Abstract
Bacterial pathogens use virulence strategies to invade epithelial barriers, but active processes of epithelial cells may also contribute to the endocytosis of microbial particles. To focus on the latter, we studied the uptake of fixed and fluorescently labeled bacterial particles in intestinal and bronchoepithelial cell cultures and found it to be enhanced in Caco-2BBe and NCI-H292 cells after treatment with tumor necrosis factor alpha and an agonist antibody against the lymphotoxin beta receptor. Confocal fluorescence microscopy, flow cytometry, and transmission electron microscopy revealed that Staphylococcus aureus and Yersinia enterocolitica were readily endocytosed, although there was scant uptake of Shigella sonnei, Salmonella enterica serovar Typhimurium, and Klebsiella pneumoniae particles. Endocytosed Staphylococcus was often associated with cytoplasmic claudin-4 vesicles; this was not found for Yersinia, suggesting that cytokine treatment upregulated two distinct endocytosis pathways. Interestingly, when Staphylococcus and Yersinia were coincubated with epithelial monolayers, the cells were unlikely to take up Yersinia unless they had also endocytosed large numbers of Staphylococcus particles, although the two bacteria were apparently processed in distinct compartments. Cytokine treatment induced an upregulation and redistribution of β1 integrin to the apical surface of NCI-H292 cells; consistent with this effect, treatment with anti-β1 integrin antibody blocked uptake of both Yersinia and Staphylococcus in NCI-H292 and Caco-2BBe cells. Our results suggest that capture of bacterial particles by mucosal epithelial cells is selective and that different endocytic mechanisms are enhanced by proinflammatory cytokines.
Chronic inflammation is associated with the production of cytokines such as tumor necrosis factor alpha (TNF-α), and lymphotoxin beta receptor ligands such as lymphotoxin α1β2 (LTα1β2) and LIGHT (16). In a seemingly disparate but equally critical role, TNF receptor (TNFR) ligands are also necessary for organogenesis and maturation of secondary lymphoid structures such as intestinal Peyer's patches and lymph nodes (5, 13, 14). The multiple effects of these cytokines on cells with essential roles in immune defense suggest that there are more complex aspects of the response to lymphotoxin/TNF ligands, including differences in the kinetics of cytokine production and the local tissue context of cytokine response. This may be especially true in the mucosal epithelium, where local cytokines may radically affect barrier integrity (3).
Along with gamma interferon (IFN-γ), TNF-α has been implicated in the compromise of epithelial barrier function, which may result in entry and colonization by pathogenic bacteria ordinarily excluded from the subepithelial mucosa (7, 27). Among the mechanisms by which TNF-α may affect barrier function are loss of cells of the monolayer to apoptosis (8), internalization of tight junction proteins with consequent loss of tight junction integrity (1), and rearrangement of the perijunctional cytoskeleton (28). Although these studies point to important alterations of paracellular junctions by TNF-α, they do not take into consideration how epithelial cells themselves may actively facilitate transmembrane entry of pathogenic bacteria under proinflammatory conditions.
Here, we compared the effects of incubating epithelial monolayers from both the bronchus and the intestine with a selection of fixed and fluorescently labeled bacterial pathogens, and we examined how cells manage these particles after culture with LT/TNFR ligands. Intriguingly, we found that cytokine treatment induced a striking increase in endocytosis of select bacteria across epithelial cell surfaces. This effect was demonstrated in both an airway cell line (NCI-H292) and an intestinal epithelial cell line (C2BBe, a subclone of Caco2). Because pathogens including Staphylococcus, Streptococcus, and Yersinia have been shown to effect invasion by exploiting epithelial surface receptors via binding to the integrin β1 subunit (9, 23), we examined how TNF-α and LT might alter β1 integrin expression in polarized epithelia. In the case of NCI-H292 cells, cytokine treatment correlated with an upregulation and redistribution of β1 integrin to the apical surface; accordingly, blockade of β1 integrin inhibited bacterial particle uptake. While the two cell lines exhibited some differences in their behavior, enhanced bacterial particle uptake under proinflammatory conditions suggests conserved mechanisms whereby epithelial cells may actively engage infectious organisms at the mucosal barrier.
MATERIALS AND METHODS
Cell culture.
Cell lines NCI-H292 (ATCC CRL-1848) and a subclone of Caco-2, C2BBe (ATCC CRL-2102), were obtained from the American Type Culture Collection and cultured using recommended medium preparations. Freshly passaged cells were seeded onto 0.4-μm-pore-size polycarbonate filter supports (Transwell filters; Corning Life Sciences) in standard media or with the added cytokine LTβR agonist antibody (5 μg/ml; R&D Systems) or recombinant TNF-α (100 ng/ml; Peprotech) or both. Treatment of cells with cytokines was generally done over a period of 7 days. In endocytosis studies, cells in Transwell cultures were cultured with or without medium including LTβR agonist and/or TNF-α for 6 days (until stable transepithelial electrical resistance values were attained), and Alexa Fluor 488-labeled Staphylococcus aureus (Invitrogen) or Alexa Fluor-labeled bacterial strains cultured in our lab were then added during the final 12 h (C2BBe) or 2 h (NCI-H292) of culture. To block β1-integrin receptors on epithelial cells, 10 μg of mouse anti-β1 (Chemicon/Millipore)/ml was added to the culture medium during the last hour of incubation before bacterial particles were introduced.
