Abstract
The currently available 7-valent pneumococcal conjugate vaccine (PCV7) elicits good immune response to and is effective against vaccine serotypes. However, its effectiveness against vaccine-related serotypes is variable. Serum samples were obtained 1 month after the last vaccination from 31 infants immunized with PCV7 at 2, 4, and 6 months of age. The sera were used to determine immunoglobulin G antibody levels to eight serotypes (seven vaccine serotypes and serotype 19A) with enzyme-linked immunosorbent assay (ELISA) and opsonic capacity against 11 serotypes (seven vaccine serotypes, serotypes 19A and 6A, and nonvaccine serotypes 5 and 7F) using a multiplexed opsonization assay. ELISA results showed antibody concentrations varied between 1.84 and 10.49 μg/ml, and all subjects had antibody concentrations of ≥0.35 μg/ml for all serotypes, including serotype 19A. In contrast, the opsonic index was detectable (i.e., opsonic index ≥ 8) in all children for the seven vaccine serotypes, 81% for serotype 6A, and merely 19% for serotype 19A. PCV7 shows good immunogenicity for vaccine serotypes in infants after a primary series. PCV7 does not elicit opsonic antibodies to serotype 19A. ELISA may thus be an inadequate surrogate assay for evaluating the response for cross-reactive serotypes in infants.
Streptococcus pneumoniae is a major human pathogen, responsible for pneumonia, meningitis, otitis media, and sepsis, especially for young children and the elderly (30). The most important virulence factor of pneumococci is the polysaccharide (PS) capsule, which shields pneumococci from host phagocytes. The shielding effect of the capsule can be neutralized by antibodies to the capsule. Pneumococci can express at least 91 different types of PS capsules (12, 28). Capsular PSs (C-PSs) from commonly found pneumococcal serotypes are included in pneumococcal vaccines to provide a broad protection with a minimal number of PSs. The 23-valent pneumococcal PS vaccine includes PSs from 23 serotypes that accounts for more than 90% of invasive pneumococcal diseases (IPDs) observed for adults (6, 14, 21, 31). In children, fewer serotypes are responsible for IPDs, and a pneumococcal 7-valent CRM197 protein conjugate vaccine (PCV7), which has been used for children in the United States since 2000, contains seven serotypes (serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F) and was designed to cover almost 90% of the IPDs in young children in the United States and Canada (10). After the use of PCV7, the incidence of IPD by the seven vaccine serotypes (VTs) has dramatically decreased but not those of non-VTs (NVTs) (3, 18, 22, 25, 39), which are chemically and serologically different from VTs.
Serotypes 6A and 19A have been labeled vaccine-related serotypes (VRTs) since they differ from serotypes 6B and 19F only slightly in capsular structures and can cross-react with antibodies to 6B and 19F. Consequently, pneumococcal conjugate vaccines had been assumed to elicit antibodies cross-reacting with and to be cross-protective against the two VRTs. However, cross-protection against serotype 6A was not universally reported (5, 20). Also, herd immunity to serotype 6A has not been evident among adults despite the significant reduction of IPDs among vaccinated children (9, 13). Further, the incidence of 19A IPD has significantly increased since 2000 in the U.S. adults and children (7, 29). Although the increased prevalence of serotype 19A IPDs suggests the ineffectiveness of PCV7 against 19A, some have noted that, before the introduction of PCV7, 19A isolates began to become antibiotic resistant and its prevalence began to increase (15). Thus, it is unclear whether PCV7 induces protective immunity against these two VRTs.
Vaccine-induced protective immunity is generally estimated by measuring antibody concentrations (i.e., as in enzyme-linked immunosorbent assay [ELISA]). However, the protective immunity can be estimated better by directly measuring opsonic capacity of vaccine-induced antibodies because the antibodies provide protection by opsonizing pneumococci for phagocytes. Nevertheless, opsonization assay (OPA) was seldom used for estimating protective immunity in young children because OPA was technically difficult to perform and required a large amount of sera. OPA technology has been greatly improved in the last several years (2, 17). For instance, multiplexed OPA permits one to measure opsonic capacities to many different serotypes with small amounts of sera from young children. To investigate the immune response to PCV7 in VRTs, we directly measured opsonic responses to all VTs and the two VRTs in young children following administration of PCV7 and compared the OPA results to antibody levels determined by ELISA. (This study was presented in part at the 6th International Symposium on Pneumococci and Pneumococcal Diseases, Reykjavik, Iceland, in 2008 [abstr. P3-057].)
MATERIALS AND METHODS
Human sera.
