Abstract
Monitoring of the kinetics of production of serum antibodies to multiple mycobacterial antigens can be useful as a diagnostic tool for the detection of Mycobacterium bovis infection as well as for the characterization of disease progression and the efficacy of intervention strategies in several species. The humoral immune responses to multiple M. bovis antigens by white-tailed deer vaccinated with BCG orally via a lipid-formulated bait (n = 5), orally in liquid form (n = 5), and subcutaneously (n = 6) were evaluated over time after vaccination and after experimental challenge with virulent M. bovis and were compared to the responses by unvaccinated deer (n = 6). Antibody responses were evaluated by using a rapid test (RT), a multiantigen print immunoassay (MAPIA), a lipoarabinomannan enzyme-linked immunosorbent assay (LAM-ELISA), and immunoblotting to whole-cell sonicate and recombinant antigen MPB83. MAPIA and RT detected minimal to no antibody responses over those at the baseline to multiple M. bovis antigens in vaccinated white-tailed deer after challenge. This was in contrast to the presence of more readily detectable antibody responses in nonvaccinated deer with more advanced disease. The LAM-ELISA results indicated an overall decrease in the level of production of detectable antibodies against lipoarabinomannan-enriched mycobacterial antigen in vaccinated animals compared to that in nonvaccinated animals after challenge. Immunoblot data were inconsistent but did suggest the occurrence of unique antibody responses by certain vaccinated groups to Ag85 and HSP70. These findings support further research toward the improvement and potential use of antibody-based assays, such as MAPIA, RT, and LAM-ELISA, as tools for the antemortem assessment of disease progression in white-tailed deer in both experimental and field vaccine trials.
Free-ranging white-tailed deer (Odocoileus virginianus) are wildlife reservoirs for bovine tuberculosis (BTb), caused by Mycobacterium bovis, in the state of Michigan (15, 18). Current management strategies, such as population reduction and decreased supplemental feeding, have effectively reduced the prevalence of disease (15). However, BTb continues to maintain a low-level presence in the wild deer population. The inclusion of effective field vaccination as part of disease management efforts in deer herds with endemic BTb would significantly aid in efforts to eradicate BTb from this potential wildlife reservoir (14, 16).
Vaccination with M. bovis bacillus Calmette-Guérin (BCG) via the oral or the parenteral route is effective in protecting white-tailed deer from disease caused by experimental M. bovis infection (14, 16). An important component of the evaluation of any vaccine candidate is gaining an understanding of the dynamics of a recipient's immunologic response to vaccination and infection over time in comparison with the dynamics of the response in unvaccinated subjects (3, 19, 22). Previous research has shown that monitoring of the kinetics of production of serum antibodies to multiple mycobacterial antigens is useful for the characterization of disease progression and the efficacy of disease treatment and as a tool for the diagnosis of M. bovis or M. tuberculosis infection in several species (11, 12, 21, 23, 24, 25).
In the present study, the humoral immune responses to multiple M. bovis antigens by white-tailed deer vaccinated with BCG via the oral and the parenteral routes were evaluated by four different assays over time after vaccination and after experimental challenge with virulent M. bovis. This information provides an understanding of the differences in the immunologic responses and disease progression in vaccinated and unvaccinated white-tailed deer infected with M. bovis and insight regarding the appropriate diagnostic tests to be used for the detection of BTb in a vaccinated population.
MATERIALS AND METHODS
Deer, vaccination, challenge, and necropsy.
Serum samples from 22 yearling white-tailed deer does were utilized for this study. These animals were part of a larger herd obtained for a vaccine efficacy trial (14). The deer originated from four BTb-free deer farms throughout the state of Iowa and were housed for the vaccination and infection studies conducted at the USDA/ARS National Animal Disease Center (NADC) in Ames, IA. All deer were housed and cared for according to the guidelines of the Association for Assessment and Accreditation for Laboratory Animal Care International. The Institutional Animal Care and Use Committee approved the protocols detailing the procedures and animal care prior to the initiation of the experiments.
At the beginning of the study, five deer voluntarily consumed 1 × 109 CFU BCG Danish strain 1331 orally via a lipid-formulated bait (oral bait group) (1, 14); five deer received 1.9 × 108 CFU BCG in culture medium orally via a catheter (oral liquid group), as described by Nol and others (14); six deer received 3.4 × 106 CFU BCG subcutaneously in the right shoulder (parenteral vaccination group); and six deer received culture medium orally via a catheter and served as unvaccinated controls (nonvaccinated animals). Mycobacterium bovis BCG Danish strain 1331 in culture and in lipid-formulated pellets was prepared by Immune Solutions Ltd. at the University of Otago, Dunedin, New Zealand, as described by Aldwell and others (1). The vaccine doses were determined by standard enumeration techniques by serial dilution plate counting on Middlebrook 7H11 medium (Becton Dickinson, Cockeysville, MD). Before vaccination and on a monthly basis throughout the study, blood was collected via the jugular vein for serologic analysis of the antibody responses. Three months after vaccination, the deer were moved from an outdoor facility to a biosafety level 3 animal building. The animals were separated into rooms with three to four animals per room, and the vaccinated deer were comingled with the unvaccinated deer. All deer were then challenged with 228 CFU of M. bovis strain 9839 (NADC designation) by the intratonsillar route (114 CFU/tonsil). This strain was originally isolated from an M. bovis-infected deer in the state of Michigan. Mycobacterium bovis strain 9839 was grown to mid-log phase on Middlebrook 7H9 medium supplemented with 10% oleic acid-albumin-dextrose complex (Difco, Detroit, MI) plus 0.05% Tween 80. Bacilli were harvested from the culture medium by pelleting the cells by centrifugation at 2,000 × g, washing the cells twice with 1 ml of phosphate-buffered saline (PBS) solution, and diluting the cells to the appropriate cell density in 2 ml of PBS. The challenge dose was determined as described above for the vaccine doses.
