Abstract
Human immunodeficiency virus type 1 (HIV-1) coinfection decreases antibodies to variant surface antigens implicated in pregnancy-associated malaria (VSA-PAM) caused by Plasmodium falciparum. The effect of HIV-1 on antibody functions that may protect mothers from pregnancy-associated malaria is unknown. Sera from multigravid pregnant women with malaria and HIV-1 coinfection (n = 58) or malaria alone (n = 29) and from HIV-1-infected (n = 102) or -uninfected (n = 54) multigravidae without malaria were analyzed for anti-VSA-PAM antibodies by flow cytometry, the ability to inhibit adhesion to chondroitin sulfate A, or to opsonize CS2-infected erythrocytes for phagocytosis by THP-1 cells. In women with malaria, anti-VSA-PAM levels correlated better with opsonic activity (r = 0.60) than with adhesion-blocking activity (r = 0.33). In univariate analysis, HIV-1 coinfection was associated with lower opsonic activity but not adhesion-blocking activity or anti-VSA-PAM levels. Malaria-infected women with anemia (hemoglobin levels of <11.0 g/dl) had lower opsonic activity than nonanemic women (P = 0.007) independent of HIV-1 status. By multivariate analysis, in malaria-infected women, anemia (but not HIV status) was associated with opsonic activity. In women without malaria, opsonic activity was not associated with either anemia or HIV-1 status. In multigravid pregnant women with malaria, impaired serum opsonic activity may contribute to anemia and possibly to the decreased immunity to pregnancy-associated malaria associated with HIV-1.
Inhabitants of regions where malaria is endemic usually develop protective immunity to malaria by adolescence; however, protection is partially abrogated in women during pregnancy (19), resulting in pregnancy-associated malaria (PAM). Approximately 25 million women fall pregnant in sub-Saharan Africa every year, many in regions where both Plasmodium falciparum malaria and human immunodeficiency virus type 1 (HIV-1) infections are endemic (31). HIV-1 infection significantly increases the prevalence and density of malaria infection in pregnant women (reviewed in reference 31; 22).
P. falciparum-infected erythrocytes (IE) express variant surface antigens (VSA) on their surface (16), which mediate adhesion to host receptors and which are major targets of the immune response (4). The clearance of IE is presumed to be mediated by antibodies specific for VSA, which may block adhesion and/or facilitate opsonic clearance by phagocytes. A unique subset of VSA, termed VSA-PAM, is expressed by malaria strains isolated from pregnant women and mediates the placental adhesion of IE via binding to chondroitin sulfate A (CSA) (9, 24, 27) and other ligands (3). Antibodies against VSA-PAM are generated in a gravidity-dependent manner (23, 27) and are associated with protection against PAM.
HIV-coinfected women have significantly lower anti-VSA-PAM antibody levels than HIV-uninfected women, and these antibodies are decreased in a CD4+ T-cell-dependent manner (20). The impairment of anti-PAM immunity does not appear to be part of a general effect of HIV infection on humoral immunity to malaria since antibody responses to most other malaria antigens studied to date are not impaired (1, 20). HIV coinfection impairs immunity to PAM in women of all gravidities. The effect of HIV on levels of total immunoglobulin G to VSA-PAM is greatest in primigravid women (20), but the relative risk of contracting malaria for HIV-infected women compared to HIV-uninfected women appears to increase with gravidity (31). We postulate that specific functional defects in antibody responses may be more relevant to an understanding of the impact of HIV infection on susceptibility to malaria and that the presence of such defects in HIV-infected multigravid women might explain their increased susceptibility to malaria. We therefore measured IgG antibodies recognizing VSA-PAM, antibody-dependent inhibition of adhesion to CSA, and opsonic activity against IE expressing VSA-PAM in sera obtained from pregnant multigravid women with and without HIV coinfection.
(This work was presented in part at the 56th Annual Meeting of the American Society of Tropical Medicine and Hygiene, Philadelphia, PA [25a].)
MATERIALS AND METHODS
Study sample.
Patient sera were collected as previously described (20). Blood was obtained in the third trimester of pregnancy from women admitted to the Queen Elizabeth Central Hospital in Blantyre, Malawi, between December 2000 and April 2004 after written, informed consent was obtained for HIV counseling and testing and after testing for malaria infection. During pregnancy, over 90% of women had taken one or more treatment courses of intermittent preventive sulfadoxine-pyrimethamine treatment (median, 2; range, 1 to 5). The hemoglobin (Hb) concentration was measured using a Coulter counter or (when this was unavailable) a Hemocue hemoglobinometer, and anemia was defined as an Hb concentration below 11.0 g/dl. Malaria infection was defined as the presence of IE upon examination of Giemsa-stained histological sections of placenta (21). Placental malaria infection was examined and staged as previously described (26), with stage 1 being parasites only, stage 2 being parasites plus pigmented leukocytes with or without malaria pigment in fibrin, stage 3 being parasites and malaria pigment in fibrin only, stage 4 being malaria pigment in fibrin only, and stage 5 being no infection. Sera were stored at −70°C before and after heat inactivation (56°C for 1 h) and transported to Australia in liquid nitrogen (20).
The present study involved the testing 87 sera from multigravid women with placental malaria, 29 of whom were HIV uninfected and 58 of whom were HIV infected, and 156 sera from multigravid pregnant women without malaria, 54 of whom were HIV uninfected and 102 of whom were HIV infected. All available sera from each group were tested. In addition, we examined serum samples from 44 primigravid women with placental malaria and without HIV infection and 14 sympatric men as negative controls (provided by Terrie Taylor, collected with informed consent from fathers of children admitted to the hospital with malaria). Age, Hb levels, CD4 T-cell count (measured by FACScan; Becton Dickinson), and birth weight data for the multigravid women are presented in Table 1. HIV-infected women gave birth to lighter babies in both the malaria-infected (on average, 258 g lighter; 2,832 ± 69.88 g versus 3,090 ± 77.45 g; P = 0.036) and malaria-uninfected (on average, 250 g lighter; 2,956 ± 62.34 g versus 3,206 ± 76.40 g; P = 0.017) cohorts. HIV infection was associated with a significantly lower Hb level in women without malaria but not in women with malaria coinfection. There was no significant difference in age between any of the groups of women, and malaria status was not associated with any difference in CD4 counts among HIV-infected women.
TABLE 1.