The serum samples used in the present study were obtained from 31 healthy infants who were monitored in the well-baby clinic at Ewha Womans University Hospital. All infants were injected with 0.5 ml of PCV7 (Prevenar; Wyeth Lederle Vaccines S.A., Louvain-la-Neuve, Belgium) intramuscularly on the anterolateral side of the thigh at 2, 4, and 6 months of age. Some children were administered other vaccines (i.e., the diphtheria-tetanus-acellular pertussis, inactivated poliovirus, hepatitis B, and/or influenza virus vaccines or others) simultaneously on the contralateral leg. Serum samples were obtained at 7 months, 4 weeks after the three-dose primary series. All sera were stored frozen at −70°C until analyzed.
ELISA.
Antipneumococcal antibodies against VTs 4, 6B, 9V, 14, 18C, 19F, and 23F and VRT 19A were measured by ELISA using both cell wall PS (CW-PS) and 22F serotype C-PS absorption, as previously described (4, 38). The ELISA was performed at the Center for Vaccine Evaluation and Study, Ewha Medical Research Institute at Ewha Womans University. Briefly, each well of a 96-well medium binding microtiter plate (Corning, Inc., Corning, NY) was coated with 100 μl of a serotype-specific pneumococcal PS antigen (American Type Culture Collection, Manassas, VA) diluted to a predetermined concentration, and plates were incubated at 37°C for 5 h in a humidified chamber. The coated plates were washed with 1× Tris-buffered saline with 0.01% Brij 35 solution. Test sera were preabsorbed with CW-PS (Statens Serum Institut, Copenhagen, Denmark) and 22F C-PS (American Type Culture Collection), and the reference standard 89-SF (provided by Carl Frasch, Center for Biologics Evaluation and Research, Food and Drug Administration, Bethesda, MD) was preabsorbed with CW-PS. Sera were serially diluted 2.5-fold in absorption solution and incubated at room temperature for 30 min. After incubation, the sera (50 μl) were transferred to the coated microtiter plates, and the plates were incubated for 2 h at room temperature. The plates were washed five times, and 100 μl of diluted alkaline phosphatase-conjugated goat anti-human immunoglobulin G (IgG; Southern Biotech, Birmingham, AL) was added to each well. After a 2-h incubation, the plates were washed five times, and 100 μl of substrate solution (diethanolamine [Sigma, St. Louis, MO] with 1 mg of p-nitrophenyl phosphate powder [Sigma]/ml) was added to each well. After a 2-h incubation at room temperature, 50 μl of 3 M NaOH was added to all wells to stop the enzyme reaction. The optical density was measured at 405 nm, and the optical density at 690 nm was subtracted. Optical densities were converted to antibody concentrations using CDC software for pneumococcal ELISA (written by Brian Plikaytis, Centers for Disease Control and Prevention, Atlanta, GA; this software can be downloaded free of charge from www.cdc.gov/ncidod/dbmd/bimb/elisa.htm). A detailed protocol can be found online (www.vaccine.uab.edu).
MOPA.
The opsonic activities of the samples were evaluated at the University of Alabama at Birmingham using the fourfold multiplexed OPA (MOPA4), as previously described (2, 33, 34, 37). Briefly, frozen aliquots of target pneumococci were thawed, washed twice with opsonization buffer B (Hanks balanced salt solution with Mg/Ca, 0.1% gelatin, and 10% fetal bovine serum) by centrifugation (12,000 × g, 2 min) and diluted to the proper bacterial density (∼2 × 105 CFU/ml of each serotype). Target strains expressing capsule types 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F were reported (2, 37). Equal volumes of four bacterial suspensions that were chosen to be analyzed together were pooled. All serum samples were incubated at 56°C for 30 min before serial dilutions in opsonization buffer B. Serially diluted serum (20 μl/well) was mixed with 10 μl of bacterial suspension in each well of round-bottom 96-well plates (Corning, Inc.). After a 30-min incubation at room temperature with shaking (Mini Orbital Shaker; Bellco Biotechnolgy, Vineland, NJ) at 700 rpm, 10 μl of 3- to 4-week-old rabbit complement (PelFreeze Biologicals, Rogers, AK) and 40 μl of HL60 cells (4 × 105 cells) were added to each well. HL60 cells were differentiated to granulocytes by culture in RPMI 1640 with 10% fetal bovine serum, 1% l-glutamine, and 0.8% dimethyl formamide at a starting density of 4 × 105 cells/ml for 5 to 6 days. Plates were incubated in a tissue culture incubator (37°C, 5% CO2) with shaking at 700 rpm. After a 45-min incubation, plates were placed on ice for 10 to 15 min, and an aliquot of the final reaction mixture (10 μl) was spotted onto four different THY agar plates (Todd-Hewitt broth with 0.5% yeast extract and 1.5% agar). When the fluid was absorbed into the agar, an equal volume of overlay agar (THY with 0.75% agar and 25 mg of 2,3,5-triphenyltetrazolium chloride/liter) containing one of the four antibiotics (optochin, spectinomycin, streptomycin, or trimethoprim) was applied to each THY agar plate (17). After an overnight incubation at 37°C, the number of bacterial colonies in the agar plates was enumerated. Opsonic indices were defined as the serum dilution that kills 50% of bacteria and were determined by linear interpolation. (A detailed protocol may be found at www.vaccine.uab.edu.)
