Abstract
Enzyme-linked immunosorbent assay (ELISA) has been widely used to evaluate antibody responses to pertussis vaccination and infection. A common reference serum is essential for the standardization of these assays. However, no internationally recognized reference serum is available. At the request of the Expert Committee on Biological Standardization (ECBS) of the World Health Organization (WHO), a set of four candidate international standards has been prepared. These candidate materials have been assessed for suitability and compared to the widely used U.S. reference pertussis antiserum (human) lot 3, lot 4, and lot 5 by 22 laboratories from 15 countries in an international collaborative study. Laboratories measured immunoglobulin G (IgG) and IgA antibodies to pertussis toxin (PT), filamentous hemagglutinin (FHA), pertactin (PRN), and fimbriae (Fim2&3) using their established immunoassays. The results of this study showed each of the four candidates to be suitable as an international standard. With the agreement of the participants, a recommendation has been made to the ECBS that the candidate material coded 06/140 be established as the First International Standard for pertussis antiserum (human), with the following assigned international units (IU): IgG anti-PT, 335 IU/ampoule; IgA anti-PT, 65 IU/ampoule; IgG anti-FHA, 130 IU/ampoule; IgA anti-FHA, 65 IU/ampoule; IgG anti-PRN, 65 IU/ampoule; and IgA anti-PRN, 42 IU/ampoule. No formal units have been proposed for anti-Fim2&3 because most assays used a mixture of fimbrial antigens. In addition, the candidate material coded 06/142 has been proposed as a WHO working preparation for characterization of assay systems.
Serological analysis by enzyme-linked immunosorbent assay (ELISA) has been widely used for evaluating antibody responses to pertussis vaccination and infection. A quantitative measurement of concentration of serum antibodies in ELISA units (EU) per ml has been shown to be important in epidemiological studies (13, 19, 23), the serodiagnosis of pertussis (1, 2, 5, 7, 17, 24), and the evaluation of responses to vaccines (3, 21, 22); however, the lack of internationally recognized reference sera has hindered interlaboratory comparisons and harmonization.
U.S. reference pertussis antiserum (human) lots 3, 4, and 5 from the U.S. Center for Biologics Evaluation and Research (CBER), Food and Drug Administration (FDA), have been widely used and have played an important role in standardization of these assays (16, 18). However, only limited quantities of these sera remain. The World Health Organization (WHO) Working Group on the standardization and control of pertussis vaccines recommended the preparation of a reference human antiserum to pertussis antigens with internationally recognized status before the supply of the U.S. preparations is exhausted (4, 25). As far as possible, continuity of unitage with that of the existing U.S. reference preparations was recommended.
A set of freeze-dried candidate reference preparations has been prepared at the National Institute for Biological Standards and Control (NIBSC; United Kingdom) from sera obtained from German plasmapheresis donors. On behalf of WHO and in collaboration with members from CBER, FDA, and the Institut für Infektiologie Krefeld GmbH, a collaborative study to compare these candidate international reference preparations with the U.S. reference preparations was organized by the NIBSC in 2007. The aims of the study were to characterize the candidate international reference preparations, to compare them to existing U.S. and in-house reference (IHR) preparations, and to define unitage for anti-PT, anti-FHA, and anti-PRN for the candidates. We report here the results of the collaborative study and additional studies evaluating the stability of the candidates.
MATERIALS AND METHODS
Participants.
Laboratories actively performing serological assays measuring antibodies to pertussis toxin (PT), filamentous hemagglutinin (FHA), pertactin (PRN), and fimbria 2 and/or 3 (Fim2&3) for the evaluation of human immunogenicity were invited to participate. A total of 22 laboratories from 15 countries, including vaccine manufacturers, diagnostic laboratories, and research facilities, participated in the study. Throughout the study, each laboratory has been identified by a randomly assigned code: numbers 1 through 22. All laboratories measured immunoglobulin G (IgG) anti-PT; but not all laboratories had assays for other antibodies.
Candidate preparations. (i) Collection and preparation of plasma samples.
Plasma samples were collected between June 2005 and November 2005 in Germany. All donors signed consent according to German law. A total of 2,500 donors of plasma or whole blood and 200 healthcare workers vaccinated with an acellular pertussis vaccine were screened for IgG anti-PT with an ELISA in a single dilution protocol. Samples with values of ≥100 EU/ml from a total of 72 donors and from two vaccinated adults were retested for the IgG anti-PT antibody concentration using previously published methods (24). According to their IgG anti-PT antibody content, two different groups of samples were defined: samples with ≥200 EU of IgG anti-PT/ml were classified as “high,” and samples with contents of ≥80 EU/ml and <200 EU/ml were classified as “low.” For the “high” IgG anti-PT pool, we collected 28 plasma bags with ∼250 ml and 4 plasma bags with ∼750 ml. For the “low” IgG anti-PT pool, we collected 46 plasma bags with ∼250 ml and 9 plasma bags with ∼750 ml.
Procedures for sample collection and preparation were carried out according to the quality manual procedures in the blood bank of the HELIOS Klinikum Krefeld, where the plasma was obtained, processed, and pooled according to the European Union and German legal requirements for plasma intended for human use. Plasmapheresis donations were frozen within a maximum time of 4 h after plasmapheresis. Whole blood was processed within 24 h after donation with an intermediate storage at 4°C, and the separated plasma was frozen within 2 h. All samples were then stored at −30°C.
Serum was prepared according to a recalcification procedure based on that used for the U.S. reference sera. In brief, the plasma was thawed at 4°C. Then, 10 μl of sterile 2 M CaCl2 per ml of plasma was added and mixed in an isolator in a pharmaceutical class A laboratory. The recalcified plasma in the bags was incubated in a stationary water bath for 30 min at 37°C. The bags were then gently shaken to dislodge the primary clots and incubated in a shaking water bath at ∼150 rpm for 120 min at 37°C. The bags were next incubated for 14 h at room temperature and subsequently centrifuged at 3,000 × g for 30 min at 4°C. The serum was transferred into a 450-ml bag, and pooling was then done by sterile connection of tubing (Terumo sterile tubing welder TSCD) using a manifold designed specifically for the present study. The pooled serum samples were transferred into sterile plastic bags (3.5 liter) and several 50-ml sterile bags (for pre-evaluation). All of the frozen serum bags (∼9.5 liters of high content and 19 liters of low content) were transported frozen to the NIBSC by WorldCourier in July 2006 and kept frozen (−20°C) until further processing.
