Abstract
Highly quantitative and high-throughput serological tests for evaluation of humoral responses to herpes simplex virus 1 (HSV-1) and HSV-2 are not available. The efficacy of luciferase immunoprecipitation system (LIPS) assays for antibody profiling and serologic diagnosis of HSV-1 and HSV-2 infection was investigated using a panel of five recombinant HSV antigens. Plasma samples from subjects seropositive for HSV-1 and/or HSV-2 or seronegative for HSV-1 and HSV-2 that had previously been analyzed by Western blotting and the Focus Plexus immunoassay were evaluated. The LIPS test measuring anti-gG1 antibody titers was 96% sensitive and 96% specific for detecting HSV-1 infection, compared with the Focus immunoassay, and was 92% sensitive and 96% specific, compared with Western blotting. The results for the anti-gG2 LIPS test for HSV-2 precisely matched those for Western blotting, with 100% sensitivity and 100% specificity, and showed robust antibody titers in all the HSV-2-infected samples that were over 1,000 times higher than those in HSV-2-negative or HSV-1-positive samples. Antibodies to three additional HSV-2 proteins, gB, gD, and ICP8, were detected in many of the HSV-1- and/or HSV-2-infected plasma samples and showed preferentially higher immunoreactivity in HSV-2-infected plasma. The titers of antibodies to these three HSV-2 antigens also significantly correlated with each other (R = 0.75 to 0.81; P < 0.0001). These studies indicate that the robust anti-gG1 and anti-gG2 antibody responses detected by LIPS assays are useful for HSV-1 and HSV-2 detection and suggest that profiling of antibody responses to a panel of HSV proteins may be useful for characterizing individual humoral responses to infection and for monitoring responses to vaccines.
Herpes simplex virus (HSV) causes cold sores, genital herpes, ocular infections, and encephalitis. HSV-1 is usually transmitted by contact with oral secretions and causes most HSV orofacial infections, while HSV-2 is usually spread by sexual contact and causes most cases of genital herpes. Seroprevalence studies indicate that about 60% of adults in the United States are infected with HSV-1, with most primary infections occurring during childhood (38). In contrast, seroprevalence rates of HSV-2 vary dramatically by geographic region, with infection rates ranging from 10 to 35% of the population (19, 27), and infection usually occurs later in life, through sexual contact (19). Up to 25% of individuals infected with HSV-2 are asymptomatic and thus pose a significant risk for transmitting virus to their sexual partners (23). In addition, acquisition of HSV-1 or HSV-2 toward the end of pregnancy carries a 30 to 50% risk of neonatal herpes (5), with the potential for prenatal morbidity (6). HSV-1 and HSV-2 also establish lifelong, latent infections in the nervous system, usually in trigeminal or dorsal root ganglia (35).
Of the approximately 80 gene products in the HSV-1 and HSV-2 genome (20), four glycoproteins, gB, gD, gH, and gL, are required for entry and infection of cells (29). gD is currently the major viral component in candidate subunit vaccines being tested for HSV-2 (32, 33). gB has also been used in candidate subunit vaccines (14, 34). Two other major vaccines under development include a replication-defective HSV-2 virus deleted for ICP8 and UL5 (16, 17, 22) and a growth-defective virus deleted for the protein kinase domain within the large subunit of ribonucleotide reductase (3, 13, 21). Serologic assays for gD and gB would be useful for studying the immune response to candidate subunit vaccines. A sensitive serologic assay for ICP8 would also be useful for identifying individuals who are infected with wild-type HSV after vaccination with a vaccine deleted for ICP8, since the vaccine would likely induce antibodies to all of the other viral proteins.
HSV-2-specific serologies have recently been developed. These serologies might be useful for diagnosing HSV-2 infections in asymptomatic individuals in high-prevalence areas who may shed the virus and transmit HSV-2 to their partners (30). Recent studies show that antiviral therapy can reduce the rates of shedding (37) and transmission of HSV-2 from symptomatic individuals to their uninfected partners (15). HSV-2 type-specific serologies might also be useful for confirming a diagnosis of genital herpes in a patient with negative HSV cultures and for determining susceptibility to HSV-2 infection, particularly in pregnant women when their male partners have histories of genital herpes, to reduce the risk of neonatal infection (5).
Commonly used serological tests, including immunofluorescence assays, Western blot assays, and enzyme-linked immunosorbent assays (ELISAs), can detect anti-HSV-1 and anti-HSV-2 antibodies for diagnosis (36); however, these assays generally do not provide highly quantitative results, and many are unable to discriminate between HSV-1 and HSV-2. gGs of HSV-1 and HSV-2 have limited sequence homologies and elicit type-specific virus responses. Serological tests based on recognition of antibodies to gG1 or gG2 are now commonly used for diagnosis (2). The Western blot assay for gG is considered the “gold standard” in HSV detection and can discriminate between HSV-1 and HSV-2 infections, but this method is time-consuming and less quantitative than other immunoassays. Other, less cumbersome gG-based tests which can discriminate between HSV-1 and HSV-2 infections are available, including ELISAs, immunoblot assays, and an immunoassay which uses beads coated with HSV-1 or HSV-2 gG antigen (Focus Technologies, Trinity Biotech USA, Biokit USA, and Fisher Scientific) (1, 24). However, these assays are less sensitive than the gG Western blot assay.