Bacterial culture.
Yersinia enterocolitica (ATCC 29913) and Klebsiella pneumoniae (ATCC 13883) were obtained from ATCC; Shigella sonnei and Salmonella enterica serovar Typhimurium were reconstituted from University of California, Riverside, medical microbiology laboratory teaching stocks, and confirmation of genus and species was achieved by the appropriate API identification kit. All cultures were stored at −80°C and subcultured to Trypticase soy broth (BD) and shaken at 200 rpm at 37°C. Overnight broth cultures were diluted 1:20, grown to mid-log phase, and harvested for labeling. Bacteria were pelleted and washed three times, fixed in phosphate-buffered saline (PBS) with 1% paraformaldehyde and 0.05% sodium azide for 30 min, washed twice more, and labeled at room temperature with stirring overnight (21 ± 1 h) with Alexa Fluor 488-TFP ester, Alexa Fluor 568-succinimidyl ester, or Alexa Fluor 647-succinimidyl ester (Invitrogen) in 0.1 M NaHCO3. Labeled particles were washed three times to remove free fluorophore and resuspended for storage at 4°C in PBS with 0.05% NaN3.
Immunofluorescence staining.
Cell cultures were fixed by using 1% paraformaldehyde-PBS and then permeabilized in 0.5% Triton X-100-PBS, washed in 0.1% Triton X-100-PBS, and blocked in 0.1% Triton X-100-PBS containing 2% bovine serum albumin. Cells were stained with antibodies to the tight junction proteins claudin-4 (Abcam) and/or zona occludens-1 (ZO-1; Zymed) and/or β1 integrin (Chemicon/Millipore) followed by secondary reagents Alexa Fluor 488-, Alexa Fluor 568-, or Alexa Fluor 647-conjugated anti-rabbit or anti-mouse antibodies (Invitrogen). DAPI (4′,6′-diamidino-2-phenylindole; Invitrogen) was used as a nuclear counterstain. Slides were mounted using ProLong Gold antifade reagent (Invitrogen).
Confocal microscopy.
Prepared slides were examined with a ×63 water immersion objective using a Zeiss Observer Z1 inverted epifluorescence microscope equipped with a BD CARV II spinning disk confocal imager, and images were collected with IPLab for Windows 4.0 software (BD Biosciences, Rockville, MD). Fluorescent images are either maximum image projections of z-stacks taken in multiple consecutive optical planes or three-dimensional snapshots following image sequence deconvolution with Volocity imaging software (Improvision, Waltham, MA).
TEM.
Cell cultures were washed twice in PBS and fixed in PBS-2% paraformaldehyde-2% glutaraldehyde. Ultrathin (80-nm) sections of cell-covered filters were prepared for transmission electron microscopy (TEM) analysis by standard methods, as previously described (12). Observations were made by using a Phillips Tecnai 12 microscope.
Flow cytometry.
Cells were plated on 24-well plates with or without inductive cytokines and cultured for 48 h. Then, during the last 12 h of incubation (C2BBe) or the last 2 h of incubation (NCI-H292), fluorescent bacterial particles were added at a ratio of 200 bacteria per cell unless otherwise indicated. Cells were detached from plates using EDTA (Gibco) and washed three times, and data were collected by using a FACSCalibur (Becton Dickinson) equipped with CellQuest Pro software (BD Bioscience). Cell viability was assessed by a trypan blue exclusion assay prior to flow cytometry analysis and confirmed by forward and side scatter. The data analysis was performed using FlowJo software (Tree Star).
RESULTS
Preferential endocytosis of S. aureus and Y. enterocolitica by epithelial cells is enhanced in the presence of proinflammatory cytokines.
Pathogenic bacteria use diverse virulence strategies such as endotoxin production, intracellular signaling, and molecular mimicry to breach epithelial defenses (6). To examine how epithelial cells might actively mediate endocytosis of bacterial pathogens, we incubated intestinal epithelial cell monolayers with selected respiratory and enteric pathogens which had been fixed, killed, and fluorescently labeled. We assessed particle uptake by using confocal microscopy for qualitative studies; for more quantitative analysis, we also used flow cytometry of monolayers that had been detached after incubation with fluorescent particles. This method enabled us to sample thousands of cells per experiment and to generate quantitative estimates not only of the proportion of cells taking up particles but also of the number of particles per cell.
In a preliminary experiment, we found that NCI-H292T cells did not survive overnight exposure to bacterial particles; time course trials revealed that 2 h of incubation with particles was sufficient to yield measurable ingestion. In the more orderly, tightly packed monolayers of columnar C2BBe intestinal cells, it was necessary to expose cell layers to particles for a minimum of 12 h or overnight in order to observe measurable differences in endocytosis among treatments.