Five months after challenge, the deer were euthanized and examined for gross and microscopic lesions, as well as for mycobacterial colonization, by culture and PCR analysis (14). For the present study, the following tissues were examined for the presence of gross lesions consistent with M. bovis infection under a scoring system described in detail by Palmer and others (16): palatine tonsil, mandibular lymph node, parotid lymph node, medial retropharyngeal lymph node, tracheobronchial lymph node, mediastinal lymph node, right cranial lung lobe, right middle and caudal lung lobes, left cranial lung lobe, left caudal lung lobe, accessory lung lobe, liver, hepatic lymph node, mesenteric lymph node, and superficial cervical lymph node. The lymph node lesion scores ranged from 0 (no lesions) to 3 (most severe). The lung lesion scores ranged from 0 (no lesions) to 5 (most severe). Each animal received a total pathology score on the basis of the sums of the lesion scores assigned to each tissue evaluated. Additional information on gross and microscopic findings, as well as culture and PCR results, can be found in the report by Nol et al. (14).
RT.
A rapid immunochromatographic assay (RT; Cervid TB STAT-PAK, Chembio Diagnostic Systems, Inc., Medford, NY) was used to detect the antibody responses in the deer. The test employs a unique cocktail of selected M. bovis antigens and a blue latex bead-based signal detection system. Serum samples were tested as described previously (12). In addition to visual reading, semiquantitative evaluation of test band intensity was performed with an in-house-developed computer-assisted optical reader designed to measure the reflectance at 624 nm. The RT densitometry results obtained at 4 months postchallenge were utilized to compare the results among the groups.
MAPIA.
The multiantigen print immunoassay (MAPIA) was performed as described by Lyashchenko et al. (11). Briefly, antigens were immobilized on a nitrocellulose membrane (Schleicher & Schuell, Keene, NH) at a protein concentration of 0.05 mg/ml with a semiautomated airbrush printing device (Linomat IV; Camag Scientific, Inc., Wilmington, DE). The membrane was cut perpendicular to the antigen bands into 4-mm-wide strips. The strips were blocked for 1 h with 1% nonfat skim milk in PBS with 0.05% Tween 20 (PBST) and were then incubated for 1 h with serum samples diluted 1:50 in blocking solution. After the strips were washed, they were incubated overnight with alkaline phosphatase-conjugated anti-deer immunoglobulin G antibody (Kirkegaard & Perry Laboratories, Gaithersburg, MD) diluted 1:500 and then received another washing step. Deer antibodies bound to printed antigens were visualized with 5-bromo-4-chloro-3-indolylphosphate-nitroblue tetrazolium (Kirkegaard & Perry Laboratories). The MAPIA antigen panel was made up of 12 defined proteins. The following recombinant antigens of M. bovis were purified to near homogeneity as polyhistidine-tagged proteins: ESAT-6 (Rv3875) and CFP10 (Rv3874), produced at the Statens Serum Institut, Copenhagen, Denmark; MPB59 (Rv1886c), MPB64 (Rv1980c), MPB70 (Rv2875), and MPB83 (Rv2873), produced at the Veterinary Sciences Division, Stormont, Belfast, United Kingdom (7); and a 16-kDa protein (Acr1, Rv3391) and a 38-kDa protein (PstS1, Rv0934), purchased from Standard Diagnostics, Seoul, South Korea. Polyprotein fusions CFP10-ESAT-6 (E6P10) and Acr1-MPB83 (16/83) were constructed as described by Lyashchenko et al. (12). M. bovis culture filtrate (MBCF) was obtained from a field strain of M. bovis (strain T/91/1378; Veterinary Sciences Division) cultured in synthetic Sauton's medium for 21 days. Bovine protein purified derivative was produced by the Veterinary Laboratories Agency (Weybridge, Addlestone, United Kingdom).
The results of the MAPIA were evaluated visually to determine the presence or the absence of bands corresponding to the antigen on the strip. Selected MAPIA results also underwent semiquantitative densitometry analysis by use of the scanned strips and Scion Image, a public-domain imaging program from the U.S. National Institutes of Health (http://rsb.info.nih.gov/nih-image/). The densitometry software produced arbitrary values of the relative absorbance for the purposes of identifying trends among treatment groups. All densitometry values were recorded after subtraction of the densitometry readings taken from the prevaccination strips. The densitometry values obtained at 2 months for MBCF and 4 months for E6P10 and 16/83 were used for comparisons among the treatment groups.
Immunoblot assay.