(ii) Safety tests.
Plasma samples were screened according to the German requirements. All samples were negative for HBsAg, anti-hepatitis C virus (anti-HCV), anti-human immunodeficiency virus type 1 (anti-HIV-1) and -2, and Treponema pallidum particle agglutination. The samples also were negative for HCV RNA and HIV RNA, as determined by reverse transcription-PCR. The bulk materials were retested in the NIBSC for HCV RNA (NAT test), anti-HIV-1 and -2, and HBsAg. All samples were found to be negative.
(iii) Lyophilization.
Four candidate reference preparations were prepared at the NIBSC from two pools of serum as detailed below.
On the day of filling, the bags were thawed at 37°C in a water bath. The material with no dilution or additions was divided into 1-ml aliquots in glass ampoules (in accordance with ISO 9187-1-2003) and then freeze-dried according to the procedure described in WHO guidelines (26). Four batches of ampoules containing lyophilized serum were prepared on separate dates: the two batches prepared from the pool with the higher anti-IgG anti-PT content were coded 06/140 and 06/146 (4,950 and 3,300 ampoules, respectively) and the two batches prepared from the pool with the lower anti-IgG anti-PT content were coded 06/142 and 06/144 (7,800 and 7,500 ampoules, respectively).
Baseline control serum.
Approximately 100 plasma donors were screened, and one plasma sample from a single donor with antibody levels for IgG and IgA to PT and FHA and antibody levels for IgG to PRN at/or below the assay detection limits was selected. This sample was dispensed into approximately 200 ampoules and freeze-dried for use as a baseline control (coded PM-06-047).
U.S. reference preparations.
Freeze-dried ampoules of U.S. reference pertussis antiserum (human) lot 3, lot 4, and lot 5 were kindly donated by the CBER, FDA.
Assay method.
ELISA was used by all laboratories in the study (14, 15, 18), except for one, which carried out only CHO cell neutralization assays. Two of the participants performed both ELISA and CHO cell neutralization assays, and one participant performed complement fixation assays. Laboratories used their own methodology, reagents, and calculation methods. The majority of the participants included in each assay at least one positive control serum from in-house sources, typically an IHR preparation.
It was recommended that ampoules of lyophilized antisera be stored at −20°C. All materials were to be reconstituted in 1 ml of sterile distilled water and, if not used fresh, reconstituted antisera were to be stored in small aliquots at −20°C: if the reconstituted antisera were to be stored for more than 30 days, a temperature of −70°C is recommended. Repeated freeze-thaw cycles of reconstituted antisera were to be avoided. If feasible, a pilot study to determine suitable dilutions was suggested.
Study design.
Preparations included in the collaborative study are listed in Table 1. In this report, the study codes A and D have been used for ampoules coded 06/140 and 06/146, respectively, which were prepared from the serum pool with the higher anti-PT IgG content. Study codes B and C have been used for ampoules coded 06/142 and 06/144, respectively, which were prepared from the serum pool with the lower anti-PT IgG content. Study code E has been used for the baseline control (PM-06-047).
TABLE 1.
Sample informationa
| Study code | Sample/references ampoule code | Description |
|---|---|---|
| A | 06/140 | Freeze-dried preparation of “high” anti-PT IgG content human serum |
| B | 06/142 | Freeze-dried preparation of “low” anti-PT IgG content human serum |
| C | 06/144 | Freeze-dried preparation of “low” anti-PT IgG content human serum |
| D | 06/146 | Freeze-dried preparation of “high” anti-PT IgG content human serum |
| E | PM-06-047 | Baseline control serum; freeze-dried preparation of human serum with “undetectable” IgG anti-PT content |
| U.S. lot 3 | U.S. reference pertussis antiserum (human) lot 3; freeze-dried reference preparation of serum with the following units: IgG anti-PT (200 EU/ml); IgA anti-PT (15 EU/ml); IgG FHA (200 EU/ml); and IgA FHA (100 EU/ml) | |
| U.S. lot 4 | U.S. reference pertussis antiserum (human) lot 4; freeze-dried reference preparation of serum with the following units: IgG anti-PRN (90 EU/ml) and IgA anti-PRN (25 EU/ml) | |
| U.S. lot 5 | U.S. reference pertussis antiserum (human) lot 5; a secondary reference with units assigned based on U.S. lots 3 and 4: IgA anti-PT (140 EU/ml); IgA anti-FHA (280 EU/ml); and IgA anti-PRN (90 EU/ml) |
For use in assays, all preparations were reconstituted with 1.0 ml of sterile water.
Each participating laboratory received three sets of ampoules comprising five samples of human serum coded by letter, together with the U.S. lot 3, 4, and 5 reference preparations (Table 1). Participants were also asked to include their IHR. Laboratories were asked to perform the three independent assays on three different days and to include all samples in each assay. For each assay, dilution curves for each reference and sample preparation were to have at least two replicates per assay and preferably at least five doses in the linear region.
Thermally accelerated degradation study.
Ampoules of each candidate international reference preparation that had been stored at temperatures of 20, 37, 45, and 56°C were transferred at 1, 3, 6, or 12 months to storage at −20°C. All ampoules which had been stored at the different temperatures and times were reconstituted and compared in the same assay with freshly reconstituted aliquots from ampoules of the same candidate which had been stored continuously at −20°C (baseline) for IgG antibodies for PT, FHA, PRN, and Fim2&3.
In-use stability study.
Ampoules of the candidate 06/140, study code A, and the candidate 06/142, study code B, were reconstituted, and aliquots were stored at −20°C for up to 128 days and at 4°C for up to 14 days. At each of several time points, stored aliquots were compared to aliquots from freshly reconstituted ampoules of the same candidate for IgG antibodies for PT, FHA, PRN, and Fim2&3.
Statistical methods.
All raw data, including plate layout, serum dilutions, and optical density values were returned to the NIBSC for analysis to ensure, as far as possible, consistent treatment.