Statistical analyses.
The analyses of serum antibody concentrations were based on logarithms of the antibody concentrations of all subjects. Geometric mean concentrations of antipneumococcal IgG antibodies and opsonic indices were evaluated, and two-sided 95% confidence intervals (CI) were determined for each pneumococcal serotype. Serum samples with opsonization indices of <8 were assigned a value of 4 for analysis purposes. The proportions of subjects achieving antipneumococcal antibody titers of ≥0.35 μg/ml and opsonic indices of ≥8 were determined, respectively. Reverse cumulative distribution curves were used to display the percentages of children that achieved different antibody concentrations to each of the seven vaccine type pneumococcal serotypes and VRTs 19A and 6A.
Ethical considerations.
The study protocol was approved by the Institutional Review Board at Ewha Womans University Hospital and was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. Informed written consent was obtained from all parents or legal guardians following a detailed explanation of the study.
RESULTS
Immune response to VTs.
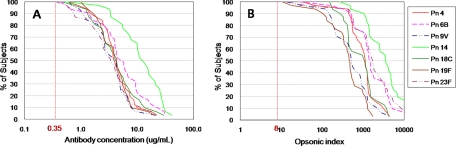
The antibody responses evaluated by ELISA and OPA against the vaccine type serotypes are shown in Table 1. Geometric mean antibody concentrations of the seven VTs ranged from 2.98 (serotype 23F) to 10.49 μg/ml (serotype 14). All subjects had an antibody concentration of ≥0.35 μg/ml for all vaccine type serotypes (Fig. 1). The geometric mean of opsonic indices for the seven VTs ranged from 360 (95% CI = 237 to 547) for serotype 19F to 3,245 (95% CI = 2,087 to 5,045) for serotype 14. The opsonic index was also detectable (i.e., ≥8) in all children for all seven VTs (Fig. 1).
TABLE 1.
Response to a primary series of the PCV7 of 31 infants for vaccine-type serotypes and VRTs 19A and 6A evaluated by antibody concentrations and opsonic activity
| Assay | Parametera | PCV7 vaccine-type serotypes
|
VRTs
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 4 | 6B | 9V | 14 | 18C | 19F | 23F | 19A | 6A | ||
| ELISA | GMC (μg/ml) | 3.93 | 4.75 | 3.19 | 10.49 | 3.86 | 3.79 | 2.98 | 1.84 | NDb |
| 95% CI | 2.98-5.17 | 3.24-6.95 | 2.41-4.22 | 7.46-14.74 | 2.77-5.37 | 2.97-4.85 | 2.11-4.19 | 1.43-2.35 | ND | |
| Amt (%) ≥ 0.35 μg/ml | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | ND | |
| OPA | GMI | 905 | 1,394 | 456 | 3,245 | 810 | 360 | 1,486 | 7 | 83 |
| 95% CI | 364-1,285 | 907-2,140 | 295-706 | 2,087-5,045 | 582-1,126 | 267-547 | 898-2,460 | 4-11 | 42-164 | |
| Amt (%) ≥ 8 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 19.4 | 81 | |
GMC, geometric mean concentration; GMI, geometric mean index.
ND, not done.
FIG. 1.
Reverse cumulative distribution curves for vaccine type serotypes generated by PCV7 in infants. (A) Antibody concentration (μg/ml); (B) opsonic index.
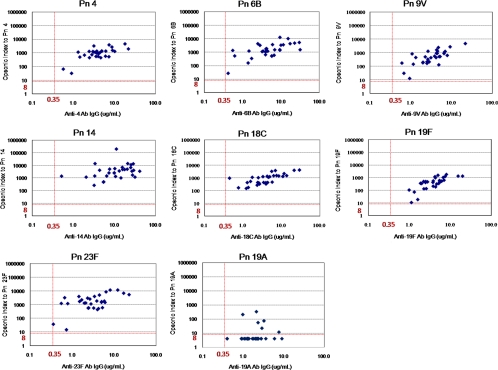
The relationship between the antibody concentration and opsonic activity for all VTs showed a good correlation. The correlation coefficient ranged from 0.65 to 0.75 for serotypes 4, 6B, 9V, 18C, and 19F. The correlation coefficients were 0.35 and 0.58 for serotypes 14 and 23F, respectively (Fig. 2).