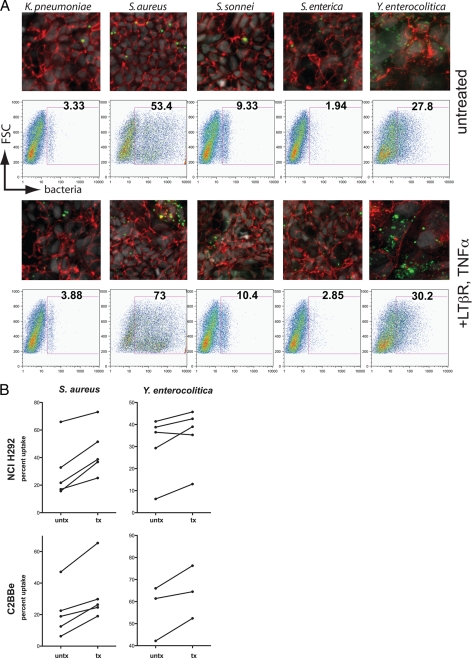
Fluorescence microscopy revealed that in C2BBe cells the quantity and pattern of uptake varied by bacterial species. S. enterica serovar Typhimurium, Shigella sonnei, and K. pneumoniae were taken up sparsely, whereas S. aureus and Y. enterocolitica appeared to be readily endocytosed. Yersinia was diffusely ingested, with many cells having taken up a few bacteria. The pattern of uptake for Staphylococcus was notably different in that a subset of cells appeared to have ingested hundreds of particles (Fig. 1A). These observations were quantitatively confirmed by flow cytometry analysis, which showed by fluorescence shift that 53.4% of cells had taken up some Staphylococcus particles and that 27.8% had ingested Yersinia. Shigella particles were sparsely taken up, whereas Klebsiella and Salmonella were largely excluded from endocytosis.
FIG. 1.
(A) Confocal spinning-disk microscopy images and flow cytometry analysis of fixed, Alexa Fluor 488-conjugated bacterial particles coincubated with C2BBe monolayers 6 days after cells were seeded onto permeable filter supports (green, bacterial particles; red, ZO-1; gray, DAPI). Flow cytometry gates indicate cells positive for uptake of fluorescently labeled bacteria. (B) Intestinal and bronchoepithelial cells cultured in the presence of LTβR agonist and TNF-α ingest more S. aureus and Y. enterocolitica particles than do untreated monolayers.
In 2004, Katakai et al. (10) reported that a combination of the proinflammatory cytokines TNF-α and lymphotoxin β induced lymphoid stromal cell differentiation. Since the epithelia overlying lymphoid tissue of the mucosa are enriched in cells specialized for antigen sampling (11, 18, 19), we wondered whether exposing epithelial cells to this particular cytokine combination could induce bacterial particle uptake in cell monolayers. We examined the effects of growing intestinal and airway epithelial cells in the presence of TNF-α and LTβR on their propensity to ingest bacterial pathogens and noted a modest but consistent increase in uptake by intestinal cells of all pathogens in the presence of cytokines (Fig. 1A), both by qualitative fluorescence microscopy and by flow cytometric quantitation. Most notable, however, was the endocytosis of S. aureus and Y. enterocolitica particles. Although the distribution of Yersinia uptake appeared regular, with many cells ingesting 1 to 10 bacterial particles, some BBe cells were observed to have ingested large numbers of staphylococci. We observed a similar pattern of uptake when these same bacterial particles were incubated with airway epithelial cells. Treatment of monolayers with LTβR agonist and TNF-α caused the greatest increases in endocytosis of Staphylococcus in both NCI-H292 (14.4% ± 2.8%, n = 5) and C2BBe (11.6% ± 2.3%, n = 5) monolayers, although cytokine treatment also increased endocytosis of Yersinia in both cell types (Fig. 1B). Although we found that treatment of cell monolayers with either TNF-α or LTβR agonist increased endocytosis, neither cytokine alone increased the uptake to the same degree as the two in combination.
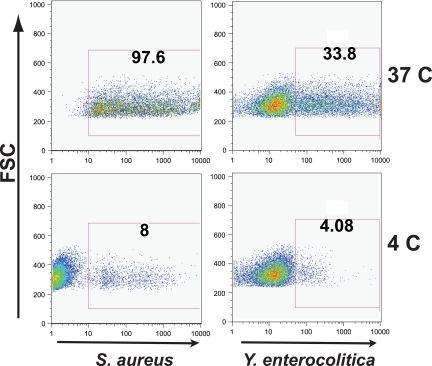
To confirm that the observed association between fluorescently labeled bacteria and epithelial cells was not due solely to adherence, experiments were repeated by incubating cells at 4°C for 1 h prior to the addition of bacterial particle suspension and maintaining that temperature throughout the particle incubation. Uptake was nearly abolished (Fig. 2), confirming that the fluorescence shift observed by flow cytometry was not due to mere adherence of fluorescent particles to cell surface moieties. To further confirm endocytosis, cell monolayers grown on permeable supports that had been incubated with fluorescent bacteria were fixed and immunostained with antibodies against the tight junction proteins claudin-4 and ZO-1. Confocal fluorescence imaging in the z dimension showed that both Staphylococcus and Yersinia were located basal to the apical cell surface markers.
FIG. 2.
Flow cytometry analysis of bacterial uptake at 37 and 4°C in NCI-H292 cells. The ingestion of both S. aureus and Y. enterocolitica is almost completely abrogated, ruling out mere adherence as the nature of the association between cells and fluorescently labeled bacteria observed by flow cytometry.