Each participating laboratory used their own design and assay format. Thus, the assays have been carried out using methods and layouts familiar to the participants and, as far as possible, these data are representative of the way in which the reference preparations will be used in practice. ELISA was carried out as a quantitative assay, using a variety of different plate layouts. A number of assay layouts included consistent placement of samples on microtiter plates. Although this offers some practical advantages, it introduces the possibility of biases which may be caused by nonrandom positioning and order of assay, as well as other factors (8, 9, 12). Where possible for the contributed data, assays have been assessed for the occurrence of any statistically significant positional or order effects.
All raw data were plotted and examined both graphically and statistically for any gross anomalies or outliers and to assess the overall consistency of the dose-response relations (10).
The dose-response curves were transformed to give approximately linear log dose-(transformed) response lines and to allow use of the methods of multiple parallel line bioassay analysis (6, 20). In the majority of assays, a four-parameter logistic curve provided a satisfactory fit to the dose-response curve; responses, expressed as proportion relative to upper and lower asymptotes, have been transformed to “logits,” and the bioassay analysis has been carried out using an in-house program (11). Where replicate titrations were performed, the classical analysis of variance has been used to provide an assessment of the linearity and parallelism of the log dose-(transformed) response lines. Where that was not the case, the linearity of the dose-response lines has been assessed graphically and the parallelism of the dose-response lines has been assessed by comparing the deviations from parallelism with the deviations from linearity. Additionally, slopes from assays within a laboratory using the same antigen have been assessed using analysis of variance to determine any consistent differences among them.
In all cases, estimates of relative activity have been determined as the displacement of linear parallel log dose-response lines using the methods of multiple parallel line analysis. The log dose-response lines for some of the IHR and other control preparations showed apparent nonparallelism to the lines for the U.S. and candidate reference preparations. To ensure that there is no effect of these preparations on the common fitted slope, data for the unique in house preparations have been omitted from the analysis. Estimates of the relative activities of the candidate reference sera are based on comparisons among the candidates and the relevant U.S. reference preparations only.
Data have been combined to give a single set of estimates for each of the three sets of ampoules assayed for each antigen in each laboratory. All comparisons among estimates of relative activity have been made using analysis of variance of the logarithms of these estimates. The intra- and interlaboratory variability for the various comparisons has been determined using these estimates. Estimates have been combined as geometric means (GM), and the variances have been summarized as geometric coefficients of variation (GCV).
A limited number of values (<0.1% of all data and <0.2% of data in any individual laboratory) were identified as “outliers” based on the occurrence of a gross discontinuity in the dose-response curve. Such values were omitted from all analysis. In some cases, a number of serum dilutions gave responses at or near the upper asymptote of the four-parameter logistic function and, in such instances, responses above a threshold, typically 95%, have been excluded from the final analysis.
Where positional effects could be assessed, these were typical of those frequently observed for this type of assay, confirming that such effects occur and that the probability levels for the classical analysis of variance may not be accurate. Moreover, the possibility of bias in the estimation of slopes and relative activity cannot be excluded.
Three laboratories contributed CHO cell assays for anti-PT antibodies, and one laboratory carried out assays by three additional methods for PT as detailed. The data from these assays were not quantitative, and results have been summarized as mean titers and as relative activities for the CHO cell assays and as positive or negative results for antibodies by the other methods.
RESULTS
Dose-response relations.
The logit transformed responses did not show significant (P < 0.05) deviations from linearity or parallelism for the majority of preparations in the majority of assays. Where apparently significant deviations from linearity were observed, these were not consistent across assays for the same antigen in the same laboratory, and the apparent significance was considered to be the result of inaccuracies in the probability levels of the statistical test consequent on the failure of the assumptions which underlie the analysis of variance. Baseline control serum, study code E, gave assay response data at or near the detection limit in many assay systems. Thus, all assay systems showed a consistent lack of response to sample E. The data for this sample have not been included in the detailed analysis.
Although the IHR and in-house control preparations, which are unique to the various laboratories, have been excluded from the final analysis, the data contributed to the present study suggest that, depending on antibody and assay system, up to ca. 30% of these preparations show consistent slope differences compared to the U.S. reference and candidate reference preparations. A small number (<5%) of assays showed consistent significant deviations from parallelism among candidate reference preparations and CBER references. In these cases, the slopes for A and D (06/140 and 06/146, pool with higher anti-PT IgG content) tended to differ consistently from the slopes for B and C (06/142 and 06/144, pool with lower anti-PT IgG content), with slopes for the IHR and/or for the CBER reference equally likely to be similar to those for A and D or to those for B and C. These differences were not concentrated within particular antibodies or laboratories. These differences also need to be considered in the context of positional effects for the assay and the possibility of bias in estimates of slope.
Comparison of the candidate reference preparations with U.S. reference preparations for the various antibodies.
Comparisons of the candidate reference preparations with the U.S. reference preparations, as appropriate for the antibody, generally showed good agreement between laboratories (Fig. 1, 2, and 3). The pooled results for the anti-PT, FHA, and PRN assays are provided in Tables 2 and 3.
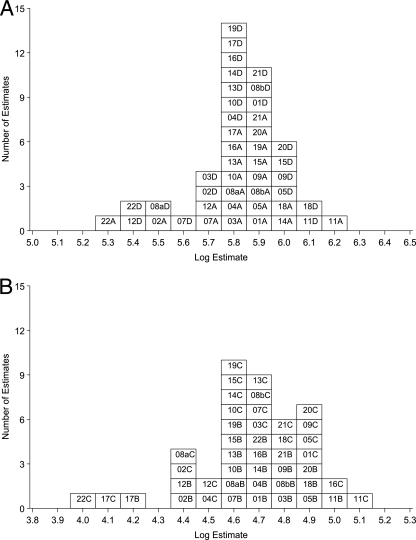
FIG. 1.
Summary for laboratory mean estimates of IgG anti-PT activity in terms of U.S. lot 3. For estimates of laboratory GM activity, the number is the lab code, followed by A or D (06/140 or 06/146) or B or C (06/142 or 06/144) to indicate the ampoule code. (A) Estimates for samples A and D in terms of U.S. lot 3; (B) estimates for samples B and C in terms of U.S. lot 3.
