Abstract
During an outbreak of parvovirus B19 we collected serum samples from 68 nonpregnant patients in the region of Antwerp (Belgium). Fifty-seven (84%) of the parvovirus B19 immunoglobulin M (IgM)-positive sera had a positive result for Epstein-Barr virus (EBV) IgM by Liaison testing, 61 (90%) had a positive result for herpes simplex virus (HSV) IgM, 20 (29%) samples were positive for cytomegalovirus IgM, and 15 (22%) had a positive result for Borrelia burgdorferi sensu lato IgM. As assay interference was suspected, sera were further investigated by using additional infectious-disease serology tests and by performing various interference elimination procedures. We could show that the EBV IgM and HSV IgM results were false positives due to aspecific IgM reactions with the solid phase. All samples were also analyzed by a modified Liaison EBV IgM assay, based on the addition of polyvinylpyrrolidone and polyvinyl alcohol to the dilution buffer, which partially eliminated this type of assay interference. Although the Liaison is a very convenient, automated immunoassay platform, this study demonstrates the potential for improvement of mainly the EBV IgM and HSV IgM tests.
Previously we reported that 5% of the positive Epstein-Barr virus (EBV) immunoglobulin M (IgM) results obtained on the Liaison platform are falsely elevated, but, while we could demonstrate the interference mechanism, we could not identify a concrete underlying (infectious) cause (2). Recently an outbreak of parvovirus B19 occurred in the region of Antwerp (Belgium), and we noticed that patients with a recent B19 infection were frequently positive for EBV IgM, sometimes with very high titers, by Liaison testing. As we suspected these EBV IgM results to be false positives, we investigated sera from recently B19-infected patients by using alternative infectious-disease serology tests and by performing various interference elimination procedures.
MATERIALS AND METHODS
Sample selection.
From March 2008 until the end of July 2008 we collected serum samples from 68 adult, nonpregnant patients that were sent in by general practitioners. From 34 patients (10 males and 24 females, 22 to 83 years old) detailed clinical information was available and an acute parvovirus B19 infection could be biologically ascertained or was very probable. In this group, at the time of presentation B19 IgM could be shown, and these patients all had reticulocytopenia (<0.2% reticulocytes), which is typical for an acute B19 infection. Moreover, in 10 patients from this group a subsequent B19 IgG seroconversion could be shown, but in this study we used only the sample from the first presentation to the general practitioner. Nonspecific symptoms (i.e., fatigue, fever) were present in 27 patients. Two patients presented with erythema infectiosum, arthralgias were present in 15 patients, and 2 patients had an asymptomatic infection.
For the other 34 patients, in which B19 IgM could be shown, exact timing of infection was not possible and/or no clinical information was available.
Infectious-disease serology assays.
Parvovirus B19-specific antibodies were determined by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (parvovirus B19 EIA, 4th generation; Biotrin International, Dublin, Ireland). This ELISA has been shown to be highly specific (3).
Assays performed on the Liaison platform (DiaSorin, Saluggia, Italy), with DiaSorin cutoffs for positivity, were for Borrelia burgdorferi sensu lato IgM (cutoff: 1.1, index), cytomegalovirus IgM (cutoff: 30 mU/liter), EBV IgM (cutoff: 40 mU/liter), varicella-zoster virus IgM (cutoff: 1.1, index), and herpes simplex virus (HSV) IgM (cutoff: 1.1, index).
On all samples HSV IgM and EBV IgM were determined by an ELISA (Enzygnost anti-HSV/IgM and Enzygnost anti-EBV/IgM II; Dade Behring/Siemens Medical Solutions Diagnostics, Marburg, Germany).
Sera positive for Borrelia burgdorferi sensu lato IgM by Liaison were also examined by immunoblotting (Borrelia afzelii Western blot; Euroimmun, Lübeck, Germany). Sera with positive cytomegalovirus IgM results by Liaison were also analyzed on a mini-VIDAS (bioMérieux, Marcy l'Etoile, France).
Interference elimination studies.
On 10 samples with enough serum available and showing positive EBV IgM and HSV IgM results by Liaison, we performed various interference elimination studies. In these methods, appropriate positive and negative control samples were used to detect any unexpected effects of the procedures. For statistical comparison of the three different sample pretreatment methods, the Wilcoxon test for paired samples was applied using Medcalc software (version 9.4; Mariakerke, Belgium).