Age, child's birth weight, and relevant clinical data for the cohorts of multigravid pregnant women participating in this studya
| Group | Mean age (yr) (SEM) (no. of women) | Mean Hb level (g/dl) (SEM) (no. of women) | Mean birth wt (g) (SEM) (no. of women) | CD4 cell count (cells/μl) (SEM) (no. of women) |
|---|---|---|---|---|
| M+ H+ | 26.96 (0.4414) (57) | 10.35 (0.2396) (58) | 2,832* (69.88) (55) | 340.3 (26.27) (55) |
| M+ H− | 28.10 (1.010) (29) | 10.84 (0.3041) (29) | 3,090* (77.45) (22) | NA |
| M− H+ | 28.79 (0.4481) (102) | 10.93† (0.1831) (99) | 2,956** (62.34) (97) | 360.1 (24.14) (90) |
| M− H− | 28.50 (0.6602) (54) | 12.21† (0.2299) (54) | 3,206** (76.40) (49) | NA |
Data represent means (standard errors of the means) and the numbers of each cohort of each respective measurement. M+, malaria infected; M−, malaria uninfected; H+, HIV infected; H−, HIV uninfected; NA, not applicable. For birth weight, P values were 0.036 (*) and 0.017 (**) by an unpaired t test. For Hb, the P value was <0.0001 (†) by an unpaired t test.
Ethical approval.
The study was approved by the College of Medicine Research Ethics Committee, University of Malawi, and by the Human Research Ethics Committee, Melbourne Health, Melbourne, Australia.
Production of CSA-binding CS2 parasites.
The P. falciparum parasite line CS2 (6) was cultured in unexpired human group O+ erythrocytes provided by the Australian Red Cross Blood Service. Cells were maintained at 1 to 12% parasitemia in RPMI-HEPES supplemented with 0.5% Albumax II, 50 μg/ml hypoxanthine, 2.5 μg/ml gentamicin, and 25 mM NaHCO3 (supplemented RPMI-HEPES). Adhesion to CSA was regularly checked and remained at a constant high level. Cultures were synchronized by gelatin flotation every 1 to 2 weeks.
Purification of parasitized erythrocytes.
Trophozoite-stage parasites were purified by Percoll (Amersham, Rydalmere, NSW, Australia) density gradient centrifugation using layers of 80%, 60%, and 40% Percoll in supplemented RPMI-HEPES. After centrifugation at 2,095 × g for 15 min, collection of the 60% layer yielded 80 to 95% pure preparations of CS2 IE, which were washed three times and resuspended in culture medium. IE were used for measurements of phagocytosis or antiadhesion assays within 3 h of preparation.
Flow cytometry.
Cultures of 5 to 10% IE were used to test sera for total IgG to VSA-PAM as described previously (20), with minor modifications. IE (0.1% hematocrit) were incubated with individual patient serum diluted at 1:20 in 1% fetal calf serum (FCS) in phosphate-buffered saline (PBS). After 30 min, IE were washed three times and incubated for 30 min with rabbit anti-human IgG (1:100; Dako, Botany, NSW, Australia), followed by donkey anti-rabbit IgG conjugated to Alexa Fluor 488 (1:500; Molecular Probes, Mount Waverley, VIC, Australia) plus 10 μg/ml ethidium bromide in PBS with 1% FCS. Samples were analyzed on a Becton Dickinson FACSCalibur flow cytometer. One thousand IE were counted, and the geometric mean fluorescence intensity for Alexa Fluor 488 was calculated as a measure of IgG binding to IE. Positive and negative controls comprising pooled sera from malaria-exposed pregnant women and sera from individual malaria-naïve Melbourne blood donors were analyzed in each assay for standardization, and antibody levels were expressed relative to controls as described previously (20). The positive-control standard serum was generated by mixing equal volumes of sera from 46 pregnant Malawian women previously shown to have high levels of reactivity against the CS2 parasite line by flow cytometry. Fifteen sera were obtained from HIV-infected multigravidae, and 31 sera were obtained from HIV-uninfected women (4 primigravid, 13 secundigravid, and 14 multigravid women). The same pooled positive-control patient serum was used in adhesion inhibition and opsonic activity assays described below.
Adhesion inhibition assays.
Sera were tested for the ability to inhibit the adhesion of IE via our previously reported methods (2), with minor modifications. Bovine tracheal CSA (10 μg/ml; Sigma, Castle Hill, NSW, Australia) in PBS was coated in 10-μl droplets onto 150-mm petri dishes (Becton-Dickinson, North Ryde, NSW, Australia) overnight. The droplet location was marked on the undersurface of the dish, and wells 10 mm in diameter were then created around the dried CSA spots using a wax pen for immunohistochemistry (Dako). Wells were blocked with 10% nonimmune human serum in supplemented RPMI-HEPES for 30 min. IE (6 to 10% parasitemia; 0.5% hematocrit) were washed and incubated in supplemented RPMI-HEPES (pH 6.8) with test and control sera at a 1-in-10 dilution in microcentrifuge tubes for 30 min at 37°C. After resuspension, 15 μl of IE was added to triplicate wells (prepared as described above), and the plate was incubated for 15 to 20 min at 37°C. Plates were carefully washed with supplemented RPMI-HEPES to remove unbound cells. Bound cells were fixed with 2% glutaraldehyde in PBS for a minimum of 2 h, stained with Giemsa for 10 min, dried, and counted by microscopy. Pooled patient serum was used as a positive control, as described above. Percent adhesion inhibition was determined relative to the positive control using the formula (sample/positive control) × 100.
Cell culture.
THP1 cells were cultured in RPMI 1640 medium supplemented with 10% FCS, 2 mM l-glutamine, and 24 μg/ml gentamicin (supplemented RPMI). Cells were passaged every 2 to 3 days by centrifugation (600 × g for 5 min), with subsequent resuspension at 2 × 104 to 4 × 104 viable cells/ml. Cell density was kept below 1 × 106 viable cells/ml at all times, and cultures were discarded after passages 12 to 15. Cells were resuspended at a concentration of 1 × 106 cells/ml, seeded at 5 × 104 cells/well in a 96-well plate, and then induced to differentiate into adherent monocytic cells by the addition of 20 nM phorbol myristate acetate for 2 to 3 days before being used in phagocytosis assays.
Colorimetric phagocytosis assay.