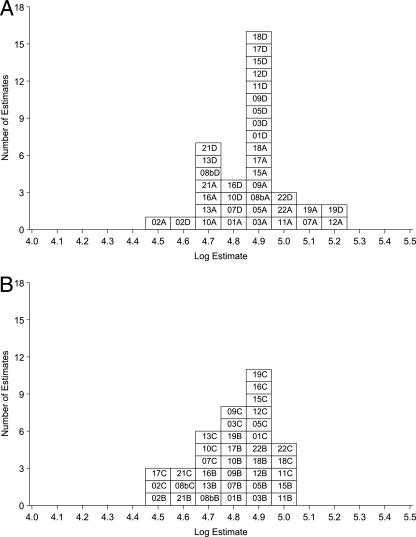
FIG. 2.
Summary for laboratory mean estimates of IgG anti-FHA activity in terms of U.S. lot 3. For estimates of laboratory GM activity, the number is the lab code, followed by A or D (06/140 or 06/146) or B or C (06/142 or 06/144) to indicate the ampoule code. (A) Estimates for samples A and D in terms of U.S. lot 3; (B) estimates for samples B and C in terms of U.S. lot 3.
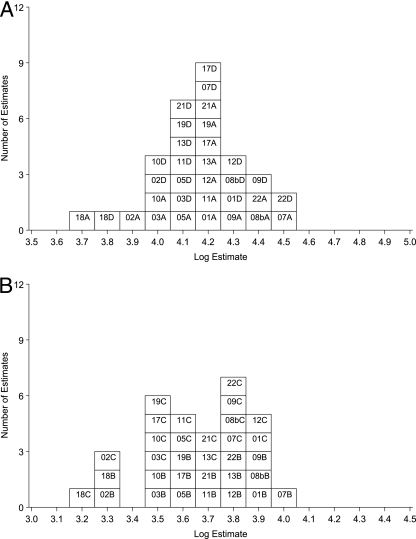
FIG. 3.
Summary for laboratory mean estimates of IgG anti-PRN activity in terms of U.S. lot 4. For estimates of laboratory GM activity, the number is the lab code, followed by A or D (06/140 or 06/146) or B or C (06/142 or 06/144) to indicate the ampoule code. (A) Estimates for samples A and D in terms of U.S. lot 4; (B) estimates for samples B and C in terms of U.S. lot 4.
TABLE 2.
Overall summary results of estimates, with 95% confidence limits, for samples A to D (IgG) in terms of U.S. lot 3 (for anti-PT and anti-FHA) and U.S. lot 4 (for anti-PRN)a
| Antibody and assay | Estimates as units of U.S. references under sample code:
|
Estimates for B and C as equivalent ml of A and D | Estimates for B and C in terms of Ab | Proposed IU for A and D (06/140 and 06/146) | |||
|---|---|---|---|---|---|---|---|
| A (06/140) | D (06/146) | B (06/142) | C (06/144) | ||||
| IgG anti-PT ELISA | 340 (315-368) [22] | 331 (320-363) [22] | 108 (100-117) [22] | 105 (94-118) [22] | 0.32 (0.30-0.34) [22] | 106 (100-113) [22] | 335 |
| IgG anti-PT CHO cell assay | 307 (140-677) [3]d | 96 (49-185) [3]e | 0.31 (0.08-1.25) [3] | NAc | NA | ||
| IgG anti-FHA ELISA | 131 (120-142) [18] | 129 (121-137) [18] | 123 (116-131) [18] | 120 (111-130) [18] | 0.94 (0.89-0.99) [18] | 122 (116-128) [18] | 130 |
| IgG anti-PRN ELISA | 65 (58-72) [16] | 64 (59-70) [16] | 39 (35-44) [16] | 38 (34-43) [16] | 0.60 (0.57-0.63) [16] | 39 (37-41) [16] | 65 |
Values are overall GM estimates of the laboratory GMs. The 95% confidence limits are given in parentheses. The numbers of estimates (one estimate per laboratory) combined are indicated in brackets.
Using proposed IU for sample A.
NA, not applicable.
Overall GM for samples A and D.
Overall GM for samples B and C.
TABLE 3.
Overall summary results of estimates, with 95% confidence limits, for samples A to D (IgA) in terms of units of U.S. lot 5a
| Antibody and assay | Estimates as units of U.S. reference lot 5 under sample code:
|
Estimates for B and C as equivalent ml of A and D | Estimates for B and C in terms of Ab | Proposed IU for A and D (06/140 and 06/146) | |||
|---|---|---|---|---|---|---|---|
| A (06/140) | D (06/146) | B (06/142) | C (06/144) | ||||
| IgA anti-PT ELISA | 63.0 (49.8-79.7) [8] | 62.4 (48.8-77.1) [8] | 20.4 (13.5-30.9) [8] | 20.1 (13.1-30.8) [8] | 0.32 (0.22-0.48) [8] | 21.1 (14.3-30.9) [8] | NAc |
| IgA anti-PT (omit lab 21) | 65.3 (50.3-84.8) [7] | 63.8 (49.7-81.9) [7] | 18.4 (12.4-27.3) [7] | 18.2 (11.9-27.9) [7] | 0.28 (0.22-0.35) [7] | 18.3 (14.5-23.1) [7] | 65 |
| IgA anti-FHA ELISA | 70.5 (54.5-91.3) [8] | 68.0 (53.8-85.8) [8] | 90.7 (74.3-110.8) [8] | 87.6 (73.3-104.8) [8] | 1.30 (1.21-1.39) [8] | 84.2 (78.5-90.4) [8] | NA |
| IgA anti-FHA (omit lab 21) | 65.7 (52.0-83.0) [7] | 63.3 (52.3-76.5) [7] | 86.7 (70.9-105.9) [7] | 83.3 (71.0-97.7) [7] | 1.33 (1.26-1.39) [7] | 86.3 (82.2-90.6) [7] | 65 |
| IgA anti-PRN ELISA | 42.8 (34.6-52.9) [5] | 41.9 (37.1-47.5) [5] | 37.5 (33.6-41.8) [5] | 38.5 (33.8-43.7) [5] | 0.90 (0.76-1.06) [5] | 37.6 (31.9-44.3) [5] | 42 |
The 95% confidence limits are given in parentheses. The numbers of estimates (one estimate per laboratory) combined are indicated in brackets.