FIG. 2.
Opsonic index (y axis) versus antibody concentration (x axis) for vaccine-type serotypes (4, 6B, 9V, 14, 18C, and 23F) and VRT 19A. The vertical and horizontal dotted lines represent the antibody titer of 0.35 μg/ml and opsonic detection limit of 8 for the OPA, respectively.
Immune response to VRT 19A.
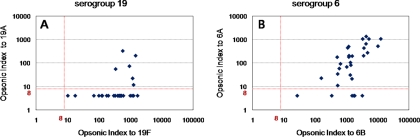
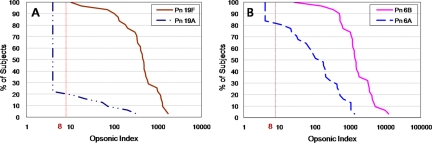
The geometric mean levels of antibodies were 3.79 μg/ml (95% CI = 2.97 to 4.85) for 19F and 1.84 μg/ml (95% CI = 1.43 to 2.35) for 19A, and all children had a titer of ≥0.35 μg/ml for both serotypes. The antibody titers evaluated by the ELISA showed a good correlation between serotype 19F and 19A (r = 0.69) (data not shown). However, the opsonic indices for 19F and 19A showed a poor correlation (Fig. 3A). While opsonic activity was detectable in all children for serotype 19F, only 6 of 31 of the children (19.4%) had an opsonic index of ≥8 for serotype 19A (Fig. 3A and 4A). Among infants with detectable cross-opsonic activity, the geometric mean antibody concentrations were 5.41 μg/ml for 19F and 2.66 μg/ml for 19A and the opsonic indices ranged 315 to 1,491 and from 12 to 323 for serotypes 19F and 19A, respectively.
FIG. 3.
Cross-opsonic capacities for serogroup 19 (A) and serogroup 6 (B). The opsonic index for the VRTs (y axis) versus opsonic index for VTs (x axis) is shown.
FIG. 4.
Reverse cumulative distribution curves of opsonic indices generated by PCV7. (A) Serotypes 19F and 19A; (B) serotypes 6B and 6A. The vertical dotted line represents the opsonic detection limit of 8 for the OPA.
In contrast to the fairly good correlation between ELISA and OPA for serotype 19F (r = 0.75), the antibody concentrations and opsonic activity for serotype 19A showed a poor correlation (r = 0.11) (Fig. 2).
Immune response to VRT 6A.
After vaccination with PCV7, opsonic activity against 6A was elicited in response to serotype 6B (Fig. 3B). The PCV7 vaccine induced opsonic indices in all infants for the VT 6B, and opsonic indices were detectable for 81% of the infants for serotype 6A (Fig. 4B). Among infants with detectable cross-opsonic antibodies, there was a good correlation between the opsonic indices for serotypes 6B and 6A (r = 0.75) (Fig. 3B).
The six infants with no detectable opsonic antibodies for serotype 6A showed high opsonic indices for serotype 6B (range, 27 to 3,205).
Immune response to NVTs 5 and 7F.
For NVTs 5 and 7F 3 and 23%, respectively, of the infants showed a detectable opsonic index (data not shown).
DISCUSSION
The children in the present study produced good immune responses in VT after a primary series of PCV7 when the responses were estimated by either OPA or ELISA. All children have antibody levels greater than 0.35 μg/ml and detectable opsonic activity (≥8) for all VTs. Also, we confirmed that there was a good correlation between the antibody concentration and opsonic activity for VT of PCV7. These results are consistent with those of other previous reports (19, 26, 32, 35, 41). Thus, PCV7 elicits strong protective immunity against the seven VTs in the children in this study as determined by both assay methods, and our study confirms usefulness of ELISA in estimating the immune protection induced for VT with PCV7.