A phagocytic assay employing PMA-differentiated THP1 cells (13, 28, 30) was modified to include colorimetric quantitation of internalized IE. The phagocytosis assay was performed as described previously (5), with minor modifications. CS2 IE were washed in cold PBS (900 × g for 2 min at 4°C) in fetal calf serum-coated Eppendorf tubes before opsonization at 1 × 108 cells/ml with 9% (vol/vol) patient sera for 1 h at room temperature. CS2 IE were also opsonized with a single batch of pooled patient sera as a positive control and internal standard against which individual patient samples were normalized (see above for details of the positive-control, pooled sera). Following opsonization, CS2 IE were washed in cold PBS and resuspended at 1 × 108 cells/ml. Opsonized and unopsonized CS2 IE were added to THP1 cells (quadruplicate wells of a 96-well plate for each serum sample seeded with 50,000 cells per well in 200 μl of supplemented RPMI) at a ratio of 20 parasites per THP1 cell and then incubated for 2 h at 37°C to allow phagocytosis. After incubation, phagocytosis was stopped by the addition of 100 μl cold PBS, aspirating the PBS, and 100 μl of 0.2% NaCl was then added for 3 min to lyse uningested erythrocytes. Lysed erythrocytes were removed by four 200-μl washes with warm supplemented RPMI, and ingested Hb was then released by lysing the THP1 cells using 100 μl 0.2 M Tris-HCl containing 6 M urea for 30 min. Hb was quantified by reactions with 100 μl of freshly made 2,7-diaminofluorene (DAF) solution (1-ml stock of 10 mg/ml DAF [Sigma] dissolved in 90% acetic acid, 0.1 ml 30% hydrogen peroxide, and 8.9 ml 6 M urea), and the colored reaction product was measured at 620 nm and quantified by reference to a standard curve of unopsonized IE derived from the same culture. The standard curve was constructed by making seven serial twofold dilutions of IE (2.5 × 107 to 3.91 × 105 cells/ml plus a zero-erythrocyte control in PBS) and adding 10 μl of each dilution to triplicate wells reserved on the assay plate. Standards were lysed in 100 μl of 0.2 M Tris-HCl containing 6 M urea and reacted with DAF as described above for the phagocytosis samples. The absorbance obtained for each phagocytosis well was converted to an equivalent number of ingested erythrocytes by reference to the standard curve and then to a phagocytic index by dividing by the number of THP-1 cells seeded (5 × 104 cells) and multiplying by 100. The opsonic activity of individual sera was defined as the phagocytic index obtained using IE opsonized with each serum sample divided by the phagocytic index obtained with the pooled patient reference serum analyzed on the same plate, multiplied by 100.
Statistical methods.
Distribution analysis showed that neither adhesion-blocking nor opsonic activity was normally distributed in these patient cohorts. Data for both assays were therefore normalized using log10 transformation prior to analysis, and parametric statistics were used to compare log-transformed data between groups. Univariate analysis, using t tests and applying Bonferroni's correction for multiple comparisons where appropriate, was used to investigate statistically significant differences in antibody levels or functions in relation to patient characteristics. Logistic regression analysis was used for estimating predictors of opsonic and antiadhesion activity. Categorical and continuous variables included in the initial model were HIV status, anemia, infant birth weight, history of febrile symptoms within the last 7 days, sulfadoxine-pyrimethamine treatment, malaria stage, presence of monocyte pigment, and presence of fibrin pigment. Backwards elimination was applied to sequentially remove nonsignificant variables until a subset of wholly significant predictors remained. A variable was considered to be significant if the P value was less than 0.05. The correlations between the logarithms of opsonic activity or adhesion with the logarithm of antibody levels were performed using Pearson's product-moment correlation with the confidence interval based on Fisher's transformation.
RESULTS
Gender- and parity-dependent immune responses to VSA-PAM.
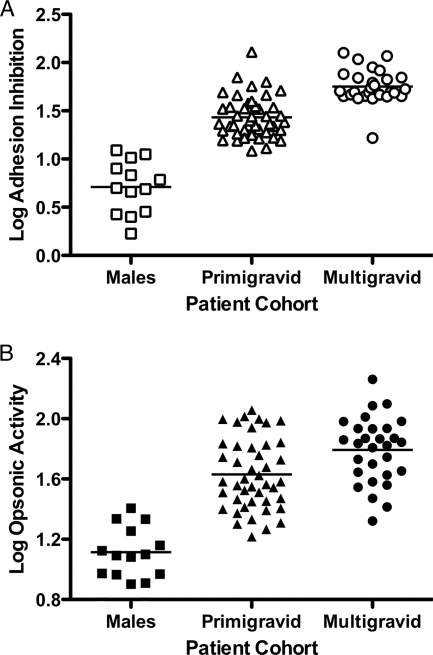
To validate the assays, we compared adhesion inhibition and opsonic activity in sera obtained from sympatric males as well as primigravid and multigravid women without HIV infection. Sera from males contained very low levels of both antiadhesion and opsonic activity, and multigravid women had significantly higher adhesion-blocking and opsonic activities than did primigravid women after deflating the significance level to account for multiple comparisons (P < 0.0001 and P = 0.004, respectively, by unpaired t test) (Fig. 1). There was a similar gender- and parity-dependent increase in this cohort in levels of antibody to VSA-PAM measured by flow cytometry, as previously described (23; data not shown).
FIG. 1.
Functional activity of antibodies to CS2-VSA is gender specific and parity dependent in both adhesion and opsonization assays. A comparison of antibody responses in males (n = 14), primigravid HIV-uninfected women (n = 44), and multigravid HIV-uninfected women (n = 29) is shown. (A) Adhesion inhibition assay. Horizontal bars represent means of each group. Differences between all groups were statistically significant (P < 0.0001 for all comparisons by unpaired t test). (B) Phagocytosis assay. CS2-IE were opsonized with patient sera and then used as targets for phagocytosis by THP1 cells. The difference in percent phagocytic index between males and primigravid HIV-uninfected women and males and multigravid HIV-uninfected and primigravid women versus multigravid HIV-uninfected women was statistically significant (P < 0.0001, P < 0.0001, and P = 0.004, respectively, by unpaired t test).
Relationship between total IgG to CS2 and functional antibody assays.