Using proposed IU for sample A.
NA, not applicable.
The pooled intralaboratory variability has been determined using the variabilities among the GM estimates for the sets of ampoules within a laboratory for any of preparations A, B, C, and D in terms of each other or in terms of the relevant U.S. reference preparation. Based on these estimated variabilities, the GCVs for individual estimates for an ampoule set range from 15 to 30%, with corresponding 95% confidence limits of 75 to 133% to 57 to 176% of the estimated value.
Preparations A (06/140) and D (06/146) were prepared from the same serum pool and should be similar. Similarly, preparations B (06/142) and C (06/144) are expected to be similar because they were prepared from the same serum pool. The data (Tables 2 to 4) support this, with no significant differences detected between dose-response lines for the paired preparations. For each antibody, the overall mean potency of the laboratory GM estimates of the relative activity of preparation D in terms of preparation A or preparation C in terms of preparation B do not differ significantly from 1, and analysis of variance showed no significant interlaboratory differences relative to the pooled intralaboratory variances.
TABLE 4.
Laboratory GM IgG anti-Fim2&3 estimates of relative activity in terms of equivalent ml of U.S. lot 3
| Sample set (code) | GM IgG anti-Fim2&3 relative activity for laboratory code:
|
Overall GMa | GCV (%) | 95% CLb | ||||
|---|---|---|---|---|---|---|---|---|
| 01 | 05 | 11 | 12 | 17 | ||||
| A (06/140) | 0.40 | 0.34 | 0.47 | 0.73 | 0.43 | 0.46c | 33 | 0.32-0.65 |
| D (06/146) | 0.36 | 0.34 | 0.48 | 0.57 | 0.45 | 0.43c | 24 | 0.33-0.58 |
| B (06/142) | 0.43 | 0.40 | 0.59 | 0.64 | 0.40 | 0.48 | 25 | 0.37-0.64 |
| C (06/144) | 0.38 | 0.39 | 0.47 | 0.69 | 0.38 | 0.45 | 29 | 0.33-0.62 |
Overall GM of laboratory GMs.
CL, confidence limits.
The combined GM for samples A and D is 0.44 ml.
The close similarity between preparations A and D, observed for all antibodies, suggests that for estimation of potency, A and D might be treated as equivalent to one another, and similarly that preparations B and C might be treated as equivalent to one another. Thus, where appropriate, estimates for A and D have been combined and estimates for B and C have been combined (Tables 2 and 3).
Based on the comparison of the IgG anti-PT and anti-FHA activity of preparations A and D with U.S. lot 3, the GM of the results were 335 EU anti-PT IgG and 130 EU anti-FHA IgG per ampoule of A or D. Based on the comparison of the IgG anti-PRN activity of preparations A and D with U.S. lot 4, the GM of the results were 65 EU IgG anti-PRN per ampoule of A or D (Table 2).
Comparison of the IgG anti-PT activity and IgG anti-FHA activity of preparations B and C with U.S. lot 3, and with A and D, gives an estimated IgG anti-PT content of 106 EU and IgG anti-FHA content of 122 EU per ampoule. Comparison of the IgG anti-PRN activity of preparations B and C with U.S. lot 4, and with A and D, gives an estimated IgG anti-PRN content of 39 EU per ampoule (Table 2).
The present study shows that the IgA anti-PT, IgA anti-FHA, and IgA anti-PRN dose-response lines for preparations A, D, B, and C do not differ significantly from the respective antibody dose-response lines for U.S. lot 5. Estimates of the IgA anti-PT activity of preparations A and D in terms of U.S. lot 5 show significant interlaboratory variability. However, if estimates from two of the eight laboratories contributing these assays are omitted, there is no significant difference between laboratories. A similar finding was obtained for estimates of the IgA anti-FHA activity of these samples. Estimates of the IgA anti-PRN activity of preparations A and D did not differ significantly among the five laboratories contributing these assays. It is therefore recommended that preparations A and D have an IgA anti-PT content of 65 EU, an IgA anti-FHA content of 65 EU, and an IgA anti-PRN content of 42 EU per ampoule based on its comparison with U.S. lot 5 (Table 3).
Comparison of the IgA anti-PT activity of preparations B and C with U.S. lot 5, and with preparations A and D, gives an estimated IgA anti-PT content of 18 EU, an estimated IgA anti-FHA content of 86 EU, and an estimated IgA anti-PRN content of 38 EU per ampoule (Table 3).
Comparison of the IgG anti-Fim activity of the candidate reference preparations A, D, B, and C with one another.
The U.S. reference lots 3, 4, and 5 do not have an assigned anti-Fim activity, and thus no unitage for the candidate preparations can be determined for anti-Fim activity using the U.S. reference lots. Although one laboratory carried out assays for Fim2 and Fim3 separately and one laboratory carried out assays for IgA anti-Fim2&3, all other participants described their assays as “IgG anti-Fim2&3.” The relative potency for IgG anti-Fim2&3 in terms of U.S. lot 3 are given in Table 4. Estimates of the IgG anti-Fim2&3 activity of preparations A, B, C, and D relative to each other (BC/AD) are consistent between laboratories, with a GM result indicating that 1.07 ampoules of A or D are equivalent to one ampoule of B or C. Potency relative to U.S. lot 3 using the mixture Fim2&3 was found to be 0.44 ml (GM) U.S. lot 3 equivalent to 1 ml of preparation A (or D) (Table 4). However, these estimates are based on assays using a mixture of antigens, and the results may differ if the reagents or methods are modified.
Stability.
Candidate preparations which had been stored at 56°C, especially for longer than 6 months, could not be readily reconstituted. Estimates showed an overall tendency for the preparations stored at the higher temperatures (e.g., ≥37°C) and for the longer times (e.g., 12 months) to have lower relative activities compared to samples stored at −20°C. Samples of the candidate 06/140, study code A, and of the candidate 06/142, study code B, which had been stored at temperatures of 20, 37, and 45°C for 12 months were assessed in greater detail in additional assays. These assays showed no significant deviations from linearity or parallelism. Based on these data from one laboratory, predicted yearly losses of activity at −20°C for each antibody for each candidate were <0.02% in most cases. These data indicate that the candidate materials are sufficiently stable to serve as international reference preparations. Confirmation of the stability after 5 (and preferably within 10) years by comparison of ampoules stored at −20°C with ampoules stored at −70°C is suggested.