Bronchial epithelial cells ingest S. aureus in a dose-dependent fashion.
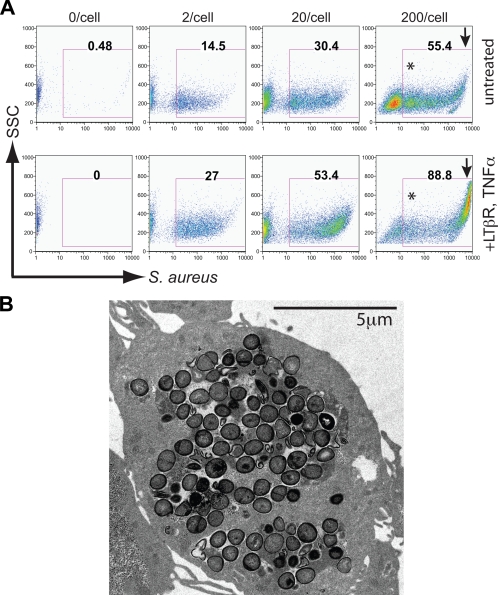
Our preliminary confocal fluorescence studies suggested that NCI-H292 cells exhibited an extraordinary capacity for endocytosis of S. aureus. To explore the capacity of this uptake, we incubated NCI-H292 cells with S. aureus in tenfold dose increases (Fig. 3A) and analyzed detached monolayers by flow cytometry. As the bacterial particle/cell ratio increased from 2:1 to 20:1, particle ingestion doubled in both treated and untreated cells. Another tenfold increase in bacterial dose resulted in a near doubling of the number of untreated cells ingesting bacteria; in the cells that had been pretreated with TNF and/or LT, we observed a substantial and discrete population of cells that took up ≥100 particles. This was deduced as follows. We determined the mean fluorescence of the least “positive” population of cells, presumed to have ingested at least one particle (asterisk in Fig. 3A). We then measured mean fluorescence of the putative high-endocytosis population and found it to have increased by more than two log cycles. This suggests a hundredfold or greater increase in the number of particles ingested. TEM analysis of sections of cell monolayers incubated with S. aureus confirmed that many cells were capable of very high-capacity endocytosis (Fig. 3B). Our TEM sections revealed multiple cells which had ingested >100 bacterial particles without lysing; these high-uptake cells were also observed by confocal microscopy. It was this high-endocytosis population that increased most dramatically with cytokine treatment at a particle/cell exposure of 200:1.
FIG. 3.
NCI-H292 endocytosis of S. aureus at tenfold increases of particle/cell ratios in untreated and TNF/LT-treated cell layers analyzed by flow cytometry (A) and TEM (B). The arrow separates a population of cells which ingested very high (≥100/cell) numbers of bacteria at high bacterial load; load ratios greater than 200/cell increased lysis/cell death. Flow cytometry data are representative of three independent experiments. The cell depicted in panel B is representative of those observed by TEM in cytokine-treated cultures at a bacterial particle/cell ratio of 200:1, and has ingested >100 Staphylococcus particles.
S. aureus and Y. enterocolitica are endocytosed via different pathways.
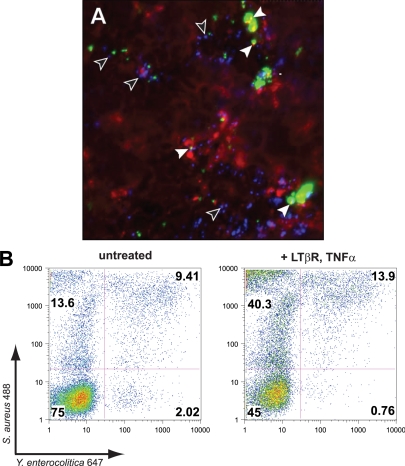
Immunofluorescence studies suggested that Staphylococcus and Yersinia were not endocytosed in the same compartments (Fig. 4A). Staphylococcus was consistently taken up in cytoplasmic vesicles colocalized with claudin-4 (filled arrowheads), a protein normally confined to epithelial tight junctions which is also a receptor for Clostridium perfringens enterotoxin (15). Yersinia particles rarely colocalized with claudin-4 (open arrowheads) or with canonical bacterial PRRs TLR2 and TLR4 (data not shown). To measure the coingestion of Staphylococcus and Yersinia particles in a more meaningful sample of cells, equivalent numbers (100 particles/cell, each) of Alexa Fluor 488-labeled Staphylococcus and Alexa Fluor 647-labeled Yersinia particles were coincubated with either untreated or TNF/LT-treated NCI-H292, and fluorescent cells were detected by flow cytometry (Fig. 4B). There were very few NCI-H292 cells that took up only Yersinia, and the cells ingesting Yersinia generally had also taken up many Staphylococcus particles. Pretreating the airway cells with TNF and LT cytokines increased the numbers of cells that ingested Y. enterocolitica, along with large numbers of S. aureus particles. Thus, while uptake of the two species of bacteria appeared to be independent, the cells most capable of bacterial particle endocytosis readily took up both bacterial species.
FIG. 4.