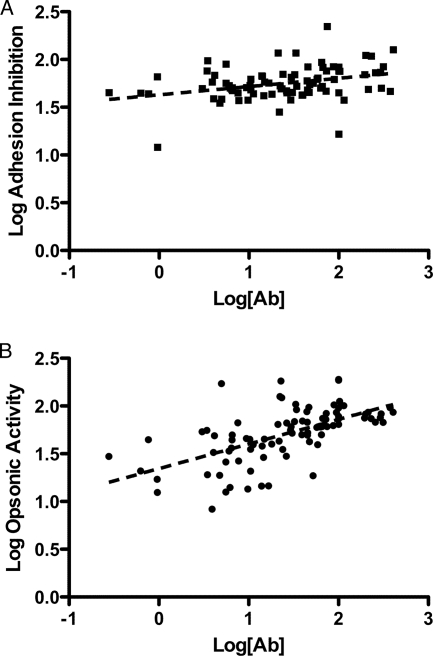
We initially examined functional activity in sera from multigravid women with malaria as these individuals would be expected to possess the highest levels of antibody to VSA-PAM. Moreover, multigravid women have the highest relative risk of malaria infection associated with HIV coinfection (31). Total VSA-PAM-specific IgG (determined by flow cytometry) correlated relatively weakly with antiadhesion activity (r = 0.33; P = 0.0019) (Fig. 2A) but showed a stronger correlation with opsonic activity (r = 0.60; P < 0.001) (Fig. 2B). The closer relationship between opsonic activity and total IgG to VSA-PAM may arise because all VSA-PAM epitopes may be targets for opsonization, whereas only a subset of epitopes participates in adhesion.
FIG. 2.
Correlation between total VSA-PAM-specific IgG and adhesion inhibition (A) or opsonic activity (B) in patient sera from multigravid women with PAM. Flow cytometry was used to measure levels of serum antibodies (Ab) specific for CS2-VSA and compared with both antiadhesion and phagocytic activities for each serum sample. Correlations were performed on log-transformed data. A weak correlation was seen between antibody level and percent adhesion inhibition (Pearson's r = 0.33). A marked correlation was seen between the antibody level and percent phagocytic index (Pearson's r = 0.60). Each point represents the mean of an experiment for a single sample performed in triplicate and standardized to the positive control for that individual experiment.
Opsonic activity is impaired by HIV infection.
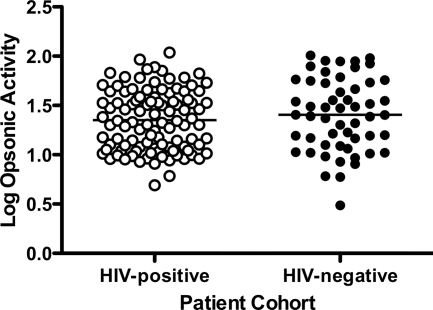
Having validated and established the characteristics of our assays, we next examined the association between HIV coinfection in multigravid women with malaria and functional antibody in their sera. Antiadhesion antibody responses did not show an association with HIV infection (P = 0.938; mean activities of 56.0 for HIV-positive women and 56.4 for HIV-uninfected women) (Fig. 3A), whereas HIV-infected women showed significantly impaired opsonic activity against CS2 IE (P = 0.045; mean activities of 45.8 for HIV-positive women and 62.1 for HIV-uninfected women) (Fig. 3B). This suggests that HIV has various effects on different aspects of anti-CS2 activity and that HIV may significantly diminish quantities of opsonizing antibodies directed against CS2 IE in multigravid women.
FIG. 3.
HIV infection reduces opsonic but not antiadhesion activity against CS2-VSA in multigravid women with PAM. Antibody function was measured and compared between HIV-infected (n = 58) (open symbols) and HIV-uninfected (n = 29) (closed symbols) women using the adhesion inhibition (A) and opsonization (B) assays. Antiadhesion activity for HIV-infected women was not statistically different from that for HIV-uninfected women (P = 0.938 by unpaired t test). The opsonic activity for HIV-infected women was significantly lower than that for HIV-uninfected women (P = 0.045 by unpaired t test). Each point represents the mean of an experiment for a single sample performed in triplicate and standardized to a positive control within an individual experiment. Horizontal bars indicate the means for each group.
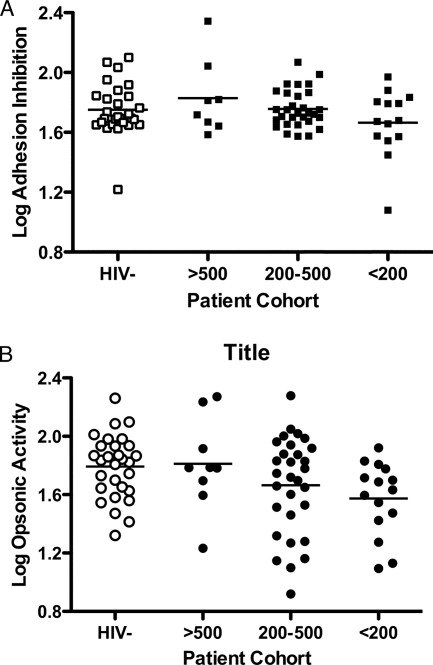
To further examine the relationship between antibody activity and HIV infection, we compared antiadhesion and opsonic activities in sera from HIV-infected women with well-preserved CD4 T-cell counts (>500 cells/μl), intermediate CD4 T-cell counts (between 200 and 500 cells/μl), and severe immunosuppression (CD4 T-cell counts of <200 cells/μl) and in sera from HIV-uninfected women. For both assays, there was a trend of decreased activity with decreased CD4 counts, but given the relatively low numbers in some of the groups, this was not statistically significant (Fig. 4). Using pairwise comparisons, however, there was a significant difference in opsonic activity between women with <200 CD4 T cells/μl and HIV-uninfected women (P = 0.0045).
FIG. 4.
CD4 T-cell count is associated with opsonic but not antiadhesion activity in HIV-infected multigravid women with PAM. Multigravid women coinfected with HIV (closed symbols) were stratified on the basis of CD4 T-cell counts into three groups, low (<200 cells/μl), intermediate (200 to 500 cells/μl), and well preserved (>500 cells/μl). Log-transformed adhesion-blocking (A) and opsonic (B) activities are shown for each group and compared to those for HIV-uninfected women (open symbols). There was no difference in adhesion-blocking activity between any of the groups (P = 0.185 by one-way ANOVA). For opsonic activity, using pairwise comparisons, only women with CD4 T-cell counts of <200 cells/μl were significantly different from women who were HIV negative (P = 0.0045 by unpaired t test).