Two different commercial reagents for interference elimination were used. (i) Heterophilic antibody interference was excluded by treating the sample, according to the manufacturer's instructions, with a nonspecific antibody-blocking tube (Scantibodies Laboratory, Santee, CA). (ii) RF absorbent (250 μl) (Dade Behring/Siemens Medical Solutions Diagnostics, Marburg, Germany), which contains sheep IgM antibodies targeted against human IgG Fc fragments, was added to 250 μl of serum, and the mixture was briefly vortexed and incubated for 1 h at room temperature. Results obtained after pretreatment with RF absorbent were multiplied by 2 to account for the dilution, except for the B19 IgM ELISA, since this is an IgG capture method.
To confirm the presence of solid phase reactive antibodies, 400 μl of serum was added to approximately 0.2 × 109 M-280 tosyl-activated beads (Dynabeads; Dynal Biotech, Oslo, Norway), vortexed, and incubated for 15 min at room temperature. After centrifugation (5 min; 2,000 × g), the supernatant was used for further analysis.
Modified Liaison EBV IgM assay.
All samples were analyzed by using a modified Liaison EBV IgM assay. This modification partially eliminates false-positive results in the Liaison EBV IgM assay (2) and is based on inhibiting aspecific IgM reactivity by blocking nonspecific binding sites on the solid phase (5, 11-13). It is performed by adding polyvinylpyrrolidone (PVP-360; Sigma-Aldrich) and polyvinyl alcohol (P8136; Sigma-Aldrich) to the EBV IgM dilution buffer (buffer A) at final concentrations of 0.1% and 0.005%, respectively, as described previously (2). The results obtained with the original EBV IgM assay were compared to those for the modified EBV IgM assay. Discrepant results were defined as differing more than 24% (three times the interassay coefficient of variation) between the original and modified assays.
RESULTS
Fifty-seven (84%) of the B19 IgM-positive sera had a positive result for EBV IgM by Liaison (range, 40 mU/liter to 1,190 mU/liter; median, 110 mU/liter). One of these samples had a borderline result by the Dade Behring EBV IgM ELISA.
Sixty-one (90%) of the B19 IgM-positive sera had a positive result for HSV IgM by Liaison (range, 1.1 to 16.7; median, 2.55). Seven of these samples had borderline results by the Dade Behring HSV IgM ELISA; three were positive.
There were no significant differences in EBV and HSV IgM positivity rates between the group in which an acute B19 infection was highly probable (83% positive EBV IgM; 88% positive HSV IgM) and the entire group of B19-positive IgM samples (84% positive EBV IgM; 90% positive HSV IgM). Similar results were seen for the 10 patients with a B19 IgG seroconversion (80% positive EBV IgM; 90% positive HSV IgM).
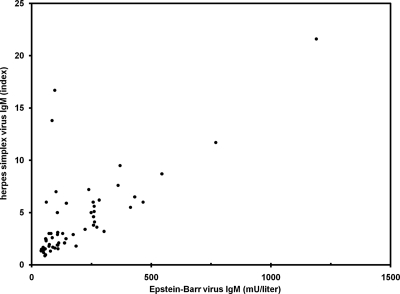
Figure 1 shows the correlation between the Liaison EBV IgM and HSV IgM titers. The significant correlation (Pearson's r = 0.82; P < 0.001) illustrates the aspecificity of both tests in the context of an acute B19 infection. As can be observed, two apparent outliers are present. One of these two samples was the one that showed the borderline positive result by EBV IgM ELISA, but it was also strongly positive by HSV IgM ELISA, suggesting a correct high titer of HSV IgM antibodies. For the other sample we could not find a straightforward explanation for the discrepancy.
FIG. 1.
A significant correlation (Pearson's r = 0.82) between the HSV IgM and EBV IgM titers by Liaison can be seen. Only parvovirus B19 IgM-positive samples which were positive for both EBV IgM and HSV IgM are shown (n = 57).
Twenty (29%) samples were positive for cytomegalovirus IgM by Liaison (range, 30 to 99 mU/liter), of which 4 were positive and 2 had borderline results by mini-VIDAS. The four samples positive by mini-VIDAS had results of 43, 55, 66, and 69 mU/liter by Liaison. The two borderline samples had results of 30 and 42 mU/liter by Liaison.
Fifteen (22%) of the B19 IgM-positive sera had a positive result for Borrelia burgdorferi sensu lato IgM by Liaison (index range, 1.1 to 4.0), of which 2 could be confirmed as positive by immunoblotting and 2 gave borderline results. The two confirmed samples had results of 4.0 and 3.7 by Liaison. The two borderline results had results of 1.9 and 1.4 by Liaison. After exclusion of these four samples, the index range narrowed down to 1.1 to 1.6.
Two samples were positive for varicella-zoster virus IgM by Liaison (indices, 1.4 and 2.0). These two samples were also positive for EBV IgM and HSV IgM by Liaison.
Interference elimination studies.