(A) C2BBe cell uptake of Y. enterocolitica (blue) and S. aureus (green), colocalized with claudin-4 (red/yellow). (B) Airway epithelial cells (NCI-H292) coincubated with equivalent numbers of S. aureus and Y. enterocolitica particles. Cytokine treatment increased the numbers of cells that took up Yersinia only, or Yersinia and Staphylococcus, but not Staphylococcus only. There were few or no airway cells which took up Yersinia only; TNF/LT treatment enhanced the tendency of cells to ingest Staphylococcus and Yersinia together. The data shown are representative of seven (immunofluorescence) and three (flow cytometry) independent experiments.
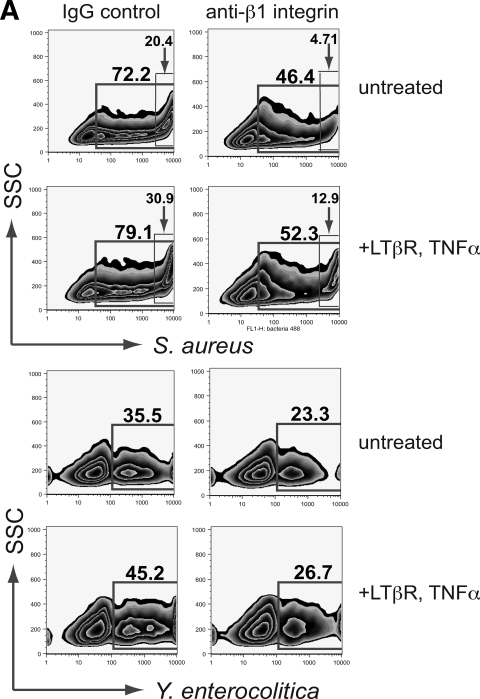
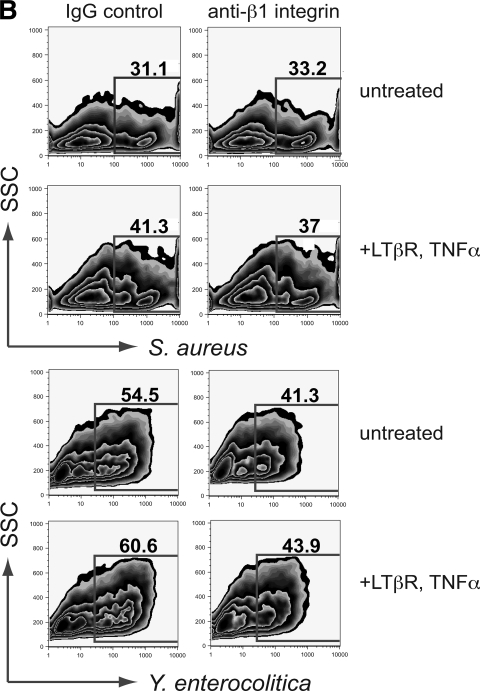
Blockade of β1 integrin reduces endocytosis of Y. enterocolitica in both intestinal and bronchial epithelium, but S. aureus uptake is blocked only in airway cells.
Yersinia spp. express invasin, a known opportunistic ligand for the epithelial α5β1 integrin receptor (2). Other pathogenic bacteria, including Staphylococcus and Streptococcus, have been shown to effect epithelial invasion by bridging to the endogenous β1 ligand fibronectin via fibronectin binding protein (25). To date, invasin-integrin and fibronectin binding protein-integrin interactions have been well studied in in vitro or in vivo experiments with live pathogens. To determine whether invasion and colonization exploiting β1 receptors can be strictly cell mediated, we blocked receptors on cultured airway and intestinal epithelial monolayers with anti-β1 antibody prior to incubation with Yersinia and Staphylococcus particles and examined by flow cytometry the effect on the ability of these cells to take up these particles. The effect of β1 blockade on ingestion of S. aureus in intestinal cells was unremarkable (Fig. 5B); however, the proportion of C2BBe cells ingesting Y. enterocolitica was reduced in both untreated and cytokine-treated cells by 13.2 and 16.7%, respectively. Interestingly, ingestion of Y. enterocolitica by airway epithelial cells was also notably reduced (Fig. 5A), although Yersinia is not typically described as a respiratory pathogen. The most dramatic β1 inhibition was of S. aureus endocytosis by NCI-H292 cells. In particular, the high uptake cell population (previously observed by increasing particle to cell ratios) was most diminished by β1 inhibition; in untreated cells, endocytosis in the high uptake (>100 bacterial particles/cell) population was reduced 4.3-fold; a 2.4-fold reduction was observed in the high-uptake population treated with proinflammatory cytokines.
FIG. 5.
Effect of blocking β1 integrin receptors on bacterial particle uptake in airway (A) and intestinal (B) epithelial cells. β1 blockade reduced uptake of S. aureus in untreated and cytokine-treated H292 cells by averages of 5.0 and 18.9%, respectively, with the most noticeable reduction in the population of cells which by fluorescence intensity and side scatter were shown to have taken up >100 particles. Uptake of Yersinia was similarly reduced, although the effect was more pronounced (12.5% reduction) in airway cells treated with TNF/LT. S. aureus uptake was not notably affected by blocking β1 integrin in C2BBe cells; blockade reduced uptake by 4.5 and 2.8% in untreated and treated cells, respectively. All data are means from at least three independent experiments.