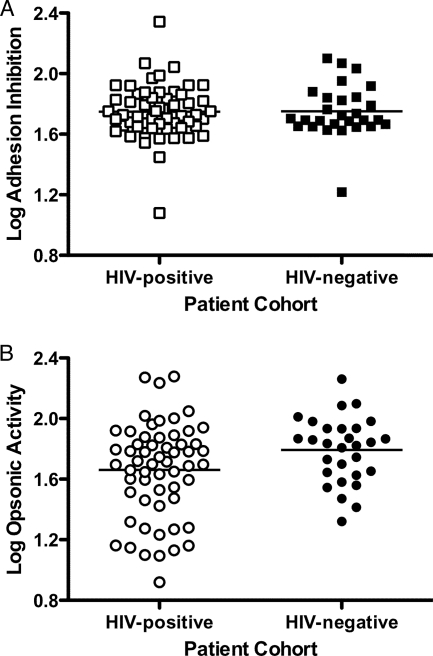
Taken together, these data suggest a significant impairment of opsonic but not adhesion-blocking activity associated with HIV infection in multigravid women with malaria. Multigravid women in an area where malaria is endemic who have malaria in their current pregnancy may be expected to have boosted levels of antibody specific for VSA-PAM at term. The effects of HIV coinfection on aspects of anti-VSA-PAM function may therefore reflect an inadequate boosting of this response. It was therefore of interest to examine opsonic activity in a cohort of multigravid women without current malaria, as it would be expected that antibody levels and function in this cohort would reflect an immunological memory of responses generated in prior pregnancies. Antibody levels are often lower in malaria-uninfected individuals than in infected individuals. As expected, there was a significantly lower level of anti-VSA-PAM in both HIV-infected (n = 102) and HIV-uninfected (n = 54) women without PAM than in the same cohorts of women with malaria (P < 0.0001 and P = 0.0094, respectively). This was also true for opsonic activity (P < 0.0001 for both comparisons). Of interest, however, in our group of multigravid women without malaria, HIV infection was not associated with lower opsonic activity (P = 0.318) (Fig. 5), suggesting that HIV infection may selectively inhibit the increase in opsonic activity toward VSA-PAM occurring during malaria infection.
FIG. 5.
HIV infection does not affect opsonic activity in multigravid pregnant women without PAM. Log opsonic activities were compared in sera from multigravid women without PAM stratified for HIV infection. There was no significant difference (P = 0.318 by unpaired t test) between HIV-infected (n = 102) (open symbols) and HIV-uninfected (n = 54) (closed symbols) women.
Association of anti-VSA PAM activity with anemia.
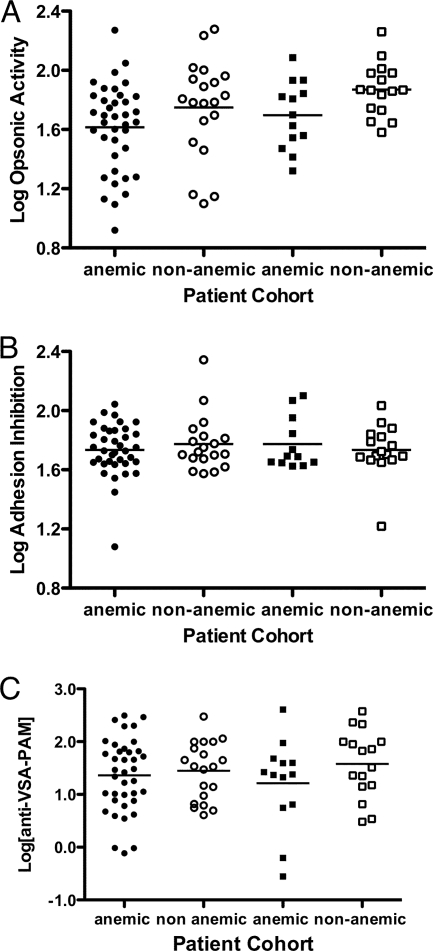
To determine whether the levels of antibodies as measured with our different assays are associated with a decreased incidence of complications in placental malaria, we examined the relationship between opsonic activity, adhesion-blocking activity, or total IgG to VSA-PAM, maternal Hb levels, and infant birth weight. There was no significant relationship between antibody levels or function and birth weight (data not shown). Among malaria-infected women, those with anemia (Hb levels of <11.0 g/dl) showed significantly lower opsonic activity than nonanemic women (P = 0.022 by one-way analysis of variance [ANOVA]) (Fig. 6A). In pairwise comparisons, after deflating the significance level to account for multiple comparisons, this difference approached significance in HIV-negative women (P = 0.03) but not in HIV-positive women (P = 0.12), suggesting that the association of anemia and low opsonic activity in these women is not due to HIV infection. Neither adhesion-blocking activity (P = 0.81 by one-way ANOVA) (Fig. 6B) nor total IgG to VSA-PAM (P = 0.51 by one-way ANOVA) (Fig. 6C) showed any significant association with anemia, suggesting that opsonic activity but not antibody level or antiadhesion activity is associated with protection from pregnancy-associated anemia in this cohort. There were no significant differences in opsonic activity or anti-VSA-PAM levels in anemic versus nonanemic women in the cohort of women without malaria (data not shown).
FIG. 6.
Opsonic but not adhesion-blocking activity or total levels of anti-VSA-PAM is associated with protection from anemia in multigravid women with PAM. Multigravid women with malaria were stratified on the basis of maternal Hb levels using the clinical definitions of anemic (Hb levels of <11 g/dl) (closed symbols) and nonanemic (Hb levels of ≥11 g/dl) (open symbols) and on the basis of HIV status (HIV infected [circles] and HIV uninfected [squares]). Data for opsonic activity (A), adhesion-blocking activity (B), and total anti-VSA-PAM (C) are shown. There was no difference in adhesion-blocking activity or total anti-VSA-PAM between groups (P = 0.81 and P = 0.51, respectively, by one-way ANOVA). There was a significant difference in opsonic activity between groups (P = 0.022 by one-way ANOVA), which, in pairwise comparisons, approached significance (after deflating the significance level to account for multiple comparisons) for HIV-uninfected women (P = 0.03 by unpaired t test) but not HIV-coinfected women (P = 0.122 by unpaired t test).
Multivariate analysis of opsonic activity in multigravid women with malaria.