In-use stability study showed that there was no evidence of loss of activity with time, although variation in estimates of relative activity was observed in some cases (data not shown). These results suggest that aliquots of the reconstituted candidate reference preparations can be used if they have been suitably stored. However, since reconstitution and storage conditions may differ between laboratories, it is recommended that laboratories carry out validation under their own conditions.
DISCUSSION
The value of accurate measurement of antibodies to Bordetella pertussis antigens by ELISA has been demonstrated for serodiagnosis (1, 2, 5, 7, 17, 24), epidemiological investigation (13, 19, 23), and evaluation of vaccine responses (3, 21, 22). A common primary reference serum is an integral component of harmonization and interlaboratory comparisons. The present study reports the evaluation of four batches of ampoules—A (06/140), B (06/142), C (06/144), and D (06/146)—that were prepared as candidate international reference preparations. The U.S. reference preparations with their assigned EU have been widely used. Hence, the antibody contents of the candidate reference preparations have been estimated in terms of the EU assigned to the relevant U.S. preparations.
Estimates of the activities of preparations A to D relative to that of U.S. lot 3 (anti-PT, anti-FHA) or U.S. lot 4 (anti-PRN) showed good agreement between laboratories. Estimates of the anti-FIM activity of preparations A to D relative to each other did not differ significantly between laboratories, and potencies for A to D relative to U.S. lot 3 broadly agreed among laboratories. The majority of assays in the present study have been carried out using designs with nonrandom assignment of preparations and dilutions to assay position and order. Differences between laboratories for estimates of IgG anti-PT, anti-FHA, and anti-PRN are generally <20%, and it is possible that, to some extent, these differences may result from positional effects. Differences among laboratories are not consistent for the antibodies to the different antigens. The overall results are summarized in Tables 2 to 4.
In general, between laboratories variances appeared to be larger for IgA assays in comparison to the IgG assays, although the absolute range of estimates (on a log scale) from smallest to largest was not generally larger. The apparently larger variances may be in part an artifact resulting from the smaller number of laboratories. Other factors that might lead to greater interlaboratory variation for IgA assays are less experience in performing IgA assay in comparison to IgG assay in the participating laboratories, relatively lower IgA antibody contents in these preparations, and differences among the specificities of the assay systems.
Suitability of a preparation to serve satisfactorily as a reference reagent requires similarity of dose-response relationships for the preparations to be compared. In the present study, the majority of log dose-response lines showed no significant deviations from parallelism for preparations A, B, C, and D and the appropriate U.S. reference preparations. However, up to 30% of the various IHRs or in-house control included in this study by the participants showed statistically significant nonparallelism compared to the U.S. and candidate reference preparations, as indicated by differences in the slopes of individual assays or consistent trends across assays (data not shown). The methods of production of the antibodies, including the specific antigens used for immunization, may produce dissimilarity among preparations. These data illustrate the importance of considering similarity when selecting reference preparations.
Seven laboratories performed assays on IgG anti-Fim using a mixture of Fim2&3 as coating antigen and, additionally, laboratory 21 used separate recombinant Fim2 and Fim3 as coating antigens. U.S. reference lots 3, 4, and 5 do not have assigned anti-Fim activity since monovalent type 2 and type 3 fimbria antigen preparations are not available. Thus, no unitage for the candidate preparations can be determined for anti-Fim activity in terms of the U.S. reference lots. However, comparison of IgG anti-Fim activity of the preparations A, B, C, and D with one another showed good interlaboratory agreement with no significant difference between laboratories. Potencies for A, B, C, and D relative to U.S. lot 3 for IgG anti-Fim2&3 showed good agreement between laboratories. The proportions of Fim2 and Fim3 may differ both in the antigens used for immunization and in the antigens used for coating plates. It should be noted that some laboratories have assigned an arbitrary value for U.S. lot 3 in their anti-Fim ELISAs (18); however, such assignments are for in-house purposes only and have no official status. In the present study, a mixture of Fim2&3 antigens was used by all except one participant and were apparently obtained from the same source. We thus provide no information here about whether the effect of different mixtures of antigens could be detected differentially by different ELISAs. When monovalent antigens become available for Fim2 and Fim3, these relationships will need to be reexamined and suitable unitage assigned.
Data for the CHO cell assays for IgG anti-PT from three laboratories give relative activities (data not shown) that are consistent with the results observed for the ELISAs, but the present study provides data from only three laboratories and is not sufficient for reliable estimation of neutralizing titer. Other assays carried out comprised one assay using a test kit for IgA anti-PT and IgA anti-FHA, one assay using immunofluorescence for detection of IgA, and one assay using complement fixation for anti-PT. These assays gave results that are broadly consistent with those obtained by ELISA and CHO cell assays.
In the present study, there was generally good quantitative agreement among the laboratories in the estimates of unitage for the candidate preparations relative to the U.S. reference sera. This agreement was observed even though there were differences in the source, purity, and characteristics of the coating antigen; the characteristics of the conjugate; the buffers used for blocking and washing; and other assay conditions that could affect consistency in estimation (8, 16). Based on information provided by the participants, at least seven sources of PT and FHA, at least four sources of PRN, and two sources of Fim antigens were used as coating antigens in the study. These data suggest that differences between the antigen sources and other reagents do not appear to have significantly affected the estimates of relative activity in the present study. Importantly, however, common reference sera and calculation methods were used for all data, suggesting that these may merit particular emphasis in harmonization activities.
Data from the present study indicate that preparation A is not distinguishable from preparation D, and that preparation B is not distinguishable from preparation C using these assay systems. This shows that the preparation of ampoules as separate batches on different days from a single pool of serum has not differentially affected activity in these assays. It is therefore recommended that for assignment of unitage in this study A and D be treated as equivalent and that B and C be treated as equivalent, and thus we have also calculated estimates based on combinations of data from the paired preparations.