The antibody responses of the deer were evaluated over time by immunoblot analysis with two sets of antigens: a whole-cell sonicate (WCS) of M. bovis strain 95-1315 and MPB83 (a kind gift from Jim McNair, AFBI, Belfast, United Kingdom). Mycobacterium bovis WCS was prepared as described by Waters et al. (23). Electrophoresis and immunoblot assays were performed by the procedures described by Waters and others (23, 25). Antigen was electrophoresed through preparative 12% (wt/vol) polyacrylamide gels. Electrophoretic transfer of the proteins onto pure nitrocellulose was accomplished with a Trans Blot cell (Bio-Rad, Hercules, CA) and sodium phosphate buffer (25 mM, pH 7.8) at 0.8 A for 90 min. After transfer, the filters were blocked with PBST and 2% (wt/vol) bovine serum albumin (BSA). After the filters were blocked, they were placed into the slot blot device and individual serum samples diluted 1:200 in PBST-BSA were added to independent slots. After 2 h of incubation at room temperature with gentle rocking, the blots were washed three times with PBST and incubated with horseradish peroxidase-conjugated anti-goat immunoglobulin G heavy and light chains (Kirkegaard & Perry Laboratories) diluted 1:20,000 in PBST-BSA for 1.5 h. The blots were again washed three times with PBST and developed for chemiluminescence in SuperSignal detection reagent (Pierce Chemical Co.). Comparisons of the reactivities of the serial serum samples to the WCS and MPB83 antigens were conducted as described by Waters et al. (23). In addition, the Scion Image program was used to obtain densitometry readings of individual lanes on each gel's radiographic image. Lane profile plots were generated for each lane. The response to the MPB83 antigen was considered positive when a band of the correct size was visually detected.
LAM-ELISA.
Lipoarabinomannan (LAM)-enriched mycobacterial antigen (PK1315) was prepared from M. bovis strain 95-1315 (PK1315) as described by Waters et al. (20, 21). Briefly, bacilli harvested from 4-week cultures were sonicated in PBS, further disrupted with 0.1- to 0.15-mm-diameter glass beads (Biospec Products, Bartlesville, OK) in a bead beater (Biospec Products), centrifuged, filtered (pore size, 0.22 μm), and digested in a 1-mg/ml proteinase K (Roche Molecular Biochemicals, Indianapolis, IN) solution (50 mM Tris, 1 mM CaCl2 buffer, pH 8.0) for 1 h at 50°C. The protein concentrations were determined (Bio-Rad), and the antigens were stored at −20°C. Immulon II 96-well microtiter plates (Dynatech, Chantilly, VA) were used. Each sample was tested in four wells, with two wells containing no antigen and two wells containing antigen. For the no-antigen wells, the wells were coated with 100 μl/well of 0.05 M carbonate-bicarbonate coating buffer (pH 9.6; C3041; Sigma-Aldrich Corp., St. Louis, MO). The antigen-coated wells received 100 μl/well of PK1315 diluted to 20 μg/ml in coating buffer. The coated plates were incubated overnight at 4°C. The plates were washed three times with 200 μl/well PBST (Sigma) and were blocked with 200 μl/well commercial milk diluent-blocking solution (diluted 1:20 with distilled H2O [Kirkegaard & Perry Laboratories]). After incubation for 1 h at 37°C in the blocking solution, the wells were washed nine times with 200 μl/well PBST. Test sera were added to the wells (100 μl/well). The test and the control sera were diluted 1:100 in PBS containing 0.1% gelatin. After incubation overnight at 4°C with the diluted test sera, the wells were washed nine times with 200 μl/well PBST and the plates were incubated for 1 h at 37°C with 100 μl/well horseradish peroxidase-conjugated protein G from a Streptococcus sp. (strain P8170; Sigma) diluted 1:2,000 in PBS with 0.1% fish gelatin. The wells were washed nine times with 200 μl/well PBST, and the plates were incubated at room temperature with 100 μl/well SureBlue reagent (Kirkegaard & Perry Laboratories). The plates were read kinetically every minute for 15 min at 650 nm by using an automated enzyme-linked immunosorbent assay (ELISA) plate reader (FlexStation 3; Molecular Devices, Sunnyvale, CA). For each well, the maximum rate of change for the enzyme (slope of the bell curve) (Vmax) was determined. For each sample, the raw Vmax value was calculated by subtracting the Vmax value for the no-antigen well from the Vmax value for the well with antigen. The raw Vmax values were also calculated for the positive and the negative controls. Finally, the Vmax for the sample/in relation to a known positive sample (Vmax s/p) was calculated by the following formula: (Vmax sample − Vmax negative control)/(Vmax positive control − Vmax negative control). Vmax s/p was the normalized number for the maximum rate of change for the enzyme, and the value was used for data analysis. The mean Vmax s/p value for the pooled data for the vaccinated animals was compared to the mean Vmax s/p value for the nonvaccinated animals.
Data analysis.