Recently, we showed that luciferase immunoprecipitation system (LIPS) assays can quantitatively measure antibody responses to cancer-associated autoantigens (8), autoantigens associated with autoimmune diseases (9, 10), and a variety of infectious agents, including hepatitis C virus, human immunodeficiency virus (HIV) (7), human T-cell leukemia virus type 1 (11), and filaria (12, 28). These assays measure antibody levels in immunoprecipitations by using fusion proteins consisting of Renilla luciferase (Ruc)-antigen produced in Cos1 cells. LIPS assays are highly sensitive and robust and are high-throughput tests that can be automated. In the present study, we tested the ability of LIPS technology to quantify and distinguish HSV-1 and HSV-2 antibodies in plasma. We show that LIPS assays for anti-gG1 and anti-gG2 antibodies are sensitive and specific tests that can discriminate between HSV-1 and HSV-2. LIPS assays for three additional HSV-2 antigens, gB, gD, and ICP8, which share sequences similar to those of HSV-1, were positive for most HSV-1- and/or HSV-2-positive plasma samples but showed higher immunoreactivity for HSV-2-infected plasma. These results indicate that profiling antibodies to HSV-1 and HSV-2 antigens by the LIPS assay is an effective test for distinguishing HSV type-specific antibodies over a dynamic range and that quantification of antibodies to other viral proteins may be useful for measuring immune responses during natural infection and after vaccination.
MATERIALS AND METHODS
Patient plasma.
Plasma samples were obtained from 53 subjects seropositive for HSV-1 and/or HSV-2 or seronegative for HSV-1 and HSV-2. Blood samples were obtained under National Institute of Allergy and Infectious Diseases Institutional Review Board-approved protocols (Bethesda, MD). Two different commercially available methods, HSV Western blot serology (University of Washington Clinical Virology Laboratory, Seattle, WA) and the Plexus HerpesSelect HSV-1 and HSV-2 immunoglobulin G immunoassay (Focus Diagnostics, Cypress, CA), were used to discriminate between HSV-1 and HSV-2 infections in plasma. The Western blot assay was considered the “gold standard,” and sensitivity and specificity were determined on the basis of Western blot results (unless otherwise noted). Plasma samples were kept at −80°C, aliquoted, and stored at 4°C. The samples were coded, and the laboratories performing the Plexus immunoassay and the LIPS assay were blinded as to the results of the HSV Western blot assays.
Generation of Ruc-antigen fusion proteins.
A mammalian Renilla luciferase (Ruc) expression vector, pREN2, was used to generate all plasmids. HSV protein fragments were amplified by PCR with gene-specific linker-primer adapters. The gG1 protein fragment, amplified from HSV-1 genomic DNA, carried amino acid (aa) residues 26 to 193. Four different protein fragments were amplified from HSV-2 genomic DNA: gG2 (aa 305 to 591), gB (aa 63 to 369), gD (aa 26 to 393), and ICP8 (aa 2 to 286). In each case, the cDNA fragments were subcloned downstream of the Ruc gene and a stop codon was inserted directly after the HSV protein-coding sequence. The HSV sequence in each plasmid construct was confirmed by DNA sequencing. Details of the nucleotide and amino acid sequences can be found in the GenBank database under accession numbers FJ457776, FJ457777, FJ457778, FJ457779, and FJ457780 for gG1, gG2, gB, gD, and ICP8, respectively. Cos-1 cells were transfected with individual Ruc expression vectors. Forty-eight hours later, the Cos1 cells were washed once with phosphate-buffered saline (PBS) and then scraped and sonicated on ice in lysis buffer (20 mM Tris, pH 7.5, 150 mM NaCl, 5 mM MgCl2, 1% Triton X-100, and 50% glycerol) with protease inhibitors (Complete protease inhibitor cocktail minitablets; Roche Diagnostics, Indianapolis, IN). The lysates were twice centrifuged for 4 min at 13,000 × g, and supernatants were collected and stored at −20°C until use. The activities (in light units [LU]/ml) of the lysates were next determined using a single-tube luminometer (20/20 from Turner Scientific) with a coelenterazine substrate mixture (Promega, Madison, WI).
LIPS analysis.
LIPS assays were performed at room temperature, using a 96-well-plate format. Master plates were constructed by diluting patient plasma samples 1:10 in assay buffer A (20 mM Tris, pH 7.5, 150 mM NaCl, 5 mM MgCl2, 1% Triton X-100) in 96-well polypropylene microtiter plates. To quantify antibody titers by the LIPS assay, 40 μl of buffer A, 10 μl of diluted human plasma (1 μl equivalent), and 50 μl of 1 × 107 LU of Ruc-antigen Cos1 cell extract, diluted in buffer A, were added to each well of polypropylene plates and incubated for 1 h at room temperature. Next, 7 μl of a 30% suspension of Ultralink protein A/G beads (Pierce Biotechnology, Rockford, IL) in PBS was added to the bottom of each well of 96-well high-throughput-screening filter plates (Millipore, Bedford, MA). One hundred microliters of the antigen-antibody reaction mixture was then transferred to filter plates and incubated for 1 h at room temperature on a rotary shaker. Proteins bound to the protein A/G beads were washed 10 times with buffer A and twice with PBS, using a BioMek FX workstation (Beckman Coulter, Fullerton, CA) with an integrated vacuum manifold. After the final wash, LU values were measured with a Berthold LB 960 Centro microplate luminometer (Berthold Technologies, Bad Wilbad, Germany), using a coelenterazine substrate mixture (Promega, Madison, WI). All the LU data shown represent the averages for two independent experiments and were corrected for background LU values of Ruc Cos-1 cell extract added to protein A/G beads but not incubated with plasma.