For VRT, the results of serotype 6A OPA correlated with those of 6B OPA, suggesting that 6B PS in PCV7 induces antibodies that cross-opsonize the 6A serotype. According to our results, infants with opsonic activity for serotype 6A were noted at 81%, which is comparable with previously reported vaccine efficacy of 76% against IPDs by 6A (39). Interestingly, there were six infants with high opsonic titers for serotype 6B but no opsonic activity for 6A. However, the results for serotype 19A, the other VRT, are strikingly different. Only a small percentage of infants (19.4%) had detectable opsonic capacity (index ≥ 8), and the opsonic index of 19A is poorly correlated with that of 19F. Furthermore, all infants showed an antibody concentration of ≥0.35 μg/ml for serotype 19A; however, the 19A specific antibody concentrations poorly correlated with the opsonic indices (Fig. 2). However, this 19A-specific antibody appears to have been induced by PCV7 since anti-Pn IgG antibody concentrations show a strong correlation between serotypes 19F and 19A (r = 0.69) (data not shown). Thus, PCV7 elicits antibodies binding to 19A, though a majority of these are functionally ineffective (nonopsonic).
Cross-protective immunity against 19A has been investigated previously. Yu et al. reported the immunologic response of vaccine-induced cross-opsonization for several conjugate vaccines (40). Antibodies induced by the experimental pentavalent CRM197 protein conjugate vaccine were found to bind the PS by an ELISA, but this was not demonstrable by OPA. However, the present study did not examine the seven-valent vaccine currently in use. Others reported cross-protection by showing that passive immunization with infant sera vaccinated with a tetanus toxoid protein conjugate vaccine in a murine pneumococcal pneumonia model showed a protective effect against serotype 19A pneumococci (16). Interestingly, the protective effect did not correlate with the anti-19A specific IgG antibody levels. These studies do suggest that the serotype 19A-specific antibody levels estimated by the ELISA do not seem to reflect the functional capacity of the antibodies for 19A serotypes.
We have shown here that PCV7 provide little immunoprotection against 19A if it was estimated with OPA and not with ELISA. Similar to the findings described here, it was recently reported that antibody responses by adults to protein conjugate vaccines for serotype 19F and 19A are highly specific and that cross-reactive IgG elicited by serogroup 19 conjugates do not seem to show opsonic response to other serotypes within serogroup 19 (8). These findings provide additional information that shuld be considered in explaining the rapid increase in prevalence of serotype 19A with the use of PCV7. Although the increase is partially due to spreading of the antibiotic resistance of 19A serotype strains (15), one should recognize that PCV7 does not induce strong cross-protective antibodies, and this weak cross-protective immunity may be partially responsible for the increased prevalence of 19A serotype. Interestingly, when the 14-valent PS vaccine was upgraded to the 23-valent vaccine, serotype 19A PS was added in addition to 19F PS because 19F was inadequate in inducing antibodies cross-reacting with serotype 19A (24).
We found that PCV7 elicits strong opsonic capacity against serotype 6A and that they are strongly correlated with opsonic capacity to serotype 6B. This finding would suggest that PCV7 should provide herd immunity against serotype 6A, unlike the published epidemiologic observations of poor herd immunity against serotype 6A (9, 13). However, the apparent absence of herd immunity to 6A can now be explained with the newly described serotype 6C, which was typed as “6A” by classical serotyping methods (28). When 6C serotype was distinguished from the 6A serotype with the use of a new serotyping method, PCV7 was found to reduce both the prevalence of nasopharyngeal carriage among children (23) and that of IPDs among adults (27) by serotype 6A but not by serotype 6C. Thus, serological evaluation of PCV7 pneumococcal immunity in serotype 6A is consistent with the epidemiologic studies of serotype 6A.
Our findings show that only a functional assay, OPA, can be a surrogate of immune protection for the VRT serotype 19A, whereas an antibody concentration evaluated by the ELISA at best can only be a correlate of immune protection. There are additional examples showing OPA to be a surrogate of protection. Immunization of human immunodeficiency virus-infected patients with PCV7 may be assessed better with OPA than with ELISA. Correlations for antibody concentrations and OPA titers were poor, suggesting nonspecific antibodies in human immunodeficiency virus-infected patients (36). Older adults generally have high levels of pneumococcal antibodies detected by ELISA, but these individuals are susceptible to pneumococcal infections. Thus, despite its usefulness among (healthy) infants, ELISA may not be a good surrogate of immune protection for the elderly or subjects with a suppressed immune status. With many technical improvements, OPA has become a practical tool for studying a large number of samples even from infants (1). It has been reported that ELISA results may overestimate or underestimate vaccine effectiveness against IPDs for several VTs and VRTs, whereas OPA results show a better correlation with actual vaccine effectiveness (11). Also, the results of functional assays are favored by the regulatory agencies. Thus, we believe that OPA can (and should) be used more often in future evaluations of pneumococcal vaccines, especially for VRTs.
Acknowledgments
This study was partially supported by a grant from the Korean Food and Drug Administration (05092KFDA341) to K.-H.K. and a grant from the National Institutes of Health (R01-AI-31473) to M.H.N.