Finally, we assessed HIV status, age, infant birth weight, and maternal anemia for their association with opsonic activity using stepwise multivariate analyses in the cohort of multigravid women with malaria (Table 2). In the multivariate analysis, the association of opsonic activity with infant birth weight and maternal age was not significant. HIV status, while significant in the univariate analysis, was no longer significant in the multivariate model; only maternal anemia remained significant (P = 0.007). The mean log opsonic activity of anemic women was 1.636 (standard error of the mean, 0.039), compared to 1.802 (standard error of the mean, 0.046) for nonanemic women, which is equivalent to mean opsonic activities of 43.21 and 63.39, respectively. Upon logistic regression, being anemic was significantly associated with a decrease in opsonic activity of 20.14 compared to being nonanemic.
TABLE 2.
Association between indicated characteristics and opsonic activity in sera of multigravid women with malaria
| Characteristic | No. of patients | % of patients | Unadjusted estimate (95% CI)
|
Adjusted estimate (95% CI)
|
||
|---|---|---|---|---|---|---|
| Coefficient | P value | Coefficient | P valuea | |||
| Total | 87 | 100 | ||||
| HIV status | ||||||
| Positive | 58 | 66.67 | 0 | |||
| Negative | 29 | 33.33 | 0.132 (0.003, 0.260) | 0.045 | NS | |
| Age (yr)b | ||||||
| ≤24 | 22 | 25.29 | 0 | |||
| 25-27 | 28 | 32.18 | −0.160 (−0.323, 0.003) | 0.055 | NS | |
| 28-30 | 17 | 19.54 | −0.047 (−0.232, 0.137) | 0.611 | NS | |
| >30 | 19 | 21.84 | −0.060 (−0.240, 0.119) | 0.506 | NS | |
| Unknown | 1 | 1.15 | ||||
| Anemiac | ||||||
| No | 51 | 58.62 | 0 | |||
| Yes | 36 | 41.38 | −0.166 (−0.287, −0.046) | 0.007 | −0.166 (−0.287, −0.046) | 0.007 |
| Birth wt (g)b | ||||||
| ≤2,650 | 21 | 24.14 | 0 | |||
| 2,651-3,000 | 23 | 26.44 | −0.005 (−0.187, 0.176) | 0.954 | NS | |
| 3,001-3,300 | 18 | 20.69 | 0.064 (−0.129, 0.257) | 0.510 | NS | |
| >3,300 | 15 | 17.24 | 0.061 (−0.143, 0.264) | 0.554 | NS | |
| Unknown | 10 | 11.49 | ||||
NS, not significant.
Cutoffs for age and birth weight were designed to divide the data set into four approximately equal groups.
Anemia was defined as Hb levels of <11.0 g/dl.
DISCUSSION
Antibody is a critical component of immunity to PAM. In pregnant women, IgG1 and IgG3 responses to VSA-PAM develop (8, 18), and total IgG to these targets has been correlated with protection from anemia and increased birth weight (29). Two main functions for antibody to VSA-PAM are suggested: blockade of adhesion to tissue-specific receptors (10) and opsonization of IE for phagocytic clearance. Adhesion-blocking antibodies have been associated with protection from anemia and premature delivery (7), whereas the protective role for opsonizing antibodies has not been explored. In the present study, we examined the relationship between antibody levels and function, HIV infection, and susceptibility to malaria complications using sera from pregnant women with and without HIV infection and malaria. Opsonic activity, like other measures of antibody immunity, showed gender and parity dependence. Moreover, opsonic activity correlated more strongly with total IgG levels to VSA-PAM than did the adhesion-blocking ability, suggesting that the opsonic clearance of IE may be a major function of antibodies generated against PAM. In univariate analyses, among multigravid women with concurrent malaria, lower opsonic activity, but not adhesion-blocking activity, was associated with anemia and HIV infection, and opsonic activity was lowest in the most immunosuppressed women. In multivariate analyses, maternal Hb remained significantly associated with opsonic activity, but HIV status did not. The strong relationship that we noted between CD4 T-cell count and opsonizing antibody and the trend for antibody to be lower in HIV-infected individuals across the whole cohort suggest that there is a relationship between HIV-related immunosuppression and opsonizing antibody, but this effect of HIV is unlikely to be a major determinant of susceptibility to malaria in pregnancy.
The strong association that we observed between anemia and opsonic activity suggests that protection from malarial anemia may be an important role of opsonizing antibody. Interestingly, in the larger group of multigravid women without malaria, neither opsonic activity nor IgG to VSA-PAM varied with HIV status or Hb levels. This may be because HIV infection impairs the maintenance of memory B-cell pools (17, 32) and therefore affects primarily the increase in circulating antibody levels, which follows an adaptive response to current infection. In children, levels of antibody responses increase during malaria infection, and antibody responses in infected children appear to protect against subsequent clinical disease (12). A similar interaction may be important in pregnancy, with opsonizing antibody being protective only in the context of present infection.
Our starting hypothesis, that HIV infection would cause a decrease in opsonizing activity, which may explain the increased susceptibility to malaria, was not confirmed. As we previously postulated (20), it is possible that HIV exerts a greater effect on antibody responses to neoantigens than on existing responses, in which case the effect of HIV on opsonizing activity in primigravid women should be investigated, as VSA-PAM would be a neoantigen in these women.
Our findings differ from those reported previously by Keen et al., who showed marked decreases in levels of opsonic activity in plasma from 23 HIV-infected compared to 23 HIV-uninfected multigravid Kenyan women (11). Those investigators used CD36−/− murine macrophages, rather than human THP-1 cells, in their phagocytic assays and manual counting instead of colorimetry to determine phagocytic indices. Samples were collected after delivery, but most women did not have placental malaria on blood film, and most of the women had well-preserved CD4 T-cell counts. None of these differences fully explain the contrasting findings with our study. No data were presented by Keen et al. on the anemia statuses of the patients used in their study. The use of murine macrophages, as opposed to human macrophages, may also introduce differences due to the different Fcγ receptors expressed on murine cells. In this regard, Tebo et al. previously reported that THP1 cells use both Fcγ receptors IIA and I to ingest malaria-infected erythrocytes (30). Murine macrophages, however, lack an FcRγ-independent activating Fcγ receptor equivalent to human Fcγ receptor IIA. This may not be an important difference, however, as Keen et al. reported similar findings using human macrophages, suggesting that species difference is not critical (11). We confirmed the expression of both Fcγ RI and RIIA on the THP1 cells used in the present study, but their relative importance in the uptake of CS2 IE opsonized with patient sera was not investigated. It is possible that HIV differentially affects phagocytosis mediated by these two receptors (15). Further studies from areas of high HIV endemicity will be of interest.