Statistical analysis.
GraphPad Prism software (San Diego, CA) was used for statistical analyses, including evaluation of test performance by measurement of area under the curve. Results for quantitative antibody titers in uninfected controls and HSV-1-positive, HSV-2-positive, and HSV-1- and HSV-2-positive samples were reported as geometric means ± 95% confidence intervals (CI). Mann-Whitney U tests were used for comparison of antibody titers in different groups, and the level of significance was set at P values of <0.05. Correlations between different antibody titers were assessed by the Spearman rank test. For calculation of sensitivity and specificity, a simple, statistically based cutoff limit for each antigen was derived from the mean value for the 14 uninfected samples plus 5 standard deviations (SD).
RESULTS
The LIPS assay is sensitive and specific for detection of anti-gG1 antibodies for serologic detection of HSV-1.
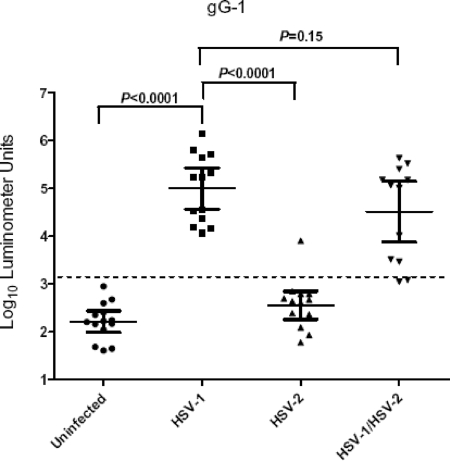
HSV gG1 glycoprotein is the standard serological marker for detecting HSV-1-specific antibodies (36). Two independent LIPS assays were used to screen 53 plasma samples in a blinded fashion, using the Ruc-gG1 fusion protein produced in Cos1 cells. On the basis of the average values for these two independent tests, the anti-gG1 antibody titers of the plasma samples ranged from 41 to 1,366,737 LU (Fig. 1). The geometric mean titer (GMT) of the anti-gG1 antibody in the 14 lowest-level responses (later identified as the 14 uninfected controls) was 165 LU (95% CI, 98 to 276) and was 600-fold lower than the GMT of 99,262 LU (95% CI, 36,670 to 268,697) for the confirmed 13 HSV-1-infected samples (Mann-Whitney U test; P < 0.0001). Anti-gG1 antibodies were absent from all but one of the HSV-2-infected plasma samples (GMT of 363 LU; 95% CI, 184 to 715) and showed low-level responses similar to those in the uninfected controls (Fig. 1). In contrast to HSV-2-positive plasma samples, HSV-1/2-positive plasma samples showed an anti-gG1 antibody GMT of 32,646 LU (95% CI, 7,540 to 141,342), which was similar to that for the HSV-1-positive infected plasma samples. The difference in mean anti-gG1 antibody titer between the singly HSV-1-positive and HSV-1/2-positive plasma samples was not statistically significant (Mann-Whitney U test; P = 0.15) (Fig. 1).
FIG. 1.
Detection of anti-HSV-1 gG1 antibodies by LIPS assays. Each symbol represents individual samples from uninfected controls or HSV-1-, HSV-2-, or HSV-1/2-positive subjects. Antibody titers in LU are plotted on a log10 scale. The dashed line represents the cutoff level for determining sensitivity and specificity for gG1 and is derived from the mean antibody titer of the 14 uninfected samples plus 5 SD. P values were calculated using the Mann-Whitney U test. The solid horizontal lines indicate the GMT of anti-gG1 antibody per group, and the vertical lines show the 95% CI.
In order to compare the sensitivity and specificity of the LIPS assay with those of the Western blot assay and the ELISA, we derived a diagnostic cutoff value (from the mean for the 14 uninfected controls plus 5 SD) equal to 1,390 LU. Assuming that Western blotting had 100% sensitivity and 100% specificity, LIPS showed 100% (13/13) sensitivity in detecting anti-gG1 antibody in HSV-1-positive plasma samples and 83% (10/12) sensitivity in detecting anti-gG1 antibody in plasma samples known to be HSV-1/2 positive (Fig. 1 and Table 1). Furthermore, the LIPS anti-gG1 test showed 100% (14/14) specificity in the uninfected controls but showed one low false positive (93% specificity; 13/14) in the HSV-2-positive group. Using a lower cutoff equal to the control mean plus 3 SD would have identified 100% of the HSV-1/HSV-2 positives and still maintained the same 93% specificity. The Plexus immunoassay for anti-gG1 antibodies missed three HSV-1/2-positive samples detected by Western blotting. Two of the three samples missed by the Plexus immunoassay were also negative by the LIPS assay. Compared to the Plexus immunoassay, the LIPS assay showed 100% (13/13) sensitivity in detecting anti-gG1 antibody in HSV-1-positive plasma samples and 92% (11/12) sensitivity in detecting anti-gG1 antibody in plasma samples known to be HSV-1/2 positive (Table 1).
TABLE 1.