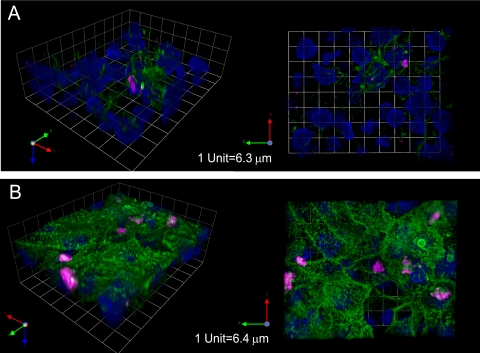
Redistribution of β1 integrin to apical surfaces of cells treated with proinflammatory cytokines.
As integrins link extracellular matrix proteins such as laminin and fibronectin to the cytoskeleton, they are normally distributed to the basolateral surfaces of polarized epithelial cells (4). It has been previously reported that β1 integrin is distributed apically in M cells of the follicle-associated epithelium specialized for antigen sampling, although not in neighboring enterocytes (2). These observations were based on comparisons to cells staining positive for the lectin UEA-1 in mouse Peyer's patches. To test whether TNF family cytokines are involved in the redistribution of β1 integrin to the apical membrane of airway epithelial cells under proinflammatory conditions, we compared β1 distribution in untreated cells to cells treated with TNF-α and LTβR agonist. Although some apical localization was observed in addition to the expected distribution at basolateral surfaces in untreated NCI-H292 cells (Fig. 6A), there was a remarkable relocalization of β1 integrin toward the apical pole in cells that were treated with TNF and LT cytokines (Fig. 6B). In addition to a notable redistribution of β1 in individual cells, samples treated with TNF and LT showed striking differences between cells expressing abundant apical β1 (major population) alongside cells expressing little or no β1 at their surface.
FIG. 6.
Spinning-disk confocal microscopy of immunostained airway epithelial cells either untreated (A) or treated with TNF-α/LTβR agonist (B). β1 integrin (green) is upregulated with cytokine treatment and blankets the apical surface of the majority of treated cells, although adjacent cells express no apical β1. Pink, S. aureus; blue, DAPI.
DISCUSSION
Studies on epithelial cells which rely on confocal fluorescence or electron microscopy imaging have only limited power to characterize the behavior of the majority of cells in a tissue sample or monolayer. Microscopy is of greatest value for close examination of specific subcellular processes, but for quantitative studies it can be limited by small sample size, even when multiple fields are sampled. In our hands, preparing adherent cultures for flow cytometry yields >80% (C2BBe) or >50% (NCI-H292) viable cells for sampling, and cells that have ingested fluorescently labeled bacteria are detectable both by fluorescence shift and increased side scatter. TEM confirmed that bacteria were ingested, and repeating incubations at 4°C abolished all but 8% of S. aureus and 4.08% of Y. enterocolitica uptake in NCI-H292 cells, a finding consistent with an endocytic process. We can therefore validate this tool as a quantitative assay for uptake of labeled bacteria by epithelial cells.
Flow cytometry also enabled us to discover that a subset of NCI-H292 cells was capable of endocytosis of very large numbers (100 or more) of S. aureus particles, and TEM provided corroborating evidence for such high-capacity uptake. We discovered that this highly Staphylococcus-endocytic population was enhanced by cytokine treatment and that it was the most sensitive of all subpopulations to the inhibition of endocytosis by blocking β1 integrin. Similarly, flow cytometric analysis of the coincubation of cells with both Staphylococcus and Yersinia particles revealed that a very large subset of intestinal epithelia preferentially endocytosed Yersinia. In contrast, in the airway epithelium, only cells that had endocytosed many Staphylococcus particles also took up Yersinia.
Sinha et al. (25) showed that Staphylococcus strains may employ “zipper type” internalization to invade epithelial cells by binding to fibronectin, which in turn ligates surface α5β1 and other cellular adhesin receptors. Our results suggest the existence of additional epithelial mechanisms whereby microbes similar to S. aureus may invade epithelial monolayers, especially in the context of inflammation. It has been recently shown that certain “carrier” strains of S. aureus, so named for their ability to delay host innate immune response and avoid clearance from the nasal epithelium, may utilize this strategy to persist in the nasal mucosa (20). This is intriguing in light of our identification of subpopulations of airway epithelial cells which internalize large numbers of Staphylococcus and Yersinia particles and is suggestive of a potential “feed-forward” mechanism that may contribute to microbial invasion or penetration of epithelial barriers. Further investigation of how particles are managed in vivo will clarify whether this seemingly maladaptive internalization may in fact actually be beneficial in mucosal tissues. Starner et al. (26) and Reibman et al. (21) have shown that CCL20, which recruits dendritic cells via its ligand CCR6, is induced in human bronchial epithelial cells by TNF-α, as well as by particulate matter. Similarly, Rumbo et al. (22) found that induction of CCL20 occurs in the intestinal epithelium after stimulation of the LTβ receptor. The activation of the LTβR pathway has been shown to be as important as that of the TNF system in the development of experimental colitis (17). By simultaneously increasing ingestion of pathogenic bacteria, epithelial cells exposed to these proinflammatory cytokines may “focus” the invading pathogens to a specific site in the epithelium where innate immune responses (e.g., chemokine release) may more effectively trigger adaptive immune responses.