The University of Alabama at Birmingham (M.H.N.) retains intellectual property rights on the target bacteria used for the MOPA. This may represent a potential conflict of interest.
Footnotes
Published ahead of print on 14 January 2009.
REFERENCES
- 1.Bogaert, D., M. Sluijter, R. De Groot, and P. W. Hermans. 2004. Multiplex opsonophagocytosis assay (MOPA): a useful tool for the monitoring of the 7-valent pneumococcal conjugate vaccine. Vaccine 224014-4020. [DOI] [PubMed] [Google Scholar]
- 2.Burton, R. L., and M. H. Nahm. 2006. Development and validation of a fourfold multiplexed opsonization assay (MOPA4) for pneumococcal antibodies. Clin. Vaccine Immunol. 131004-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2008. Invasive pneumococcal disease in children 5 years after conjugate vaccine introduction-eight states, 1998-2005. MMWR Morb. Mortal. Wkly. Rep. 57144-148. [PubMed] [Google Scholar]
- 4.Concepcion, N. F., and C. E. Frasch. 2001. Pneumococcal type 22f polysaccharide absorption improves the specificity of a pneumococcal-polysaccharide enzyme-linked immunosorbent assay. Clin. Diagn. Lab. Immunol. 8266-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dagan, R., N. Givon-Lavi, O. Zamir, M. Sikuler-Cohen, L. Guy, J. Janco, P. Yagupsky, and D. Fraser. 2002. Reduction of nasopharyngeal carriage of Streptococcus pneumoniae after administration of a 9-valent pneumococcal conjugate vaccine to toddlers attending day care centers. J. Infect. Dis. 185927-936. [DOI] [PubMed] [Google Scholar]
- 6.Drinkovic, D., C. G. Wong, S. L. Taylor, S. A. Roberts, and A. J. Morris. 2001. Pneumococcal bacteraemia and opportunities for prevention. N. Z. Med. J. 114326-328. [PubMed] [Google Scholar]
- 7.Farrell, D. J., K. P. Klugman, and M. Pichichero. 2007. Increased antimicrobial resistance among nonvaccine serotypes of Streptococcus pneumoniae in the pediatric population after the introduction of 7-valent pneumococcal vaccine in the United States. Pediatr. Infect. Dis. J. 26123-128. [DOI] [PubMed] [Google Scholar]
- 8.Fernsten, P., B. Hu., and X. Yu. 2008. Specificity of the opsonic response to serotype 19A and 19F conjugate vaccines, abstr. P3-065. In ISPPD-6, 6th International Symposium on Pneumococci and Pneumococcal Diseases. 8 to 12 June 2008. Reykjavik, Iceland.
- 9.Haber, M., A. Barskey, W. Baughman, L. Barker, C. G. Whitney, K. M. Shaw, W. Orenstein, and D. S. Stephens. 2007. Herd immunity and pneumococcal conjugate vaccine: a quantitative model. Vaccine 255390-5398. [DOI] [PubMed] [Google Scholar]
- 10.Hausdorff, W. P., J. Bryant, P. R. Paradiso, and G. R. Siber. 2000. Which pneumococcal serogroups cause the most invasive disease: implications for conjugate vaccine formulation and use, part I. Clin. Infect. Dis. 30100-121. [DOI] [PubMed] [Google Scholar]
- 11.Henckaerts, I., N. Durant, D. De Grave, L. Schuerman, and J. Poolman. 2007. Validation of a routine opsonophagocytosis assay to predict invasive pneumococcal disease efficacy of conjugate vaccine in children. Vaccine 252518-2527. [DOI] [PubMed] [Google Scholar]
- 12.Henrichsen, J. 1995. Six newly recognized types of Streptococcus pneumoniae. J. Clin. Microbiol. 332759-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hicks, L. A., L. H. Harrison, B. Flannery, J. L. Hadler, W. Schaffner, A. S. Craig, D. Jackson, A. Thomas, B. Beall, R. Lynfield, A. Reingold, M. M. Farley, and C. G. Whitney. 2007. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998-2004. J. Infect. Dis. 1961346-1354. [DOI] [PubMed] [Google Scholar]
- 14.Hogg, G. G., J. E. Strachan, and R. A. Lester. 2000. Invasive pneumococcal disease in the population of Victoria. Med. J. Aust. 173(Suppl.)S32-S35. [DOI] [PubMed] [Google Scholar]