Comparative performance levels of the LIPS assay, Western blot analysis, and the Plexus immunoassay for serologic diagnosis of HSV infectiona
| Assays and infection group | % (no. of samples detected/no. positive)
|
|
|---|---|---|
| Sensitivity | Specificity | |
| Western blot vs Plexus for HSV-1 (gG1) | ||
| Uninfected | 100 (14/14) | |
| HSV-1 | 100 (13/13) | |
| HSV-2 | 100 (14/14) | |
| HSV-1/2 | 75 (9/12) | |
| LIPS vs Western blot for HSV-1 (gG1) | ||
| Uninfected | 100 (14/14) | |
| HSV-1 | 100 (13/13) | |
| HSV-2 | 93 (13/14) | |
| HSV-1/2 | 83 (10/12) | |
| LIPS vs Plexus for HSV-1 (gG1) | ||
| Uninfected | 100 (14/14) | |
| HSV-1 | 100 (13/13) | |
| HSV-2 | 93 (13/14) | |
| HSV-1/2 | 92 (11/12) | |
| Western blot vs Plexus for HSV-2 (gG2) | ||
| Uninfected | 93 (13/14) | |
| HSV-1 | 100 (13/13) | |
| HSV-2 | 100 (14/14) | |
| HSV-1/2 | 100 (12/12) | |
| LIPS vs Western blot for HSV-2 (gG2) | ||
| Uninfected | 100 (14/14) | |
| HSV-1 | 100 (13/13) | |
| HSV-2 | 100 (14/14) | |
| HSV-1/2 | 100 (12/12) | |
| LIPS vs Plexus for HSV-2 (gG2) | ||
| Uninfected | 93 (13/14) | |
| HSV-1 | 100 (13/13) | |
| HSV-2 | 100 (14/14) | |
| HSV-1/2 | 100 (12/12) | |
For LIPS assays, the cutoff limit for calculating sensitivity and specificity for each antigen was derived from the mean for the 14 uninfected samples plus 5 SD.
The LIPS assay is highly sensitive and specific in detecting anti-gG2 antibody for serologic diagnosis of HSV-2 infection.
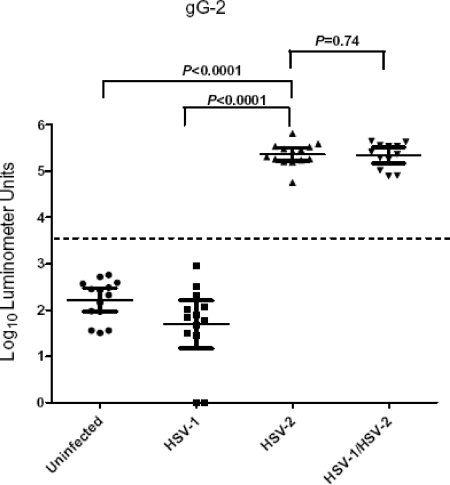
Detection of anti-gG2 antibody, the standard test for distinguishing HSV-2-specific antibody (36), was evaluated with the 53 plasma samples by using LIPS assays. While the GMT of the anti-gG2 antibody for the 14 uninfected control samples was 164 LU (95% CI, 92 to 294), the 12 plasma samples known to be HSV-2 positive had a 1,400-fold-higher GMT, 227,931 LU (95% CI, 163,843 to 317,088) (Fig. 2). The difference in anti-gG2 antibody titer between the uninfected control samples and the samples from the HSV-2-positive patients was highly significant (P < 0.0001). The 13 HSV-1-positive plasmas had low anti-gG2 antibody titers, similar to those in uninfected control samples (Fig. 2). The GMT of the anti-gG2 antibody in the HSV-1/2-positive plasma samples was 219,463 LU, which was not statistically different from those in the HSV-2-positive plasma samples (P = 0.74).
FIG. 2.
Detection of anti-HSV-2 gG2 antibodies by LIPS assays. Each symbol represents individual samples from uninfected controls or HSV-1-, HSV-2-, or HSV-1/2-positive subjects. Antibody titers in LU are plotted on a log10 scale. The dashed line represents the cutoff level for determining sensitivity and specificity for gG2 and is derived from the mean antibody titer of the 14 uninfected samples plus 5 SD. P values were calculated using the Mann-Whitney U test. The solid horizontal lines indicate the GMT of anti-gG2 antibody per group, and the vertical lines show the 95% CI.
Comparison of the sensitivity and specificity results for the anti-gG2 LIPS test with those for the Western blot assay, using a diagnostic cutoff of 1,281 LU for the LIPS assay (derived from the mean for the 14 uninfected control samples plus 5 SD), showed that the LIPS assay had 100% (14/14) sensitivity in detecting anti-gG2 antibody for the HSV-2-positive plasma samples and 100% (12/12) sensitivity in detecting HSV-1/2-positive plasma samples (Fig. 2 and Table 1). The anti-gG2 LIPS test showed 100% (14/14) specificity in the uninfected controls and 100% specificity in the HSV-1-positive plasma samples. While the result from the LIPS assay exactly matched the Western blotting results for detecting anti-gG2 positive plasma, comparison of the LIPS assay with the Plexus immunoassay revealed one false positive in the uninfected group by the Plexus immunoassay (Table 1). This Western blot-negative sample was negative by the LIPS assay, with an anti-gG2 antibody titer of only 262 LU.