The number of vaccinated animals (pooled) with lesions was compared to the number of nonvaccinated animals with lesions by Fisher's exact test (two by two, one sided; Proc FREQ program of SAS software, version 9.1; SAS Institute, Cary, NC). Differences determined by the one-sided Fisher's exact test to have P values less than or equal to 0.1 were considered significant. The total pathology scores derived from lymph node lesions, lung lesions, and lymph node and lung lesions combined for vaccinated and nonvaccinated animals were also compared by using the Kruskall-Wallis nonparametric one-way analysis of variance, followed by the Wilcoxon rank-sum test (SAS software, version 9.1; SAS Institute). Differences were determined to be significant when P values were less than or equal to 0.1. Correlations between the relative densities obtained from the RT bands and pathology scores were analyzed by using Spearman's rank test (Proc FREQ, SCORR, program of SAS software, version 9.1, SAS Institute). LAP-ELISA data were analyzed by repeated-measures analysis of variance (Statview software, version 5.0; SAS Institute). When significant (P ≤ 0.1) effects were detected, Fisher's protected least-significant-difference method was used to compare the data for the different treatment groups on specific sampling dates. A P value of ≤0.1 was considered significant.
RESULTS
Pathology.
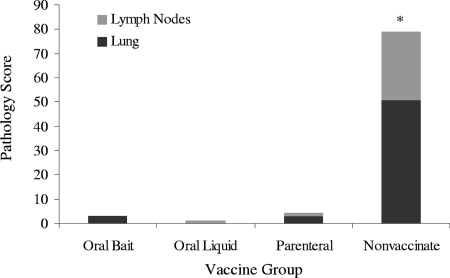
Five of the 16 vaccinated animals developed lesions consistent with tuberculosis, but none of these animals received a total pathology score of greater than 3. All six nonvaccinated animals had lesions in one or more tissues, and the total pathology scores ranged from 5 to 30 (Table 1). The number of vaccinated deer with lesions was significantly lower than the number of nonvaccinated deer with lesions (Fisher's exact test, P < 0.0062). The total lung pathology score, the total lymph node pathology score, and the lung and lymph node pathology scores combined were all significantly higher for the nonvaccinated group than for the vaccinated group (P = <0.001, P < 0.001, and P = 0.002, respectively, based on the Wilcoxon rank-sum test) (Fig. 1).
TABLE 1.
Total pathology scores and antibody responses to M. bovis antigens as measured by MAPIA and RT densitometry in vaccinated and nonvaccinated deer after M. bovis challengea
| Vaccination group and animal identifier | Relative antibody density over that at the baseline determined by:
|
Total pathology score (avg) | |||
|---|---|---|---|---|---|
| MAPIA
|
RT | ||||
| E6P10 | 16/83 | MBCF | |||
| Oral bait | |||||
| 402 | 0 | 0 | 41b,c | 0 | 0 |
| 404 | 0 | 15b | 0c | 0 | 0 |
| 406 | 0c | 0c | 5b,c | 0 | 0 |
| 410 | 0c | 0c | 0c | 0 | 2 |
| 418 | 0c | 0c | 13c | 0 | 1 |
| Totald | 0/5 | 1/5 | 3/5 | 0/5 | 3 (0.6) |
| Oral liquid | |||||
| 14 | 0 | 0 | 0 | 0 | 0 |
| 20 | 38b | 0 | 0 | 0 | 0 |
| 81 | 0 | 0 | 0 | 0 | 0 |
| 84 | 0 | 0 | 0 | 0 | 1 |
| 416 | 0 | 0 | 0 | 0 | 0 |
| Total | 1/5 | 0/5 | 0/5 | 0/5 | 1 (0.2) |
| Parenteral | |||||
| 1 | 0 | 0c | 0b,c | 87b | 0 |
| 23 | 0c | 0c | 63b,c | 0 | 0 |
| 26 | 0c | 0c | 0b,c | 0 | 1 |
| 59 | 0c | 0 | 83b | 0 | 0 |
| 73 | 0c | 16c | 0 | 0 | 3 |
| 91 | 0 | 0 | 0 | 0 | 0 |
| Total | 0/6 | 1/6 | 2/6 | 1/6 | 4 (0.7) |
| Nonvaccinated animals | |||||
| 3 | 94 | 16 | 96 | 32 | 16 |
| 19 | 10c | 3c | 45c,e | 20 | 5 |
| 22 | 12 | 28 | 96 | 39 | 11 |
| 24 | 0c | 0c | 0c,e | 0 | 5 |
| 57 | 0 | 0 | 90 | 27 | 19 |
| 422 | 0 | 45 | 85 | 90 | 30 |
| Total | 3/6 | 4/6 | 5/6 | 5/6 | 86 (14) |
Measurements were made 4 months after intratonsillar infection with 228 CFU of M. bovis for E6P10, 16/83, and RT and at 2 months postinfection for MBCF.
The antibody titer was over that at the baseline 1 to 3 months postvaccination, prior to M. bovis challenge.
Antibody was detected in the baseline sample.
Number of animals with antibodies/total number of animals tested.
Antibody was detected in unvaccinated animals 1 to 3 months prior to M. bovis challenge.
FIG. 1.
Gross pathology scores for selected lymph node and lung tissue samples for individual treatment groups. Scoring took place 5 months after intratonsillar infection with 228 CFU of M. bovis. The lymph node lesion scores ranged from 0 (no lesions) to 3 (most severe). The lung lesion scores ranged from 0 (no lesions) to 5 (most severe). Each animal received pathology scores on the basis of the sums of the lesion scores assigned to each tissue evaluated. *, the total combined pathology score for nonvaccinated animals was significantly greater than the pooled score for vaccinated animals.
RT.