Our parent study was designed primarily to recruit HIV-infected women to examine factors influencing mother-to-child HIV transmission. HIV-uninfected women were recruited if they had peripheral blood malaria infection, together with matched controls for these women. The prevalence of malaria upon peripheral blood examination was 9.5%, which is lower than that reported in previous studies from the same site (25). Together, these factors may have introduced biases into our study, affecting the relationship between HIV and malarial immunity. For example, because all HIV-1-infected women but only HIV-uninfected women with smear-positive malaria and their controls were recruited, the proportions of women differed by gravidity between the HIV-1-infected and -uninfected groups. In the population of women screened for this study, HIV-1 prevalence was not significantly associated with factors that may influence malaria exposure, such as education, residence, and employment (14). Moreover, to minimize possible confounding, we tested all available samples from our patient groups and compared multigravid women with and without HIV-1 separately for those with current malaria infection and those without. It may be that primigravid study participants, who have a greater malaria risk and are more likely to deliver infants with malaria-related low birth weight, would show a different pattern of responses.
The role of opsonizing antibodies in facilitating the clearance of IE by phagocytic cells clearly requires further study independently of any deficiencies of such responses associated with HIV infection. We observed a strong association between low levels of opsonizing antibodies and maternal anemia. A deficit in antibodies that facilitate the clearance of infected erythrocytes may be highly relevant to the development of this important complication of malaria. Most severe anemia occurs in children, and the defective clearance of IE could play a pathogenic role in its development. Assays of functional antibody immunity, together with examination of phagocytic cell activity from malaria-infected individuals, may provide critical insights into disease pathogenesis and provide useful surrogates of vaccine efficacy suitable for use in field studies.
Acknowledgments
We thank Terrie Taylor for providing the male plasma samples, Aphrodite Caragounis for technical assistance, James Blaszak for early assistance with establishing opsonic activity assays, Tim Spelman for statistical advice, and James Beeson for advice with adhesion inhibition assays. We thank the pregnant women who kindly participated in this study, who were recruited as part of NIH RO1 award number AI 49084.
This work was supported by NHMRC project grant 400090 to S.J.R. and A.J.
We do not have a commercial or other association that might pose a conflict of interest.
Footnotes
Published ahead of print on 7 January 2009.
REFERENCES
- 1.Ayisi, J., O. Branch, A. Rafi-Janajreh, A. M. Van Eijk, F. Ter Kuile, D. Rosen, P. Kager, D. Lanar, A. Barbosa, D. Kaslow, B. Nahlen, and A. Lal. 2003. Does infection with human immunodeficiency virus affect antibody responses to Plasmodium falciparum antigenic determinants in asymptomatic pregnant women? J. Infect. 46164-172. [DOI] [PubMed] [Google Scholar]
- 2.Beeson, J., E. Mann, S. Elliott, V. Lema, E. Tadesse, M. Molyneux, G. Brown, and S. Rogerson. 2004. Antibodies to variant surface antigens of Plasmodium falciparum-infected erythrocytes and adhesion inhibitory antibodies are associated with placental malaria and have overlapping and distinct targets. J. Infect. Dis. 189540-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beeson, J., S. Rogerson, B. Cooke, J. Reeder, W. Chai, A. Lawson, M. Molyneux, and G. Brown. 2000. Adhesion of Plasmodium falciparum-infected erythrocytes to hyaluronic acid in placental malaria. Nat. Med. 686-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bull, P., B. Lowe, M. Kortok, C. Molyneux, C. Newbold, and K. Marsh. 1998. Parasite antigens on the infected red cell surface are targets for naturally acquired immunity to malaria. Nat. Med. 4358-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan, H. T., K. Kedzierska, J. O'Mullane, S. M. Crowe, and A. Jaworowski. 2001. Quantifying complement-mediated phagocytosis by human monocyte-derived macrophages. Immunol. Cell Biol. 79429-435. [DOI] [PubMed] [Google Scholar]
- 6.Cooke, B., S. Rogerson, G. Brown, and R. Coppel. 1996. Adhesion of malaria-infected red blood cells to chondroitin sulfate A under flow conditions. Blood 884040-4044. [PubMed] [Google Scholar]
- 7.Duffy, P. E., and M. Fried. 2003. Antibodies that inhibit Plasmodium falciparum adhesion to chondroitin sulfate A are associated with increased birth weight and the gestational age of newborns. Infect. Immun. 716620-6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elliott, S., A. Brennan, J. Beeson, E. Tadesse, M. Molyneux, G. Brown, and S. Rogerson. 2005. Placental malaria induces variant-specific antibodies of the cytophilic subtypes immunoglobulin G1 (IgG1) and IgG3 that correlate with adhesion inhibitory activity. Infect. Immun. 735903-5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fried, M., and P. Duffy. 1996. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science 2721502-1504. [DOI] [PubMed] [Google Scholar]
- 10.Fried, M., F. Nosten, A. Brockman, B. Brabin, and P. Duffy. 1998. Maternal antibodies block malaria. Nature 395851-852. [DOI] [PubMed] [Google Scholar]
- 11.Keen, J., L. Serghides, K. Ayi, S. N. Patel, J. Ayisi, A. van Eijk, R. Steketee, V. Udhayakumar, and K. C. Kain. 2007. HIV impairs opsonic phagocytic clearance of pregnancy-associated malaria parasites. PLoS Med. 4e181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kinyanjui, S. M., T. Mwangi, P. C. Bull, C. I. Newbold, and K. Marsh. 2004. Protection against clinical malaria by heterologous immunoglobulin G antibodies against malaria-infected erythrocyte variant surface antigens requires interaction with asymptomatic infections. J. Infect. Dis. 1901527-1533. [DOI] [PubMed] [Google Scholar]