Antibody responses to three other HSV-1 and HSV-2 proteins.
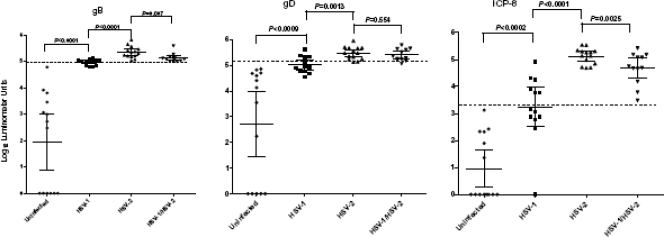
Previously, we demonstrated that the LIPS assay can be easily employed to evaluate whole proteomes. For example, we profiled humoral responses to the entire HIV proteome, including minor accessory proteins, such as TAT and p6, in HIV-infected patients (7). To determine how effective LIPS assays would be in detecting antibody responses to additional HSV-1 and HSV-2 proteins, we evaluated antibodies to HSV-2 gB, gD, and ICP8. The HSV-2 gB, gD, and ICP8 protein fragments used for LIPS assays share 92%, 84%, and 89% amino acid identity with the corresponding protein fragments of HSV-1. LIPS assays detected high titers of antibody to gB, gD, and ICP8 in most of the HSV-1- and/or HSV-2-infected plasma samples (Fig. 3). The GMTs of the anti-ICP8 antibodies in the HSV-1-, HSV-2-, and HSV-1/2-positive samples were 1,740 LU, 133,017 LU, and 48,228 LU, respectively, all significantly (ranging from 1,000 to 130,000 times) higher than those of the anti-ICP8 antibodies (GMT of 9 LU; 95% CI, 2 to 45) in the uninfected controls. In contrast to the low background level observed with the anti-ICP8 test, there were several high titers for anti-gD, and to a lesser extent anti-gB, antibodies in uninfected controls. Three of these uninfected plasma samples with elevated anti-gB antibody titers were among seven plasma samples that were also positive for anti-gD antibodies; they were negative for anti-gG and anti-ICP-8 antibodies. These antibody responses might reflect true immunoreactivity resulting from a nonproductive HSV infection or antibody cross-reactivity to HSV antigens. Regardless of the results for some of the individual plasma samples, the GMTs of anti-gB and anti-gD antibodies in the uninfected controls were more than 1,000-fold lower than those in the HSV-1-, HSV-2-, or HSV1/2-positive plasma samples.
FIG. 3.
Detection of anti-ICP8, -gB, and -gD antibodies by LIPS assays. Each symbol represents individual samples from uninfected controls or HSV-1-, HSV-2-, or HSV-1/2-positive subjects. Antibody titers in LU are plotted on a log10 scale. The dashed line represents the cutoff level for determining sensitivity and specificity for each vial antigen and is derived from the mean antibody titer of the 14 uninfected samples plus 5 SD. P values were calculated using the Mann-Whitney U test. The solid lines indicate the antibody GMT per group, and the vertical lines show the 95% CI.
Anti-ICP8, anti-gB, and anti-gD antibody titers, which were measured using HSV-2 fusion proteins, were significantly higher in HSV-2-positive plasma samples than in HSV-1-positive plasma samples (P values of <0.0001, <0.0001, and <0.0013, respectively) (Fig. 3). There was no overlap between the highest anti-ICP8, anti-gB, or anti-gD antibody titers in the negative-control plasma samples and the lowest antibody titers in the HSV-2 or HSV1/2-positive plasma samples. While the titers of anti-gD antibodies were similar for HSV-2- and HSV-1/2-positive plasma samples, the differences in titer between anti-gB and anti-ICP8 antibodies were significantly higher in HSV-2-positive plasma samples than in HSV-1/2-positive plasma samples.
With a diagnostic cutoff derived from the mean for the 14 uninfected control samples plus 5 SD, the gB, gD, and ICP8 tests showed 100% specificity and sensitivities of 89% (35/39), 69% (27/39), and 82% (32/39), respectively. Further analysis of anti-gB, anti-gD, and anti-ICP8 antibody titers measured by LIPS assays revealed significant correlations between the three different antigens. By the Spearman rank test, the correlation between anti-gB and anti-ICP8 antibodies (R = 0.81; P < 0.0001) was essentially identical to the correlation between anti-gD and anti-ICP8 antibodies (R = 0.81; P < 0.0001). The correlation between anti-gB and anti-gD antibodies was slightly lower than that for the other two proteins (R = 0.75; P < 0.0001).
DISCUSSION
This study demonstrates that LIPS assays can robustly measure titers of antibodies to a panel of HSV-1 and HSV-2 antigens with high diagnostic sensitivity and specificity. While Western blotting is the “gold standard” serological test for distinguishing HSV-1- from HSV-2-positive serum or plasma samples, the test is labor-intensive and difficult to use in high-throughput testing. In contrast, the high-throughput microtiter and robotic LIPS format tested here make this approach feasible for rapid screening of large numbers of plasma samples. While the anti-gG2 LIPS assay showed 100% sensitivity and specificity, the anti-gG1 test was slightly less sensitive and specific. It is likely that additional improvements to the gG1 test, including use of a different gG1 recombinant antigen or adjustment of the cutoff values, might further enhance performance.