- 15.Hwa, C. E., S. H. Kim, B. W. Eun, S. J. Kim, N. H. Kim, J. Lee, and H. J. Lee. 2008. Streptococcus pneumoniae serotype 19A in children, South Korea. Emerg. Infect. Dis. 14275-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jakobsen, H., V. D. Sigurdsson, S. Sigurdardottir, D. Schulz, and I. Jonsdottir. 2003. Pneumococcal serotype 19F conjugate vaccine induces cross-protective immunity to serotype 19A in a murine pneumococcal pneumonia model. Infect. Immun. 712956-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim, K. H., J. Yu, and M. H. Nahm. 2003. Efficiency of a pneumococcal opsonophagocytic killing assay improved by multiplexing and by coloring colonies. Clin. Diagn. Lab. Immunol. 10616-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klugman, K. P., S. A. Madhi, R. E. Huebner, R. Kohberger, N. Mbelle, and N. Pierce. 2003. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N. Engl. J. Med. 3491341-1348. [DOI] [PubMed] [Google Scholar]
- 19.Lucero, M. G., V. E. Dulalia, R. N. Parreno, D. M. Lim-Quianzon, H. Nohynek, H. Makela, and G. Williams. 2004. Pneumococcal conjugate vaccines for preventing vaccine-type invasive pneumococcal disease and pneumonia with consolidation on X-ray in children under two years of age. Cochrane Database Syst. Rev. 2004CD004977. [DOI] [PubMed] [Google Scholar]
- 20.Mbelle, N., R. E. Huebner, A. D. Wasas, A. Kimura, I. Chang, and K. P. Klugman. 1999. Immunogenicity and impact on nasopharyngeal carriage of a nonavalent pneumococcal conjugate vaccine. J. Infect. Dis. 1801171-1176. [DOI] [PubMed] [Google Scholar]
- 21.McIntyre, P. B., R. E. Gilmour, G. L. Gilbert, A. M. Kakakios, and C. M. Mellis. 2000. Epidemiology of invasive pneumococcal disease in urban New South Wales, 1997-1999. Med. J. Aust. 173(Suppl.)S22-S26. [DOI] [PubMed] [Google Scholar]
- 22.Messina, A. F., K. Katz-Gaynor, T. Barton, N. Ahmad, F. Ghaffar, D. Rasko, and G. H. McCracken, Jr. 2007. Impact of the pneumococcal conjugate vaccine on serotype distribution and antimicrobial resistance of invasive Streptococcus pneumoniae isolates in Dallas, TX, children from 1999 through 2005. Pediatr. Infect. Dis. J. 26461-467. [DOI] [PubMed] [Google Scholar]
- 23.Nahm, M. H., J. Lin, J. A. Finkelstein, and S. I. Pelton. 2009. Increase in the prevalence of the newly discovered pneumococcal serotype 6c in the nasopharynx after introduction of pneumococcal conjugate vaccine. J. Infect. Dis. 199:320-325. [DOI] [PMC free article] [PubMed]
- 24.Nahm, M. H., J. V. Olander, and M. Magyarlaki. 1997. Identification of cross-reactive antibodies with low opsonophagocytic activity for Streptococcus pneumoniae. J. Infect. Dis. 176698-703. [DOI] [PubMed] [Google Scholar]
- 25.O'Brien, K. L., L. H. Moulton, R. Reid, R. Weatherholtz, J. Oski, L. Brown, G. Kumar, A. Parkinson, D. Hu, J. Hackell, I. Chang, R. Kohberger, G. Siber, and M. Santosham. 2003. Efficacy and safety of seven-valent conjugate pneumococcal vaccine in American Indian children: group randomised trial. Lancet 362355-361. [DOI] [PubMed] [Google Scholar]
- 26.Oosterhuis-Kafeja, F., P. Beutels, and P. Van Damme. 2007. Immunogenicity, efficacy, safety, and effectiveness of pneumococcal conjugate vaccines (1998-2006). Vaccine 252194-2212. [DOI] [PubMed] [Google Scholar]
- 27.Park, I. H., M. R. Moore, J. J. Treanor, S. I. Pelton, T. Pilishvili, B. Beall, M. A. Shelly, B. E. Mahon, and M. H. Nahm. 2008. Differential effects of pneumococcal vaccines against serotypes 6A and 6C. J. Infect. Dis. 1981818-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park, I. H., D. G. Pritchard, R. Cartee, A. Brandao, M. C. Brandileone, and M. H. Nahm. 2007. Discovery of a new capsular serotype (6C) within serogroup 6 of Streptococcus pneumoniae. J. Clin. Microbiol. 451225-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pelton, S. I., H. Huot, J. A. Finkelstein, C. J. Bishop, K. K. Hsu, J. Kellenberg, S. S. Huang, R. Goldstein, and W. P. Hanage. 2007. Emergence of 19A as virulent and multidrug resistant pneumococcus in Massachusetts following universal immunization of infants with pneumococcal conjugate vaccine. Pediatr. Infect. Dis. J. 26468-472. [DOI] [PubMed] [Google Scholar]
- 30.Plotkin, S. A., and W. A. Orenstein. 2004. Vaccines, 4th ed. Saunders, Philadelphia, PA.