- 13.Kumaratilake, L., A. Ferrante, T. Jaeger, and S. Morris-Jones. 1997. The role of complement, antibody, and tumor necrosis factor alpha in the killing of Plasmodium falciparum by the monocytic cell line THP-1. Infect. Immun. 655342-5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwiek, J. J., V. Mwapasa, A. P. Alker, A. S. Muula, H. E. Misiri, M. E. Molyneux, S. J. Rogerson, F. M. Behets, and S. R. Meshnick. 2008. Socio-demographic characteristics associated with HIV and syphilis seroreactivity among pregnant women in Blantyre, Malawi, 2000-2004. Malawi Med. J. 2080-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leeansyah, E., B. Wines, S. Crowe, and A. Jaworowski. 2006. The mechanism underlying defective Fc-gamma receptor-mediated phagocytosis by HIV-1 infected human monocyte-derived macrophages. J. Immunol. 1781096-1104. [DOI] [PubMed] [Google Scholar]
- 16.Leech, J., J. Barnwell, L. Miller, and R. Howard. 1984. Identification of a strain-specific malarial antigen exposed on the surface of Plasmodium falciparum-infected erythrocytes. J. Exp. Med. 1591567-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malaspina, A., S. Moir, S. M. Orsega, J. Vasquez, N. J. Miller, E. T. Donoghue, S. Kottilil, M. Gezmu, D. Follmann, G. M. Vodeiko, R. A. Levandowski, J. M. Mican, and A. S. Fauci. 2005. Compromised B cell responses to influenza vaccination in HIV-infected individuals. J. Infect. Dis. 1911442-1450. [DOI] [PubMed] [Google Scholar]
- 18.Megnekou, R., T. Staalsoe, D. Taylor, R. Leke, and L. Hviid. 2005. Effects of pregnancy and intensity of Plasmodium falciparum transmission on immunoglobulin G subclass responses to variant surface antigens. Infect. Immun. 734112-4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menendez, C. 1995. Malaria during pregnancy: a priority area of malaria research and control. Parasitol. Today 11178-183. [DOI] [PubMed] [Google Scholar]
- 20.Mount, A., V. Mwapasa, S. Elliott, J. Beeson, E. Tadesse, V. Lema, M. Molyneux, S. Meshnick, and S. Rogerson. 2004. Impairment of humoral immunity to Plasmodium falciparum malaria in pregnancy by HIV infection. Lancet 3631860-1867. [DOI] [PubMed] [Google Scholar]
- 21.Mwapasa, V., S. Rogerson, M. Molyneux, E. Abrams, D. Kamwendo, V. Lema, E. Tadesse, E. Chaluluka, P. Wilson, and S. Meshnick. 2004. The effect of Plasmodium falciparum malaria on peripheral and placental HIV-1 RNA concentrations in pregnant Malawian women. AIDS 181051-1059. [DOI] [PubMed] [Google Scholar]
- 22.Ned, R., J. Moore, S. Chaisavaneeyakorn, and V. Udhayakumar. 2005. Modulation of immune responses during HIV-malaria co-infection in pregnancy. Trends Parisitol. 21284-291. [DOI] [PubMed] [Google Scholar]
- 23.Ricke, C., T. Staalsoe, K. Koram, B. Akanmori, E. Riley, T. Theander, and L. Hviid. 2000. Plasma antibodies from malaria-exposed pregnant women recognize variant surface antigens on Plasmodium falciparum-infected erythrocytes in a parity-dependent manner and block parasite adhesion to chondroitin sulfate A. J. Immunol. 1653309-3316. [DOI] [PubMed] [Google Scholar]
- 24.Rogerson, S. J., S. C. Chaiyaroj, K. Ng, J. C. Reeder, and G. V. Brown. 1995. Chondroitin sulfate A is a cell surface receptor for Plasmodium falciparum-infected erythrocytes. J. Exp. Med. 18215-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rogerson, S. J., E. Chaluluka, M. Kanjala, P. Mkundika, C. Mhango, and M. E. Molyneux. 2000. Intermittent sulfadoxine-pyrimethamine in pregnancy: effectiveness against malaria morbidity in Blantyre, Malawi, in 1997-99. Trans. R. Soc. Trop. Med. Hyg. 94549-553. [DOI] [PubMed] [Google Scholar]
- 25a.Rogerson, S. J., L. Fernandes, F. Yosaatmadja, V. Mwapasa, M. Molyneux, E. Tadesse, S. Meshnick, and A. Jaworowski. 2007. HIV infection impairs opsonic phagocytosis of Plasmodium falciparum-infected erythrocytes, abstr. no. 693. In American Society of Tropical Medicine and Hygiene (ASTMH) 56th Annual Meeting, 4 to 8 November 2007, Philadelphia, PA.
- 26.Rogerson, S. J., E. Pollina, A. Getachew, E. Tadesse, V. M. Lema, and M. E. Molyneux. 2003. Placental monocyte infiltrates in response to Plasmodium falciparum malaria infection and their association with adverse pregnancy outcomes. Am. J. Trop. Med. Hyg. 68115-119. [PubMed] [Google Scholar]
- 27.Salanti, A., M. Dahlback, L. Turner, M. Nielsen, L. Barfod, P. Magistrado, A. Jensen, T. Lavstsen, M. Ofori, K. Marsh, L. Hviid, and T. Theander. 2004. Evidence for the involvement of VAR2CSA in pregnancy-associated malaria. J. Exp. Med. 2001197-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwenk, R., L. Asher, I. Chalom, D. Lanar, P. Sun, K. White, D. Keil, K. Kester, J. Stoute, D. Heppner, and U. Krzych. 2003. Opsonization by antigen-specific antibodies as a mechanism of protective immunity induced by Plasmodium falciparum circumsporozoite protein-based vaccine. Parasite Immunol. 2517-25. [DOI] [PubMed] [Google Scholar]
- 29.Staalsoe, T., C. Shulman, J. Bulmer, K. Kawuondo, K. Marsh, and L. Hvild. 2004. Variant surface antigen-specific IgG and protection against clinical consequences of pregnancy-associated Plasmodium falciparum malaria. Lancet 363283-289. [DOI] [PubMed] [Google Scholar]
- 30.Tebo, A., P. Kremsner, and A. Luty. 2002. Fcγ receptor-mediated phagocytosis of Plasmodium falciparum-infected erythrocytes in vitro. Clin. Exp. Immunol. 130300-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ter Kuile, F., M. Parise, F. Verrhoeff, V. Udhayakumar, R. Newman, A. Van Eijk, S. Rogerson, and R. Steketee. 2004. The burden of co-infection with human immunodeficiency virus type I and malaria in pregnant women in sub-Saharan Africa. Am. J. Trop. Med. Hyg. 741-54. [PubMed] [Google Scholar]
- 32.Titanji, K., A. De Milito, A. Cagigi, R. Thorstensson, S. Grutzmeier, A. Atlas, B. Hejdeman, F. Kroon, L. Lopalco, A. Nilsson, and F. Chiodi. 2006. Loss of memory B cells impairs maintenance of long-term serologic memory during HIV-1 infection. Blood 1081580-1587. [DOI] [PubMed] [Google Scholar]