Despite the high titers of antibody to gG1 detected by the LIPS assay, which were often 600-fold higher than those in uninfected plasma, several HSV-1/2-positive plasma samples failed to show positive anti-gG1 HSV-1 antibody responses above the cutoff value (derived from the mean for the uninfected controls plus 5 SD). Interestingly, two of the three HSV-1/2-positive plasma samples that scored negative by the Plexus immunoassay also yielded negative results by the LIPS assay. One possible explanation for these results is that initial infection with HSV-2 affords some protection against HSV-1 infection (25) and results in lower titers of antibodies against HSV-1-specific proteins, such as gG1. In addition, the cross-reactivity of the HSV-1 and HSV-2 antigens might result in lower titers of antibodies to the second HSV infection due to the phenomenon of “original antigenic sin” (18), in which the immune system responds less effectively to a closely related antigen after the initial recognition of the primary antigen. It is also possible that these two samples represent false positives in Western blot analysis.
A study using several HSV-1/2 ELISAs found that these assays show less than optimum HSV diagnostic performance compared to Western blotting (26). In this study, LIPS assays generated fewer false negatives and false positives than the Plexus immunoassay. While LIPS assays use recombinant gG produced in mammalian cells, the Plexus immunoassay uses recombinant antigen produced by baculovirus expression in insect cells. It is possible that differences in conformation-specific epitopes or posttranslational modifications, such as glycosylation, in mammalian versus insect cells might account for the disparate results.
The ease and simplicity of the LIPS format allowed the rapid development and evaluation of antibody responses to other HSV proteins. In addition to the glycoproteins gB and gD, two well-studied antibody targets (14, 34), we also found relatively high titers of antibody to ICP8, a single-stranded DNA binding protein. The high titers of antibody to gB (and possibly gD) that we detected using the LIPS assay may be related to a prior observation that detection of anti-gB antibodies by immunoprecipitation is more sensitive than detection by immunoblot analysis (31). While gG, gD, and gB are structural proteins and are abundant both in virions and on virus-infected cells, ICP8 is a nonstructural protein and is not present in virions (4). Nonetheless, we were able to detect antibodies to this nonstructural protein in all of the HSV-2-positive individuals. While the HSV-1 and HSV-2 ICP8 antigens used in the LIPS assay are highly conserved (89% amino acid identity), there were marked quantitative differences to these antigens in the plasma samples tested. This strain specificity is reminiscent of the reduced LIPS serologic responses to related filarial antigens in subjects infected with related worms (12, 28).
Highly quantitative measurements of titers of antibodies to specific viral proteins are important for the development of subunit vaccines. The use of replication-defective vaccines, which induce antibody responses to nearly all of the viral proteins, often results in difficulties in determining whether or not patients develop asymptomatic infection after vaccination. Two such replication-defective vaccines under development are HSV-2 dl5-29, which is deleted for only ICP8 and UL5 (16, 17, 22), and ICP10ΔPK, which is missing 339 aa from the protein kinase domain of the large subunit of ribonucleotide reductase (3, 13, 21). In the case of HSV-2 dl5-29, our ability to easily measure titers of antibody to ICP8, despite the fact that it is a nonstructural protein, should allow future vaccine studies with animals to determine if asymptomatic infection has occurred after vaccination. We are currently using LIPS assays to follow antibody titers in guinea pig vaccine and challenge studies (Y. Hoshino, L. Pesnicak, K. Dowdell, P. Burbelo, D. M. Knipe, S. E. Straus, and J. I. Cohen, unpublished results). The ability to rapidly quantify serial levels of antibodies to a large panel of viral proteins may further our understanding of the maturation and differences in immune response in individuals with herpes simplex infections that vary in severity (from asymptomatic to frequently recurrent), duration, or responsiveness to antiviral therapy. Evaluation of the serologic response to the complete proteomes of HSV-1 and HSV-2 may offer even-more-accurate diagnostic assays or help to define additional targets for vaccine development.
In summary, our results suggest that HSV LIPS assays are very effective in high-throughput screening for discrimination of HSV-1 and HSV-2 seropositivity and should be useful for monitoring antibody responses to a variety of HSV antigens in vaccine trials.
Acknowledgments
This study was supported by the intramural research programs of the National Institute of Dental and Craniofacial Research and the National Institute of Allergy and Infectious Diseases.
This project has been funded in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract N01-CO-12400.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Published ahead of print on 7 January 2009.
