Spitzer N, editors. New Encyclopedia of Neuroscience. 4. Elsevier; Oxford: 2008. in press. [Google Scholar]
- Smith PG, Bruckert JW, Mills E. Reinnervation of Muller’s smooth muscle by atypical sympathetic pathways following neonatal ganglionectomy in the rat: structural and functional investigations of enhanced neuroplasticity. Neuroscience. 1987;23:781–793. doi: 10.1016/0306-4522(87)90095-9. [DOI] [PubMed] [Google Scholar]
- Smith PG, Fan Q. Sympathetic nerve trajectories to rat orbital targets: role of connective tissue pathways. J Comp Neurol. 1996;365:69–78. doi: 10.1002/(SICI)1096-9861(19960129)365:1<69::AID-CNE6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Smith PG, Warn JD, Steinle JJ, Krizsan-Agbas D, Hasan W. Modulation of parasympathetic neuron phenotype and function by sympathetic innervation. Auton Neurosci. 2002;96:33–42. doi: 10.1016/s1566-0702(01)00371-x. [DOI] [PubMed] [Google Scholar]
- Sroka K. On the genesis of myocardial ischemia. Zeitschrift fur Kardiologie. 2004;93:768–783. doi: 10.1007/s00392-004-0137-6. [DOI] [PubMed] [Google Scholar]
- Tseng WY, Tsao CF, Ko CC, Huang HT. Local capsaicin application to the stellate ganglion and stellatectomy attenuate neurogenic inflammation in rat bronchi. Auton Neurosci. 2001;94:25–33. doi: 10.1016/S1566-0702(01)00361-7. [DOI] [PubMed] [Google Scholar]
- Ungerer M, Hartmann F, Karoglan M, Chlistalla A, Ziegler S, Richardt G, Overbeck M, Meisner H, Schomig A, Schwaiger M. Regional in vivo and in vitro characterization of autonomic innervation in cardiomyopathic human heart. Circulation. 1998;97:174–180. doi: 10.1161/01.cir.97.2.174. [DOI] [PubMed] [Google Scholar]
- Ungerer M, Parruti G, Bohm M, Puzicha M, DeBlasi A, Erdmann E, Lohse MJ. Expression of beta-arrestins and beta-adrenergic receptor kinases in the failing human heart. Circ Res. 1994;74:206–13. doi: 10.1161/01.res.74.2.206. [DOI] [PubMed] [Google Scholar]
- Warn JD, Fan Q, Smith PG. Decreased neuronal nitric oxide synthase-immunoreactivity and NADPH-diaphorase activity in rat pterygopalatine ganglion parasympathetic neurons and cerebrovascular innervation following long-term sympathectomy. Neurosci Lett. 1997;232:25–28. doi: 10.1016/s0304-3940(97)00566-1. [DOI] [PubMed] [Google Scholar]
- Weihe E, Schutz B, Hartschuh W, Anlauf M, Schafer MK, Eiden LE. Coexpression of cholinergic and noradrenergic phenotypes in human and nonhuman autonomic nervous system. J Comp Neurol. 2005;492:370–379. doi: 10.1002/cne.20745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel GT, Brown JH. Presynaptic modulation of acetylcholine release from cardiac parasympathetic neurons. Am J Physiol. 1985;248:H33–39. doi: 10.1152/ajpheart.1985.248.1.H33. [DOI] [PubMed] [Google Scholar]
- Yang B, Slonimsky JD, Birren SJ. A rapid switch in sympathetic neurotransmitter release properties mediated by the p75 receptor. Nat Neurosci. 2002;5:539–545. doi: 10.1038/nn0602-853. [DOI] [PubMed] [Google Scholar]