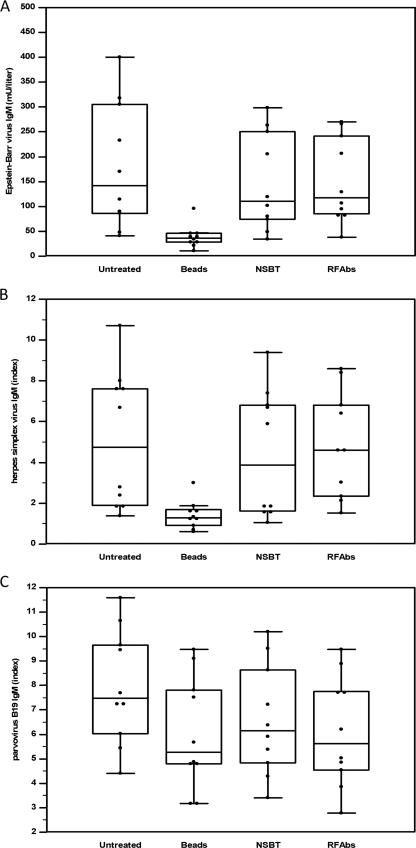
When the 10 selected samples with unlabeled beads were preincubated, a strong reduction in the EBV IgM and HSV IgM titers was obtained, compared with pretreatment using a nonspecific antibody-blocking tube or RF absorbent (P < 0.001). These three pretreatments did not have significantly different effects on the B19 IgM titers. Figure 2 shows the box-and-whisker plots from the different sample pretreatments. These results confirm the presence of solid phase reactive antibodies as the cause of the false-positive EBV IgM and HSV IgM titers.
FIG. 2.
Box-and-whisker plots comparing the three different sample pretreatment methods. Significant effects from the preincubation with unlabeled beads on EBV IgM titers (A) and HSV IgM titers (B) can be seen. These three pretreatments did not have significantly different effects on the B19 IgM titers (C). The slightly, but statistically significantly, lower results (P < 0.01) for the pretreated samples (beads, nonspecific antibody-blocking tube [NSBT], and RF absorbent [RFAbs]) compared to results for the untreated samples in panel C were not considered relevant in this experiment.
Modified Liaison EBV IgM assay.
For 53 of the EBV IgM-positive samples (93%), markedly different results were obtained with the original and modified EBV IgM assays. An average titer reduction of 48% was obtained, and although no positive EBV IgM results became negative, nine results became borderline (i.e., between 20 and 40 mU/liter).
DISCUSSION
Parvovirus B19 infection has been reported to produce false-positive reactions in various infectious-disease serology assays (7-10). In the present study we observed a very high frequency of false-positive Liaison EBV IgM and HSV IgM results, which we showed were caused by aspecific IgM reactions with the solid phase. Also, false-positive results in the cytomegalovirus IgM and Borrelia burgdorferi sensu lato IgM tests were seen, albeit at lower frequencies.
Although no diagnostic system is free from false-positive results, the strikingly high frequency of false-positive results in the Liaison EBV IgM and HSV IgM assays during an acute B19 infection raises questions about the overall specificity of these two tests. In 2005 an initial analytical evaluation of the EBV IgM on Liaison was published (4). Unfortunately, in this study only healthy controls were used and sera from patients with other infectious diseases (e.g., B19) were not evaluated. Further information on assay specificity can be found in the Liaison EBV IgM and HSV IgM assay inserts, which state that “as a rule, the presence of potentially cross-reactive antibodies does not interfere in the assay.” This statement is correct in the sense that specific B19 IgM antibodies will probably not cross-react in the EBV IgM and HSV IgM assays, but it is misleading considering the data presented here. This occurrence of false-positive EBV IgM results has apparently also been noticed by the manufacturer since a recently modified version of the Liaison EBV IgM assay insert (version of 25 June 25 2008) mentions a warning for possible false-positive results in acute rubella virus infections. It is likely that the same type of interference previously described by us and reconfirmed in this study also causes false-positive EBV IgM results in acute rubella virus infections (of 20 samples strongly positive for rubella virus IgM, 15 were EBV IgM positive [our unpublished observations]).
After having noticed that B19 frequently causes false-positive results, we analyzed the six samples with false-positive EBV IgM results from our previous comparative study (2) and found that only one of these samples had a high titer of B19 IgM antibodies. Tests for rubella virus IgM antibodies were negative for these six patients. It is probable that other (infectious) causes might induce false-positive EBV IgM results by Liaison.