REFERENCES
- 1.Ashley, R. L. 2002. Performance and use of HSV type-specific serology test kits. Herpes 938-45. [PubMed] [Google Scholar]
- 2.Ashley, R. L., J. Militoni, F. Lee, A. Nahmias, and L. Corey. 1988. Comparison of Western blot (immunoblot) and glycoprotein G-specific immunodot enzyme assay for detecting antibodies to herpes simplex virus types 1 and 2 in human sera. J. Clin. Microbiol. 26662-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aurelian, L., H. Kokuba, and C. C. Smith. 1999. Vaccine potential of a herpes simplex virus type 2 mutant deleted in the PK domain of the large subunit of ribonucleotide reductase (ICP10). Vaccine 171951-1963. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Ze'ev, A., R. Abulafia, and S. Bratosin. 1983. Herpes simplex virus and protein transport are associated with the cytoskeletal framework and the nuclear matrix in infected BSC-1 cells. Virology 129501-507. [DOI] [PubMed] [Google Scholar]
- 5.Brown, Z. A., J. Benedetti, R. Ashley, S. Burchett, S. Selke, S. Berry, L. A. Vontver, and L. Corey. 1991. Neonatal herpes simplex virus infection in relation to asymptomatic maternal infection at the time of labor. N. Engl. J. Med. 3241247-1252. [DOI] [PubMed] [Google Scholar]
- 6.Brown, Z. A., S. Selke, J. Zeh, J. Kopelman, A. Maslow, R. L. Ashley, D. H. Watts, S. Berry, M. Herd, and L. Corey. 1997. The acquisition of herpes simplex virus during pregnancy. N. Engl. J. Med. 337509-515. [DOI] [PubMed] [Google Scholar]
- 7.Burbelo, P. D., K. H. Ching, T. L. Mattson, J. S. Light, L. R. Bishop, and J. A. Kovacs. 2007. Rapid antibody quantification and generation of whole proteome antibody response profiles using LIPS (luciferase immunoprecipitation systems). Biochem. Biophys. Res. Commun. 352889-895. [DOI] [PubMed] [Google Scholar]
- 8.Burbelo, P. D., R. Goldman, and T. L. Mattson. 2005. A simplified immunoprecipitation method for quantitatively measuring antibody responses in clinical sera samples by using mammalian-produced Renilla luciferase-antigen fusion proteins. BMC Biotechnol. 522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burbelo, P. D., S. Groot, M. C. Dalakas, and M. J. Iadarola. 2008. High definition profiling of autoantibodies to glutamic acid decarboxylases GAD65/GAD67 in stiff-person syndrome. Biochem. Biophys. Res. Commun. 3661-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burbelo, P. D., H. Hirai, H. Leahy, A. Lernmark, S. A. Ivarsson, M. J. Iadarola, and A. L. Notkins. 2008. A new luminescence assay for autoantibodies to mammalian cell-prepared insulinoma-associated protein 2. Diabetes Care 311824-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burbelo, P. D., E. Meoli, H. P. Leahy, J. Graham, K. Yao, U. Oh, J. E. Janik, R. Mahieux, F. Kashanchi, M. J. Iadarola, and S. Jacobson. 2008. Anti-HTLV antibody profiling reveals an antibody signature for HTLV-I-associated myelopathy/tropical spastic paraparesis (HAM/TSP). Retrovirology 596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burbelo, P. D., R. Ramanathan, A. D. Klion, M. J. Iadarola, and T. B. Nutman. 2008. Rapid, novel, specific, high-throughput assay for diagnosis of Loa loa infection. J. Clin. Microbiol. 462298-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casanova, G., R. Cancela, L. Alonzo, R. Benuto, C. Magana Mdel, D. R. Hurley, E. Fishbein, C. Lara, T. Gonzalez, R. Ponce, J. W. Burnett, and G. J. Calton. 2002. A double-blind study of the efficacy and safety of the ICP10deltaPK vaccine against recurrent genital HSV-2 infections. Cutis 70235-239. [PubMed] [Google Scholar]
- 14.Corey, L., A. G. Langenberg, R. Ashley, R. E. Sekulovich, A. E. Izu, J. M. Douglas, Jr., H. H. Handsfield, T. Warren, L. Marr, S. Tyring, R. DiCarlo, A. A. Adimora, P. Leone, C. L. Dekker, R. L. Burke, W. P. Leong, S. E. Straus, et al. 1999. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: two randomized controlled trials. JAMA 282331-340. [DOI] [PubMed] [Google Scholar]
- 15.Corey, L., A. Wald, R. Patel, S. L. Sacks, S. K. Tyring, T. Warren, J. M. Douglas, Jr., J. Paavonen, R. A. Morrow, K. R. Beutner, L. S. Stratchounsky, G. Mertz, O. N. Keene, H. A. Watson, D. Tait, and M. Vargas-Cortes. 2004. Once-daily valacyclovir to reduce the risk of transmission of genital herpes. N. Engl. J. Med. 35011-20. [DOI] [PubMed] [Google Scholar]
- 16.Da Costa, X. J., C. A. Jones, and D. M. Knipe. 1999. Immunization against genital herpes with a vaccine virus that has defects in productive and latent infection. Proc. Natl. Acad. Sci. USA 966994-6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Da Costa, X. J., L. A. Morrison, and D. M. Knipe. 2001. Comparison of different forms of herpes simplex replication-defective mutant viruses as vaccines in a mouse model of HSV-2 genital infection. Virology 288256-263. [DOI] [PubMed] [Google Scholar]