- 31.Raz, R., G. Elhanan, Z. Shimoni, R. Kitzes, C. Rudnicki, Y. Igra, A. Yinnon, et al. 1997. Pneumococcal bacteremia in hospitalized Israeli adults: epidemiology and resistance to penicillin. Clin. Infect. Dis. 241164-1168. [DOI] [PubMed] [Google Scholar]
- 32.Rennels, M. B., K. M. Edwards, H. L. Keyserling, K. S. Reisinger, D. A. Hogerman, D. V. Madore, I. Chang, P. R. Paradiso, F. J. Malinoski, and A. Kimura. 1998. Safety and immunogenicity of heptavalent pneumococcal vaccine conjugated to CRM197 in United States infants. Pediatrics 101604-611. [DOI] [PubMed] [Google Scholar]
- 33.Romero-Steiner, S., C. E. Frasch, G. Carlone, R. A. Fleck, D. Goldblatt, and M. H. Nahm. 2006. Use of opsonophagocytosis for serological evaluation of pneumococcal vaccines. Clin. Vaccine Immunol. 13165-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romero-Steiner, S., D. Libutti, L. B. Pais, J. Dykes, P. Anderson, J. C. Whitin, H. L. Keyserling, and G. M. Carlone. 1997. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae using differentiated HL-60 cells. Clin. Diagn. Lab. Immunol. 4415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shinefield, H. R., S. Black, P. Ray, I. Chang, N. Lewis, B. Fireman, J. Hackell, P. R. Paradiso, G. Siber, R. Kohberger, D. V. Madore, F. J. Malinowski, A. Kimura, C. Le, I. Landaw, J. Aguilar, and J. Hansen. 1999. Safety and immunogenicity of heptavalent pneumococcal CRM197 conjugate vaccine in infants and toddlers. Pediatr. Infect. Dis. J. 18757-763. [DOI] [PubMed] [Google Scholar]
- 36.Tarrago, D., J. Casal, J. Ruiz-Contreras, J. T. Ramos, P. Rojo, H. Snippe, and W. T. Jansen. 2005. Assessment of antibody response elicited by a 7-valent pneumococcal conjugate vaccine in pediatric human immunodeficiency virus infection. Clin. Diagn. Lab. Immunol. 12165-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang, D., R. L. Burton, M. H. Nahm, and S. J. Soong. 2008. A four-parameter logistic model for estimating titers of functional multiplexed pneumococcal opsonophagocytic killing assay. J. Biopharm. Stat. 18307-325. [DOI] [PubMed] [Google Scholar]
- 38.Wernette, C. M., C. E. Frasch, D. Madore, G. Carlone, D. Goldblatt, B. Plikaytis, W. Benjamin, S. A. Quataert, S. Hildreth, D. J. Sikkema, H. Kayhty, I. Jonsdottir, and M. H. Nahm. 2003. Enzyme-linked immunosorbent assay for quantitation of human antibodies to pneumococcal polysaccharides. Clin. Diagn. Lab. Immunol. 10514-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whitney, C. G., T. Pilishvili, M. M. Farley, W. Schaffner, A. S. Craig, R. Lynfield, A. C. Nyquist, K. A. Gershman, M. Vazquez, N. M. Bennett, A. Reingold, A. Thomas, M. P. Glode, E. R. Zell, J. H. Jorgensen, B. Beall, and A. Schuchat. 2006. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: a matched case-control study. Lancet 3681495-1502. [DOI] [PubMed] [Google Scholar]
- 40.Yu, X., B. Gray, S. Chang, J. I. Ward, K. M. Edwards, and M. H. Nahm. 1999. Immunity to cross-reactive serotypes induced by pneumococcal conjugate vaccines in infants. J. Infect. Dis. 1801569-1576. [DOI] [PubMed] [Google Scholar]
- 41.Zangwill, K. M., D. P. Greenberg, C. Y. Chiu, P. Mendelman, V. K. Wong, S. J. Chang, S. Partridge, and J. I. Ward. 2003. Safety and immunogenicity of a heptavalent pneumococcal conjugate vaccine in infants. Vaccine 211894-1900. [DOI] [PubMed] [Google Scholar]