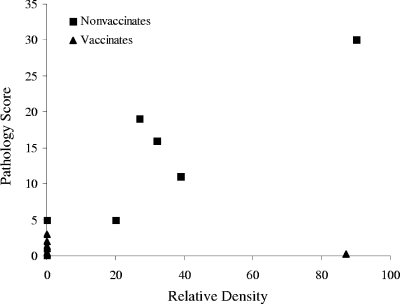
Among the 16 vaccinated deer, only 1 animal developed detectable antibodies, as determined by RT, at any time during the study. One of the six deer in the parenteral vaccination group developed a response, as determined by RT, starting at 3 months postvaccination and remained reactive for 4 months after challenge. In contrast, five of six deer in the nonvaccinated group developed detectable antibodies, as determined by RT, by 4 months after M. bovis challenge. Two of those animals developed a response by 2 months postchallenge, and four deer did so by 3 months postchallenge. None of the nonvaccinated deer developed antibodies detectable by RT before M. bovis challenge. The relative densities obtained from the RT responses for all deer at 4 months postchallenge are summarized in Table 1. The relative densities of the RT responses in nonvaccinated deer at 4 months postchallenge were positively correlated (Spearman's R = 0.60; P = 0.003) with the total combined pathology scores for the same group (Fig. 2). Interestingly, deer 19 and 24, the two animals in the nonvaccinated group with the lowest pathology scores, had the lowest densitometry readings in that group as well. However, these two deer still achieved higher pathology scores than any of the vaccinated deer (Table 1).
FIG. 2.
Positive correlation between relative densities determined by RT and disease severity in vaccinated and nonvaccinated deer shown in terms of the pathology scores. Sera were collected at 4 months after challenge with a total of 228 CFU of M. bovis strain 9839. Statistical analysis was conducted by using Spearman's rank test (R = 0.60; P = 0.003).
MAPIA.
The following proteins were recognized by the animals in this study challenge, as determined by MAPIA, and are reported here in terms of the numbers of deer producing detectable antibodies to the following antigens after M. bovis challenge: 16/83 (n = 19), MBCF (n = 17), E6P10 (n = 5), CFP10 (n = 4), bovine protein purified derivative (n = 3), the 16-kDa protein (n = 2), MPB83 (n = 1), and ESAT-6 (n = 1). Although these data varied among both individuals and on the basis of the treatment group, three antigens (16/83, MBCF, and E6P10) were deemed the most useful for evaluation of the responses of vaccinated and nonvaccinated deer after M. bovis challenge. The relative densities measured from the responses of the deer in the individual treatment groups to 16/83 and E6P10 at 4 months postinfection and to MBCF at 2 months postinfection are summarized in Table 1. The overall numbers of vaccinated animals and nonvaccinated animals producing detectable antibodies to the three antigens over the 4-month postinfection period, as determined by MAPIA, are reported in Table 2.
TABLE 2.
Numbers of vaccinated and nonvaccinated animals with detectable antibodies to E6P10, 16/83, and MBCF determined by MAPIA 1 to 4 months after intratonsillar infection with 228 CFU of M. bovis
| Group | Antigen | No. of animals with detectable antibodies/total no. of animals at the following time (mo) postinfection:
|
|||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Vaccinateda | E6P10 | 1/16 | 1/16 | 1/16 | 1/16 |
| 16/83 | 1/16 | 2/16 | 1/16 | 2/16 | |
| MBCF | 5/16 | 5/16 | 5/16 | 5/16 | |
| Nonvaccinated | E6P10 | 0/6 | 2/6b | 2/6c | 3/6b |
| 16/83 | 0/6 | 4/6b | 3/6c | 4/6b | |
| MBCF | 3/6 | 5/6b | 4/6c | 4/6b | |
None of the vaccinated animals responded to more than one antigen throughout the 4-month postinfection period.
Four of six nonvaccinated animals produced detectable antibodies to more than one antigen at 2 and 4 months postinfection.
Two of six nonvaccinated animals produced detectable antibodies to more than one antigen at 3 months postinfection.
Relative to the baseline levels (i.e., the preexisting responses, prior to vaccination), four of six deer in the nonvaccinated group had produced detectable antibodies to at least two of the three most useful antigens at 2 and at 4 months postinfection (Table 2). None of the 16 vaccinated deer produced detectable antibodies to more than one of the antigens throughout the postinfection period (Table 2). Six of the eight vaccinated animals that did produce detectable antibodies to one of the three most useful antigens had also done so before challenge (data not shown). In the oral bait vaccination group, zero of five, one of five, and three of five deer had detectable antibodies to E6P10 and 16/83 (4 months postchallenge) and to MBCF (2 months postchallenge), respectively (Table 1). One of five deer in the oral liquid vaccination group had detectable antibodies to E6P10 postchallenge. However, none of the deer in the oral liquid group had levels of antibodies to 16/83 or MBCF over the baseline levels at any time postinfection (Table 1). In the parenteral vaccination group, zero of six, one of six, and two of six deer responded to E6P10, 16/83, and MBCF, respectively (Table 1). In contrast, three of six, four of six, and five of six of the animals in the nonvaccinated group developed antibodies to E6P10, 16/83, and MBCF, respectively (Tables 1 and 2).
Immunoblots.