- 18.Davenport, F. M., A. V. Hennessy, and T. Francis, Jr. 1953. Epidemiologic and immunologic significance of age distribution of antibody to antigenic variants of influenza virus. J. Exp. Med. 98641-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleming, D. T., G. M. McQuillan, R. E. Johnson, A. J. Nahmias, S. O. Aral, F. K. Lee, and M. E. St. Louis. 1997. Herpes simplex virus type 2 in the United States, 1976 to 1994. N. Engl. J. Med. 3371105-1111. [DOI] [PubMed] [Google Scholar]
- 20.Gupta, R., T. Warren, and A. Wald. 2007. Genital herpes. Lancet 3702127-2137. [DOI] [PubMed] [Google Scholar]
- 21.Gyotoku, T., F. Ono, and L. Aurelian. 2002. Development of HSV-specific CD4+ Th1 responses and CD8+ cytotoxic T lymphocytes with antiviral activity by vaccination with the HSV-2 mutant ICP10DeltaPK. Vaccine 202796-2807. [DOI] [PubMed] [Google Scholar]
- 22.Hoshino, Y., S. K. Dalai, K. Wang, L. Pesnicak, T. Y. Lau, D. M. Knipe, J. I. Cohen, and S. E. Straus. 2005. Comparative efficacy and immunogenicity of replication-defective, recombinant glycoprotein, and DNA vaccines for herpes simplex virus 2 infections in mice and guinea pigs. J. Virol. 79410-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koutsky, L. A., R. L. Ashley, K. K. Holmes, C. E. Stevens, C. W. Critchlow, N. Kiviat, C. M. Lipinski, P. Wolner-Hanssen, and L. Corey. 1990. The frequency of unrecognized type 2 herpes simplex virus infection among women. Implications for the control of genital herpes. Sex. Transm. Dis. 1790-94. [DOI] [PubMed] [Google Scholar]
- 24.Leach, C. T., R. L. Ashley, J. Baillargeon, and H. B. Jenson. 2002. Performance of two commercial glycoprotein G-based enzyme immunoassays for detecting antibodies to herpes simplex viruses 1 and 2 in children and young adolescents. Clin. Diagn. Lab. Immunol. 91124-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Looker, K. J., and G. P. Garnett. 2005. A systematic review of the epidemiology and interaction of herpes simplex virus types 1 and 2. Sex. Transm. Infect. 81103-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morrow, R. A., and D. Friedrich. 2003. Inaccuracy of certain commercial enzyme immunoassays in diagnosing genital infections with herpes simplex virus types 1 or 2. Am. J. Clin. Pathol. 120839-844. [DOI] [PubMed] [Google Scholar]
- 27.Nahmias, A. J., F. K. Lee, and S. Beckman-Nahmias. 1990. Sero-epidemiological and -sociological patterns of herpes simplex virus infection in the world. Scand. J. Infect. Dis. Suppl. 6919-36. [PubMed] [Google Scholar]
- 28.Ramanathan, R., P. D. Burbelo, S. Groot, M. J. Iadarola, F. A. Neva, and T. B. Nutman. 2008. A luciferase immunoprecipitation systems assay enhances the sensitivity and specificity of diagnosis of Strongyloides stercoralis infection. J. Infect. Dis. 198444-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reske, A., G. Pollara, C. Krummenacher, B. M. Chain, and D. R. Katz. 2007. Understanding HSV-1 entry glycoproteins. Rev. Med. Virol. 17205-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rooney, J. F., J. M. Felser, J. M. Ostrove, and S. E. Straus. 1986. Acquisition of genital herpes from an asymptomatic sexual partner. N. Engl. J. Med. 3141561-1564. [DOI] [PubMed] [Google Scholar]
- 31.Simmonds, P., I. W. Smith, and J. F. Peutherer. 1987. Detection of antibody to viral proteins following primary infection with herpes simplex virus. J. Med. Virol. 23191-205. [DOI] [PubMed] [Google Scholar]
- 32.Stanberry, L. R., S. L. Spruance, A. L. Cunningham, D. I. Bernstein, A. Mindel, S. Sacks, S. Tyring, F. Y. Aoki, M. Slaoui, M. Denis, P. Vandepapeliere, and G. Dubin. 2002. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N. Engl. J. Med. 3471652-1661. [DOI] [PubMed] [Google Scholar]
- 33.Straus, S. E., B. Savarese, M. Tigges, A. G. Freifeld, P. R. Krause, D. M. Margolis, J. L. Meier, D. P. Paar, S. F. Adair, D. Dina, et al. 1993. Induction and enhancement of immune responses to herpes simplex virus type 2 in humans by use of a recombinant glycoprotein D vaccine. J. Infect. Dis. 1671045-1052. [DOI] [PubMed] [Google Scholar]
- 34.Straus, S. E., A. Wald, R. G. Kost, R. McKenzie, A. G. Langenberg, P. Hohman, J. Lekstrom, E. Cox, M. Nakamura, R. Sekulovich, A. Izu, C. Dekker, and L. Corey. 1997. Immunotherapy of recurrent genital herpes with recombinant herpes simplex virus type 2 glycoproteins D and B: results of a placebo-controlled vaccine trial. J. Infect. Dis. 1761129-1134. [DOI] [PubMed] [Google Scholar]
- 35.Wagner, E. K., and D. C. Bloom. 1997. Experimental investigation of herpes simplex virus latency. Clin. Microbiol. Rev. 10419-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wald, A., and R. Ashley-Morrow. 2002. Serological testing for herpes simplex virus (HSV)-1 and HSV-2 infection. Clin. Infect. Dis. 35S173-S182. [DOI] [PubMed] [Google Scholar]
- 37.Wald, A., L. Corey, R. Cone, A. Hobson, G. Davis, and J. Zeh. 1997. Frequent genital herpes simplex virus 2 shedding in immunocompetent women. Effect of acyclovir treatment. J. Clin. Investig. 991092-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu, F., M. R. Sternberg, B. J. Kottiri, G. M. McQuillan, F. K. Lee, A. J. Nahmias, S. M. Berman, and L. E. Markowitz. 2006. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA 296964-973. [DOI] [PubMed] [Google Scholar]