The present study shows that the parameters of the dose-response curves of preparations A, B, C, and D for both the IgG anti-PT and the IgG anti-FHA do not differ significantly from that of U.S. lot 3, and estimates of the activity of these samples in terms of U.S. lot 3 are consistent between laboratories. Similarly, the parameters of the IgG anti-PRN dose-response curves of preparations A, B, C, and D do not differ significantly from that of U.S. lot 4, and estimates of the activity of these samples in terms of U.S. lot 4 are consistent between laboratories. The candidate materials have also been shown to have satisfactory predicted stability.
A recommendation (with the agreement of the participants) was made to the Expert Committee on Biological Standardization (ECBS) of the WHO in 2008 that preparation A (ampoule code 06/140) be established as the First International Standard for pertussis antiserum (human) with assigned international units (IU) per ampoule as follows: IgG anti-PT, 335 IU/ampoule; IgA anti-PT, 65 IU/ampoule; IgG anti-FHA, 130 IU/ampoule; IgA anti-FHA, 65 IU/ampoule; IgG anti-PRN, 65 IU/ampoule; and IgA anti-PRN, 42 IU/ampoule.
These proposed assigned IU values are based on the estimates for A (06/140) determined in comparison with the relevant U.S. reference preparation so that, as far as possible, continuity between studies can be maintained. It is additionally proposed that the candidate preparation coded 06/142 (sample B, lower anti-PT activity) be made available as a WHO working preparation for pertussis antiserum (human) that might be suitable for characterization of assay systems. Preparations D and C also appear suitable to serve as international standards, and it is recommended that these materials be retained.
The recommendation was accepted by the ECBS in October 2008 and, beginning in 2009, these reference preparations will be available from the NIBSC for distribution. They can be used to assist in the standardization of immunoassays used to measure human antibodies to B. pertussis in vaccine studies of products in current distribution, as well as those under development, for refinement of serological methods used for diagnosis or surveillance and, potentially, for development of assays for measurement of antibodies to antigens other than the ones evaluated in this collaborative study.
Acknowledgments
We acknowledge the CBER, FDA, Rockville, MD, for provision of ampoules of U.S. standard pertussis antiserum, human, lots 3, 4, and 5. We thank the study participants for their contributions: María Eugenia Rodriguez, CINDEFI-CONICET, National University of La Plata, La Plata, Argentina; Lyn Gilbert Centre for Infectious Diseases and Microbiology Westmead Hospital, Westmead, New South Wales, Australia; Valérie Haezebroeck, GlaxoSmithKline Biologicals, Rixensart, Belgium; Waldely de Oliveira Dias, Instituto Butantan Centro de Biotecnologia, Sao Paulo, Brazil; Raymond Tsang and Irene Martin, National Microbiology Laboratory, Public Health Agency, Winnipeg, Manitoba, Canada; Scott Halperin, Canadian Center for Vaccinology, IWK Health Center, Halifax, Nova Scotia, Canada; Shumin Zhang, National Institute for the Control of Pharmaceutical and Biological Products, Beijing, People's Republic of China; Charlotte Sørensen, Statens Serum Institute, Copenhagen, Denmark; Qiushui He, Pertussis Reference Laboratory, National Public Health Institute, Turku, Finland; N. Guiso and E Njamkepo, Institut Pasteur, National Center of Reference of Pertussis and other Bordetelloses, Paris, France; C. H. Wirsing von Konig and Marion Riffelmann, Institut fuer Hygiene und Labormedizin, HELIOS Klinikum Krefeld, Krefeld, Germany; Anna Giammanco, Department of Hygiene and Microbiology, University of Palermo, Palermo, Italy; D. Notermans and Bert Elvers, National Institute of Public Health and the Environment (RIVM), Centre for Infectious Disease Control, Laboratory for Infectious Diseases and Perinatal Screening, Bilthoven, The Netherlands; Guy Berbers, Laboratory for Infectious Diseases and Screening, RIVM, Bilthoven, The Netherlands; Hans Hallander and Margaretha Ljungman, Swedish Institute for Infectious Disease Control, Solna, Sweden; Timothy G. Harrison, RSIL, HPA, Centre for Infections, London, United Kingdom; Annette Crowley-Luke, Immunoassay Laboratory, CEPR, HPA, Salisbury, United Kingdom; Kathryn Edwards and Sandy Yoder, Vanderbilt University Medical Center, Nashville, TN; Michael E. Pichichero, University of Rochester School of Medicine and Dentistry, New York, NY; ChrisAnna M. Mink, UCLA Center for Vaccine Research, Torrance, California; Linda Han, State Laboratory Institute, Jamaica Plain, MA; Robert B. Belshe and Kathleen Lottenbach, St. Louis University, Center for Vaccine Development, St. Louis, MO; and Steve Hildreth, Sanofi Pasteur, Inc., Swiftwater, PA.
Footnotes
Published ahead of print on 24 December 2008.
REFERENCES
- 1.André, P., V. Caro, E. Njamkepo, A. M. Wendelboe, A. Van Rie, and N. Guiso. 2008. Comparison of serological and real-time PCR assays to diagnose Bordetella pertussis infection in 2007. J. Clin. Microbiol. 461672-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baughman, A. L., K. M. Bisgard, K. M. Edwards, D. Guris, M. D. Decker, K. Holland, B. D. Meade, and F. Lynn. 2004. Establishment of diagnostic cutoff points for levels of serum antibodies to pertussis toxin, filamentous hemagglutinin, and fimbriae in adolescents and adults in the United States. Clin. Diagn. Lab. Immunol. 111045-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cherry, J. D., J. Gornbein, U. Heininger, and K. Stehr. 1998. A search for serologic correlates of immunity to Bordetella pertussis cough illnesses. Vaccine 161901-1906. [DOI] [PubMed] [Google Scholar]
- 4.Corbel, M. J., J. G. Kreeftenberg, and I. Knezevic. 2004. WHO Working Group on the standardisation and control of pertussis vaccines—report of a meeting held on 6-7 May 2003 in Ferney Voltaire, France. Vaccine 22293-300. [DOI] [PubMed] [Google Scholar]
- 5.de Melker, H. E., F. G. Versteegh, M. A. Conyn-Van Spaendonck, L. H. Elvers, G. A. Berbers, A. van Der Zee, and J. F. Schellekens. 2000. Specificity and sensitivity of high levels of immunoglobulin G antibodies against pertussis toxin in a single serum sample for diagnosis of infection with Bordetella pertussis. J. Clin. Microbiol. 38800-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finney, D. J. 1978. Statistical methods in biological assays, 3rd ed. Charles Griffin, London, United Kingdom.