Specific bands of reactivity to M. bovis WCS at ∼10 kDa, ∼15 kDa, ∼20 to 25 kDa, ∼32 kDa, ∼34 to 35 kDa, ∼42 kDa, ∼60 kDa, ∼68 to 70 kDa, ∼74 to 76 kDa, ∼90 kDa, and ∼125 kDa were detected by immunoblotting throughout the study. The most reactive proteins were at the ∼20- to 25-kDa level, as all the deer produced antibodies to these antigens at some time during the study, including before vaccination.
Differences in antibody responses to proteins within specific size ranges were noted among the vaccination groups. During the first 3 months after M. bovis challenge, four of five animals in the oral bait vaccination group, two of five in the oral liquid vaccination group, five of six in the parenteral vaccination group, and five of six in the nonvaccinated group had responses to antigen at approximately the 32-kDa level greater than those detected at the baseline. However, by 4 months postchallenge, five of six nonvaccinated animals continued to produce antibody responses to these proteins, whereas two of five, two of five, and three of six deer in the oral bait, oral liquid, and parenteral vaccination groups, respectively, maintained antibody responses (data not shown). Of the two deer in the oral bait vaccination group that maintained antibodies to the 32-kDa protein, one deer produced a very weak response. One of the two deer in the oral liquid vaccination group had a weak response, whereas the other deer (deer 84) had a very strong response and was the only deer in that group to have lesions. Unlike in the other vaccination groups, all three deer in the parenteral vaccination group produced strong antibody responses to the 32-kDa protein. Two of the three deer did not sustain lesions by the end of the study, and the third deer had a very low pathology score. The deer in the nonvaccinated group (deer 24) that did not respond to the 32-kDa protein was one of two deer that had the lowest pathology scores (score, 5). The other nonvaccinated deer with a low pathology score (deer 19) had only a weak response to the 32-kDa protein relative to the responses of the other four animals that responded.
All of the deer in the oral bait and the parenteral vaccination groups showed antibody responses to proteins at the 68- to 70-kDa level at some time in the 4 months postchallenge. At 4 months postchallenge, four of five of the deer in the oral bait vaccination group and four of six deer in the parenteral vaccination group still showed the production of antibodies to these proteins. Only one animal in each of the other two groups developed a response to these antigens. All of the deer in the parenteral vaccination group deer and two of the deer in the oral bait vaccination group had already developed antibodies to these proteins after vaccination.
Regarding the MPB83-specific immunoblot assay, there were no patterns in antibody response to this antigen among the vaccine groups. Three of five deer in the oral bait vaccination group became positive for this antigen at different time points after vaccination, of which only one developed an increased response at 1 month postchallenge, which persisted to 4 months postchallenge. None of the deer in the oral liquid vaccination group developed antibodies to MPB83 detectable by immunoblotting after vaccination or challenge; however, the prevaccination serum of one of the animals did have a response, but the animals was negative by every test thereafter. One of five deer in the parenteral vaccination group became weakly positive for MPB83 at 1 month postvaccination, and this response disappeared by 2 weeks after challenge. One of six deer in the nonvaccinated group developed detectable antibodies to MPB83 by immunoblotting 2 weeks after challenge and maintained this response until 4 months postchallenge.
LAM-ELISA.
Mean Vmax s/p values obtained from pooled data for vaccinated animals and nonvaccinated animals indicated a trend of increasing detectable antibodies to LAM-enriched mycobacterial antigens in the nonvaccinated group after M. bovis challenge, whereas the vaccinated animals had an initial increase followed by a consistent decrease in detectable antibodies. The mean Vmax s/p values were not significantly different between the two groups until the final sampling point, which was at 16 weeks after infection (P = 0.002, based on Fisher's protected least-significant-difference method) (Fig. 3).
FIG. 3.
LAM-ELISA results as mean Vmax s/p values for nonvaccinated animals versus the mean value for vaccinated animals. The mean Vmax s/p values were calculated for nonvaccinated animals and vaccinated animals at each time point relative to the times of vaccination and challenge. Standard errors are reported for each mean. The Vmax s/p was the normalized number for the maximum rate of change for the enzyme. PV, postvaccination; PC, postchallenge; *, the mean Vmax s/p value for vaccinated animals was significantly lower than the mean Vmax s/p value for nonvaccinated animals.
DISCUSSION
Our MAPIA, RT, and LAM-ELISA results indicate that vaccinated white-tailed deer, which developed fewer and less severe lesions than nonvaccinated animals after M. bovis infection, as described here and by Nol et al. (14), generally harbor lower and/or diminishing levels of detectable circulating antibodies to M. bovis antigens than nonvaccinated deer with more advanced disease. This finding is consistent with those of previous studies, in which associations between the severity of disease caused by M. bovis infection and the levels of antibody production were noted (5, 10, 12, 23, 26).
Using RT and/or MAPIA, we were generally able to distinguish vaccinated and M. bovis-challenged deer with minimal lesion pathology from nonvaccinated and challenged deer with more advanced lesion development. In the present study, with one exception, none of the vaccinated deer responded by RT or produced detectable antibodies to more than one of the three most useful antigens by MAPIA. One of the two nonvaccinated animals that did not develop a detectable antibody response after infection to at least two of the three antigens analyzed by MAPIA did react by RT postinfection. The single nonvaccinated animal that tested negative by both assays was one of the two animals with the lowest lesion scores. It is possible that, given time, this animal would have reacted by these tests as the disease increased in severity; it is also possible that this animal had some resistance to M. bovis infection and may not have experienced a rapid development of pathology. These tests could serve as antemortem tools to aid researchers with the evaluation of the efficacy of a vaccine against M. bovis infection in deer. With this scenario, detection of the production of high levels of antibodies to M. bovis antigens would be indicative of vaccine failure.