Endocytosis of Y. enterocolitica by intestinal epithelial cells has been shown to be enhanced in cells upregulating β1 integrin at their apical surfaces (23). Whether invasion by this member of the Enterobacteriaceae is also enhanced in nonintestinal epithelia has not been demonstrated prior to the present study. We found that expression of β1 integrin was enhanced by culturing bronchial epithelial cells with TNFR ligands and that much of the protein was directed to the apical pole. This may be coordinated with increased expression of the endogenous α5β1 ligand, fibronectin, which occurs under proinflammatory conditions (24), although because of the distribution of the proteins, its significance is not clear. Apical β1 expression correlated with an increased tendency for airway epithelia to ingest both S. aureus and Y. enterocolitica; integrin binding may be directly responsible for capture of the bacteria, although the specific binding partner differs on each type of particle.
Interestingly, we observed S. aureus, but not Y. enterocolitica, colocalized in cytoplasmic vesicles with claudin-4, a protein that is ordinarily a key component of paracellular tight junctions. Previous studies on tight junction protein changes in response to inflammatory cytokines had not described any role in particle uptake; however, given the presence of the tight junction at the apical pole of the polarized epithelium, such a mechanism might be plausible, especially for uptake of such large particles. Although a specific mechanism remains to be identified, this suggests that S. aureus may be ingested by at least two distinct endocytosis mechanisms, which could help to explain its more abundant uptake in both C2BBe and NCI-H292 cells.
In summary, these results suggest that epithelial cells of the intestine and airways are not passive victims during invasion of the mucosa by bacterial pathogens. Events such as redistribution of tight junction and cellular adhesion proteins may in fact be beneficial responses to enable a kind of immune surveillance function of epithelial cells.
Acknowledgments
This study was supported by a Grand Challenges in Global Health grant from the Bill and Melinda Gates Foundation and the Foundation for the National Institutes of Health and grants AI63426 and AI73689 from the National Institutes of Health.
We thank Steven McDaniel for his TEM expertise and David Carter for his generous advice regarding confocal fluorescence microscopy.
Footnotes
Published ahead of print on 7 January 2009.
REFERENCES
- 1.Bruewer, M., A. Luegering, T. Kucharzik, C. A. Parkos, J. L. Madara, A. M. Hopkins, and A. Nusrat. 2003. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J. Immunol. 1716164-6172. [DOI] [PubMed] [Google Scholar]
- 2.Clark, M. A., B. H. Hirst, and M. A. Jepson. 1998. M-cell surface β1 integrin expression and invasin-mediated targeting of Yersinia pseudotuberculosis to mouse Peyer's patch M cells. Infect. Immun. 661237-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clayburgh, D. R., L. Shen, and J. R. Turner. 2004. A porous defense: the leaky epithelial barrier in intestinal disease. Lab. Investig. 84282-291. [DOI] [PubMed] [Google Scholar]
- 4.Coconnier, M. H., M. F. Bernet-Camard, and A. L. Servin. 1994. How intestinal epithelial cell differentiation inhibits the cell-entry of Yersinia pseudotuberculosis in colon carcinoma Caco-2 cell line in culture. Differentiation 5887-94. [DOI] [PubMed] [Google Scholar]
- 5.Drayton, D. L., S. Liao, R. H. Mounzer, and N. H. Ruddle. 2006. Lymphoid organ development: from ontogeny to neogenesis. Nat. Immunol. 7344-353. [DOI] [PubMed] [Google Scholar]
- 6.Finlay, B. B., and P. Cossart. 1997. Exploitation of mammalian host cell functions by bacterial pathogens. Science 276718-725. [DOI] [PubMed] [Google Scholar]
- 7.Fleckenstein, J. M., and D. J. Kopecko. 2001. Breaching the mucosal barrier by stealth: an emerging pathogenic mechanism for enteroadherent bacterial pathogens. J. Clin. Investig. 10727-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gitter, A. H., K. Bendfeldt, J. D. Schulzke, and M. Fromm. 2000. Leaks in the epithelial barrier caused by spontaneous and TNF-alpha-induced single-cell apoptosis. FASEB J. 141749-1753. [DOI] [PubMed] [Google Scholar]
- 9.Jett, B. D., and M. S. Gilmore. 2002. Internalization of Staphylococcus aureus by human corneal epithelial cells: role of bacterial fibronectin-binding protein and host cell factors. Infect. Immun. 704697-4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katakai, T., T. Hara, M. Sugai, H. Gonda, and A. Shimizu. 2004. Lymph node fibroblastic reticular cells construct the stromal reticulum via contact with lymphocytes. J. Exp. Med. 200783-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keita, A. V., E. Gullberg, A. C. Ericson, S. Y. Salim, C. Wallon, A. Kald, P. Artursson, and J. D. Soderholm. 2006. Characterization of antigen and bacterial transport in the follicle-associated epithelium of human ileum. Lab. Investig. 86504-516. [DOI] [PubMed] [Google Scholar]