- 7.Fry, N. K., O. Tzivra, Y. T. Li, A. McNiff, N. Doshi, P. A. Maple, N. S. Crowcroft, E. Miller, R. C. George, and T. G. Harrison. 2004. Laboratory diagnosis of pertussis infections: the role of PCR and serology. J. Med. Microbiol. 53519-525. [DOI] [PubMed] [Google Scholar]
- 8.Gaines Das, R. E. 1999. Assessment of assay precision: a case study of an ELISA for antipertussis antibody. Biologicals 27125-131. [DOI] [PubMed] [Google Scholar]
- 9.Gaines Das, R. E., and A. Meager. 1995. Evaluation of assay designs for assays using microtitre plates: results of a study of in vitro bioassays and immunoassays for tumour necrosis factor (TNF). Biologicals 23285-297. [DOI] [PubMed] [Google Scholar]
- 10.Gaines Das, R. E., and L. R. Rice. 1985. SCAN, an exploratory program for preliminary analysis of bioassay and immunoassay data. Comput. Methods Programs Biomed. 2125-33. [DOI] [PubMed] [Google Scholar]
- 11.Gaines Das, R. E., and M. S. Tydeman. 1982. Iterative weighted regression analysis of logit responses: a computer program for analysis of bioassays and immunoassays. Comput. Programs Biomed. 1513-22. [DOI] [PubMed] [Google Scholar]
- 12.Gaines Das, R. E., M. P. Rose, and J. M. Zanelli. 1992. International collaborative study by in vitro bioassays of the first International Standard for porcine inhibin. J. Reprod. Fertil. 96803-814. [DOI] [PubMed] [Google Scholar]
- 13.Giammanco, A., A. Chiarini, P. A. Maple, N. Andrews, R. Pebody, N. Gay, R. M. Olander, F. Fivet-Groyne, S. Baron, A. Tischer, S. Swidsinski, J. Schellekens, and E. Reizenstein. 2003. European Sero-Epidemiology Network: standardisation of the assay results for pertussis. Vaccine 22112-120. [DOI] [PubMed] [Google Scholar]
- 14.Kreeftenberg, J. G. 1988. Collaborative study on the candidate reference materials JNIH-3, JNIH-4, and JNIH-5 for the assay of acellular pertussis vaccines. WHO report BS/88.1586. World Health Organization, Geneva, Switzerland.
- 15.Lopez, A. L., E. Pineda, A. Garakian, and J. D. Cherry. 1998. Effect of heat inactivation of serum on Bordetella pertussis antibody determination by enzyme-linked immunosorbent assay. Diagn. Microbiol. Infect. Dis. 3021-24. [DOI] [PubMed] [Google Scholar]
- 16.Lynn, F., G. F. Reed, and B. D. Meade. 1996. Collaborative study for the evaluation of Enzyme-linked immunosorbent assays used to measure human antibodies to Bordetella pertussis antigens. Clinic. Diagn. Lab. Immunol. 3689-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marchant, C. D., A. M. Loughlin, S. M. Lett, C. W. Todd, L. H. Wetterlow, R. Bicchieri, S. Higham, P. Etkind, E. Silva, and G. R. Siber. 1994. Pertussis in Massachusetts, 1981-1991: incidence, serologic diagnosis, and vaccine effectiveness. J. Infect. Dis. 1691297-1305. [DOI] [PubMed] [Google Scholar]
- 18.Meade, B. D., A. Deforest, K. M. Edwards, T. A. Romani, F. Lynn, C. H. O'Brien, C. B. Swartz, G. F. Reed, and M. A. Deloria. 1995. Description and evaluation of serologic assays used in a multicenter trial of acellular pertussis vaccines. Pediatrics 96570-575. [PubMed] [Google Scholar]
- 19.Okada, K. K., Ueda, K. Morokuma, Y. Kino, K. Tokugawa, and S. Nishima. 2004. Seroepidemiologic study on pertussis, diphtheria, and tetanus in the Fukuoka area of southern Japan: seroprevalence among persons 0 to 80 years old and vaccination program. Jpn. J. Infect. Dis. 5767-71. [PubMed] [Google Scholar]
- 20.SAS Institute, Inc. 1989. SAS/STAT user's guide, version 6, 4th ed. SAS Institute, Inc., Cary, NC.
- 21.Storsaeter, J., H. O. Hallander, L. Gustafsson, and P. Olin. 1998. Levels of anti-pertussis antibodies related to protection after household exposure to Bordetella pertussis. Vaccine 161907-1916. [DOI] [PubMed] [Google Scholar]
- 22.Storsaeter, J., H. O. Hallander, L. Gustafsson, and P. Olin. 2003. Low levels of antipertussis antibodies plus lack of history of pertussis correlate with susceptibility after household exposure to Bordetella pertussis. Vaccine 213542-3549. [DOI] [PubMed] [Google Scholar]
- 23.Ward, J. I., J. D. Cherry, S. J. Chang, S. Partridge, W. Keitel, M. K. Edwards, Lee, J. Treanor, D. P. Greenberg, S. Barenkamp, D. I. Bernstein, R. Edelman, et al. 2006. Bordetella pertussis infections in vaccinated and unvaccinated adolescents and adults, as assessed in a national prospective randomized Acellular Pertussis Vaccine Trial (APERT). Clin. Infect. Dis. 43151-157. [DOI] [PubMed] [Google Scholar]
- 24.Wirsing von König, C. H., D. Gounis, S. Laukamp, H. Bogaerts, and H. J. Schmitt. 1999. Evaluation of a single-sample serological technique for diagnosing pertussis in unvaccinated children. Eur. J. Clin. Microbiol. Infect. Dis. 18341-345. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization. 2005. Potency assays for acellular pertussis vaccines. WHO Tech. Rep. Ser. 92712. [Google Scholar]
- 26.World Health Organization. 2006. Recommendations for the preparation, characterization, and establishment of international and other biological reference standards. WHO Tech. Rep. Ser. 93296-107. [Google Scholar]