Antibody-based tests could be used to evaluate the status of BTb in a free-ranging, vaccinated herd through the testing of live animals. The tests could identify severely affected animals that should be removed from the population to prevent shedding of the organism and the further spread of infection. Vaccine-protected animals with no or minimal lesion development would remain in the herd, as it has been suggested that such vaccinated deer experience minimal pathological changes postinfection and, even though they are infected, are not considered an important source of M. bovis to the environment for transmission to other animals (4).
The present study was limited to the evaluation of the humoral immune responses of small numbers of vaccinated and unvaccinated white-tailed deer in the first 4 to 5 months after challenge with M. bovis. Studies involving large wildlife species at a high biosafety level often suffer from a low power and a short duration due to the great expense, logistical issues, and animal welfare considerations. Although obstacles exist, it would be extremely useful to conduct follow-up experiments with larger sample sizes and of a longer duration that monitor the postchallenge antibody responses in both vaccinated and unvaccinated deer. BCG-vaccinated white-tailed deer may acquire long-term protection (>1 year) from the development of severe disease, as has been shown in red deer (4). Whether white-tailed deer continue producing similar results by MAPIA, RT, and LAM-ELISA after a year or longer following vaccination and M. bovis challenge would be of great interest and utility to a field vaccination program.
The results of immunoblotting with the WCS antigen suggest that the captive deer used in this study had prior exposure to environmental mycobacteria, although we did not isolate these organisms from the deer's tissues. Ongoing studies evaluating the responses of captive and free-ranging deer to various WCS antigens (i.e., from M. kansasii, M. avium, M. avium subsp. paratuberculosis, and M. bovis) indicate that deer are constantly exposed to antigens, presumably ubiquitous mycobacteria present in their environment, that elicit antibody reactive to mycobacterial WCS (6; W. R. Waters, personal observation). Obviously, preexisting responses complicate the analysis of specific responses to vaccination and infection and likely affect vaccine efficacy and disease progression in both experimental and field situations (2, 22).
Proteins at the 32-kDa and the 68- to 70-kDa levels elicited relatively unique antibody response patterns among the treatment groups, as detected by immunoblotting. The 32-kDa protein most likely corresponds to the immunodominant antigen 85 (Ag85) complex. The Ag85 complex is a member of the mycolyltransferase family found in all mycobacteria and represents a major fraction of the proteins secreted in culture filtrates of M. bovis, including BCG (17, 27). The Ag85 complex has effectively been used in the form of a DNA vaccine to boost the protective effects of BCG vaccination in mice and cattle. Although it is unclear why the nonvaccinated animals had prolonged responses to the Ag85 complex relative to the lengths of the responses of the other groups, particularly the orally vaccinated groups, it is possible that mucosal exposure to this antigen prior to challenge leads to a reduction in the antibody response over time after challenge and may correspond to a lack of lesion development. Alternatively, effective mucosal vaccination may limit the antigen load detected by the host in the form of antibody production. That the animals in the parenteral vaccination group did not respond in this manner could be a result of the more effective sensitization to the Ag85 complex afforded by vaccination by the parenteral route compared to that afforded by vaccination by the oral routes. The protein(s) in the 68- to 70-kDa range probably corresponds to a heat shock protein (HSP70) that has been shown to be immunogenic (8, 9). It is unclear why the animals in the parenteral and oral bait vaccination groups responded more consistently to this antigen than the animals in the other groups. More research needs to be conducted in order to understand the significance of the responses to both sets of proteins by BCG-vaccinated white-tailed deer.
The animals in this study did not produce readily detectable antibody responses to MPB83 by immunoblotting or MAPIA. This is in contrast to the observations from previous studies with cervids in which MPB83 was found to be immunodominant in these tests (6, 13, 23). However, when this antigen was combined with the 16-kDa protein (also known as Acr1), it was the most readily detected antigen by MAPIA in this study and was one of the most readily detected antigens in other studies as well (6, 13, 23, 24). These results highlight the variability of the responses by deer, as well as the variability of the responses by many other host species, to mycobacterial antigens on both an individual and a herd basis. These types of findings are not limited to M. bovis infections and are observed in the face of a variety of mycobacterial infections. This could be due to genetic differences among individuals and herds, as well as environmental factors. The results of this study and others highlight the importance of the use of a multiantigen approach, and a continued search for effective antigens is needed to develop more sensitive and specific serologic assays for the evaluation of vaccines and disease progression, as well as the general diagnosis of M. bovis infection in all species. (6, 13, 21, 23).