The consequences of these false-positive results may be important: 30 patients (83%) with certainly a high probability of acute B19 infection had a positive EBV IgM result at the time of presentation; on the other hand, only 3 patients from this EBV IgM-positive group had no detectable EBV nuclear antigen IgG, which means that in the majority (90%) of these patients an acute EBV infection was unlikely. Generally, EBV IgM results should always be interpreted in conjunction with those for EBV nuclear antigen IgG (6). Preferably, a follow-up sample to show an IgG seroconversion or significant IgG titer change should be taken. This advice was recently added to the new assay insert for the Liaison EBV IgM (version of 25 June 2008).
Prevention of these false-positive results could be achieved by including in the assays various blocking reagents which compete with nonspecific adsorption of proteins to the solid phase (1, 5, 11). Unfortunately, the modification of the Liaison EBV IgM assay that we previously proposed, i.e., adding polyvinylpyrrolidone and polyvinyl alcohol to the dilution buffer (2), could only partially decrease the false-positivity rate in acute B19 infections. Either further work needs to be done on this aspect of assay modification or additional fundamental changes by Diasorin (e.g., change of solid phase) are needed to improve the performance of especially the EBV IgM and HSV IgM assays.
In conclusion we can say that, although the Liaison is a very convenient, automated immunoassay platform, this study demonstrates that there is still a major opportunity for improvement of mainly the EBV IgM and HSV IgM tests.
Acknowledgments
We thank A. Vereecken and G. Salembier for their support to this study.
Footnotes
Published ahead of print on 30 December 2008.
REFERENCES
- 1.Barrett, D. A., M. S. Hartshome, M. A. Hussain, P. N. Shaw, and M. C. Davies. 2001. Resistance to nonspecific protein adsorption by poly(vinyl alcohol) thin films adsorbed to a poly(styrene) support matrix studied using surface plasmon resonance. Anal. Chem. 735232-5239. [DOI] [PubMed] [Google Scholar]
- 2.Berth, M., and E. Bosmans. 2008. Prevention of assay interference in infectious-disease serology tests done on the Liaison platform. Clin. Vaccine Immunol. 15891-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Enders, M., S. Helbig, A. Hunjet, H. Pfister, C. Reichhuber, and M. Motz. 2007. Comparative evaluation of two commercial enzyme immunoassays for serodiagnosis of human parvovirus B19 infection. J. Virol. Methods 146409-413. [DOI] [PubMed] [Google Scholar]
- 4.Feng, Z., Z. Li, B. Sui, G. Xu, and T. Xia. 2005. Serological diagnosis of infectious mononucleosis by chemiluminescent immunoassay using capsid antigen p18 of Epstein-Barr virus. Clin. Chim. Acta 35477-82. [DOI] [PubMed] [Google Scholar]
- 5.Haycock, J. W. 1993. Polyvinylpyrrolidone as a blocking agent in immunochemical studies. Anal. Biochem. 208397-399. [DOI] [PubMed] [Google Scholar]
- 6.Hess, R. D. 2004. Routine Epstein-Barr virus diagnostics from the laboratory perspective: still challenging after 35 years. J. Clin. Microbiol. 423381-3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jenkerson, S. A., M. Beller, J. P. Middaugh, and D. D. Erdman. 1995. False positive rubeola IgM tests. N. Engl. J. Med. 3321103-1104. [DOI] [PubMed] [Google Scholar]
- 8.Jones, J. W., J. V. Pether, and R. W. Frost. 1994. Human parvovirus B19. Hard to differentiate from infectious mononucleosis. BMJ 308595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Navalpotro, D., C. Gimeno, and D. Navarro. 2006. Concurrent detection of human herpesvirus type 6 and measles-specific IgMs during acute exanthematic human parvovirus B19 infection. J. Med. Virol. 781449-1451. [DOI] [PubMed] [Google Scholar]
- 10.Ratnam, S., G. Tipples, C. Head, M. Fauvel, M. Fearon, and B. J. Ward. 2000. Performance of indirect immunoglobulin M (IgM) serology tests and IgM capture assays for laboratory diagnosis of measles. J. Clin. Microbiol. 3899-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodda, D. J., and H. Yamazaki. 1994. Poly(vinyl alcohol) as a blocking agent in enzyme immunoassays. Immunol. Investig. 23421-428. [DOI] [PubMed] [Google Scholar]
- 12.Studentsov, Y. Y., M. Schiffman, H. D. Strickler, G. Y. Ho, Y. Y. Pang, J. Schiller, R. Herrero, and R. D. Burk. 2002. Enhanced enzyme-linked immunosorbent assay for detection of antibodies to virus-like particles of human papillomavirus. J. Clin. Microbiol. 401755-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waterboer, T., P. Sehr, and M. Pawlita. 2006. Suppression of nonspecific binding in serological Luminex assays. J. Immunol. Methods. 309200-204. [DOI] [PubMed] [Google Scholar]