- 12.Kelley, R. O., R. A. Dekker, and J. G. Bluemink. 1973. Ligand-mediated osmium binding: its application in coating biological specimens for scanning electron microscopy. J. Ultrastruct. Res. 45254-258. [DOI] [PubMed] [Google Scholar]
- 13.Koni, P. A., and R. A. Flavell. 1998. A role for tumor necrosis factor receptor type 1 in gut-associated lymphoid tissue development: genetic evidence of synergism with lymphotoxin beta. J. Exp. Med. 1871977-1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuprash, D. V., A. V. Tumanov, D. J. Liepinsh, E. P. Koroleva, M. S. Drutskaya, A. A. Kruglov, A. N. Shakhov, E. Southon, W. J. Murphy, L. Tessarollo, S. I. Grivennikov, and S. A. Nedospasov. 2005. Novel tumor necrosis factor-knockout mice that lack Peyer's patches. Eur. J. Immunol. 351592-1600. [DOI] [PubMed] [Google Scholar]
- 15.Lo, D., W. Tynan, J. Dickerson, M. Scharf, J. Cooper, D. Byrne, D. Brayden, L. Higgins, C. Evans, and D. J. O'Mahony. 2004. Cell culture modeling of specialized tissue: identification of genes expressed specifically by follicle-associated epithelium of Peyer's patch by expression profiling of Caco-2/Raji cocultures. Int. Immunol. 1691-99. [DOI] [PubMed] [Google Scholar]
- 16.Locksley, R. M., N. Killeen, and M. J. Lenardo. 2001. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104487-501. [DOI] [PubMed] [Google Scholar]
- 17.Mackay, F., J. L. Browning, P. Lawton, S. A. Shah, M. Comiskey, A. K. Bhan, E. Mizoguchi, C. Terhorst, and S. J. Simpson. 1998. Both the lymphotoxin and tumor necrosis factor pathways are involved in experimental murine models of colitis. Gastroenterology 1151464-1475. [DOI] [PubMed] [Google Scholar]
- 18.Neutra, M. R., N. J. Mantis, and J. P. Kraehenbuhl. 2001. Collaboration of epithelial cells with organized mucosal lymphoid tissues. Nat. Immunol. 21004-1009. [DOI] [PubMed] [Google Scholar]
- 19.Neutra, M. R., E. Pringault, and J. P. Kraehenbuhl. 1996. Antigen sampling across epithelial barriers and induction of mucosal immune responses. Annu. Rev. Immunol. 14275-300. [DOI] [PubMed] [Google Scholar]
- 20.Quinn, G. A., and A. M. Cole. 2007. Suppression of innate immunity by a nasal carriage strain of Staphylococcus aureus increases its colonization on nasal epithelium. Immunology 12280-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reibman, J., Y. Hsu, L. C. Chen, B. Bleck, and T. Gordon. 2003. Airway epithelial cells release MIP-3α/CCL20 in response to cytokines and ambient particulate matter. Am. J. Respir. Cell Mol. Biol. 28648-654. [DOI] [PubMed] [Google Scholar]
- 22.Rumbo, M., F. Sierro, N. Debard, J. P. Kraehenbuhl, and D. Finke. 2004. Lymphotoxin beta receptor signaling induces the chemokine CCL20 in intestinal epithelium. Gastroenterology 127213-223. [DOI] [PubMed] [Google Scholar]
- 23.Schulte, R., S. Kerneis, S. Klinke, H. Bartels, S. Preger, J. P. Kraehenbuhl, E. Pringault, and I. B. Autenrieth. 2000. Translocation of Yersinia enterocolitica across reconstituted intestinal epithelial monolayers is triggered by Yersinia invasin binding to beta1 integrins apically expressed on M-like cells. Cell. Microbiol. 2173-185. [DOI] [PubMed] [Google Scholar]
- 24.Sheppard, D. 2003. Functions of pulmonary epithelial integrins: from development to disease. Physiol. Rev. 83673-686. [DOI] [PubMed] [Google Scholar]
- 25.Sinha, B., P. P. Francois, O. Nusse, M. Foti, O. M. Hartford, P. Vaudaux, T. J. Foster, D. P. Lew, M. Herrmann, and K. H. Krause. 1999. Fibronectin-binding protein acts as Staphylococcus aureus invasin via fibronectin bridging to integrin α5β1. Cell. Microbiol. 1101-117. [DOI] [PubMed] [Google Scholar]
- 26.Starner, T. D., C. K. Barker, H. P. Jia, Y. Kang, and P. B. McCray, Jr. 2003. CCL20 is an inducible product of human airway epithelia with innate immune properties. Am. J. Respir. Cell Mol. Biol. 29627-633. [DOI] [PubMed] [Google Scholar]
- 27.Turner, J. R. 2006. Molecular basis of epithelial barrier regulation: from basic mechanisms to clinical application. Am. J. Pathol. 1691901-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang, F., W. V. Graham, Y. Wang, E. D. Witkowski, B. T. Schwarz, and J. R. Turner. 2005. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am. J. Pathol. 166409-419. [DOI] [PMC free article] [PubMed] [Google Scholar]