In conclusion, white-tailed deer that were protected from severe disease due to vaccination with BCG, either orally or parenterally, produced either no detectable antibody responses or decreasing antibody levels, as determined by MAPIA, RT, and LAM-ELISA, compared to the antibody responses and the antibody levels of nonvaccinated deer inoculated with M. bovis. These results support the potential of antibody-based assays as useful indicators of vaccine efficacy in experimental vaccine trials as well as for the monitoring of disease in vaccinated free-ranging deer populations. In addition, although the immunoblotting data provided limited insight regarding vaccine-induced protection against disease, they do encourage the further investigation of the immune responses to the Ag85 complex and HSP70 in deer vaccinated with BCG or similarly attenuated M. bovis constructs. More research on the development of serologic assays for the detection of immune responses to vaccination and M. bovis infection is needed. In addition, more expansive, longer-term studies on the safety and efficacy of BCG against M. bovis infection in white-tailed deer are imperative, so that vaccination may ultimately be used for the management of wild deer populations affected by BTb.
Acknowledgments
We express our appreciation to M. Howard, P. Lasley, R. Lyon, B. Olthoff, J. Pollock, and S. Zimmerman for their assistance in the laboratory as well as with the animals. We also thank D. Ewing, T. Holtz, T. Krausman, J. Steffan, and R. Whipple for animal care. We are grateful to P. Andersen and J. McNair for kindly providing the antigens for MAPIA.
Footnotes
Published ahead of print on 7 January 2009.
REFERENCES
- 1.Aldwell, F. E., D. L. Keen, N. A. Parlane, M. A. Skinner, G. W. de Lisle, and B. M. Buddle. 2003. Oral vaccination with Mycobacterium bovis BCG in a lipid formulation induces resistance to pulmonary tuberculosis in brushtail possums. Vaccine 2270-76. [DOI] [PubMed] [Google Scholar]
- 2.Buddle, B. M., B. J. Wards, F. E. Aldwell, D. M. Collins, and G. W. de Lisle. 2002. Influence of sensitisation to environmental mycobacteria on subsequent vaccination against bovine tuberculosis. Vaccine 201126-1133. [DOI] [PubMed] [Google Scholar]
- 3.Buddle, B. M., D. N. Wedlock, M. Denis, and M. A. Skinner. 2005. Identification of immune response correlates for protection against bovine tuberculosis. Vet. Immunol. Immunopathol. 10845-51. [DOI] [PubMed] [Google Scholar]
- 4.Griffin, J. F. T., C. G. Mackinotsh, and C. R. Rodgers. 2006. Factors influencing the protective efficacy of a BCG homologous prime-boost vaccination regime against tuberculosis. Vaccine 24835-845. [DOI] [PubMed] [Google Scholar]
- 5.Griffin, J. F., J. P. Cross, D. N. Chinn, C. R. Rodgers, and G. S. Buchan. 1994. Diagnosis of tuberculosis due to Mycobacterium bovis in New Zealand red deer (Cervus elaphus) using a composite blood test and antibody assays. N. Z. Vet. J. 42173-179. [DOI] [PubMed] [Google Scholar]
- 6.Harrington, N. P., O. P. Surujballi, J. F. Prescott, J. R. Duncan, W. R. Waters, K. Lyashchenko, and R. Greenwald. 2008. Antibody responses of cervids (Cervus elaphus) following experimental Mycobacterium bovis infection and the implications for immunodiagnosis. Clin. Vaccine Immunol. 151650-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lightbody, K. A., R. A. Skuce, S. D. Neill, and J. M. Pollock. 1998. Mycobacterial antigen-specific antibody responses in bovine tuberculosis: an ELISA with potential to confirm disease status. Vet. Rec. 142295-300. [DOI] [PubMed] [Google Scholar]
- 8.Lowrie, D. B., C. L. Silva, M. J. Colston, S. Ragno, and R. E. Tascon. 1997. Protection against tuberculosis by a plasmid DNA vaccine. Vaccine 15834-838. [DOI] [PubMed] [Google Scholar]
- 9.Lowrie, D. B., R. E. Tascon, V. L. Bonato, V. M. Lima, L. H. Faccioli, E. Stavropoulos, M. J. Colston, R. G. Hewinson, K. Moelling, and C. L. Silva. 1999. Therapy of tuberculosis in mice by DNA vaccination. Nature 400269-271. [DOI] [PubMed] [Google Scholar]
- 10.Lyashchenko, K., A. O. Whelan, R. Greenwald, J. M. Pollock, P. Andersen, R. G. Hewinson, and H. M. Vordermeier. 2004. Association of tuberculin-boosted antibody responses with pathology and cell-mediated immunity in cattle vaccinated with Mycobacterium bovis BCG and infected with M. bovis. Infect. Immun. 722462-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lyashchenko, K. P., M. Singh, R. Colangeli, and M. L. Gennaro. 2000. A multi-antigen print immunoassay for the serological diagnosis of infectious diseases. J. Immunol. Methods 24291-100. [DOI] [PubMed] [Google Scholar]
- 12.Lyashchenko, K. P., R. Greenwald, J. Esfandiari, J. H. Olsen, R. Ball, G. Dumonceaux, F. Dunker, C. Buckley, M. Richard, S. Murray, J. B. Payeur, P. Andersen, J. M. Pollock, S. Mikota, M. Miller, D. Sofranko, and W. R. Waters. 2006. Tuberculosis in elephants: antibody responses to defined antigens of Mycobacterium tuberculosis, potential for early diagnosis and monitoring of treatment. Clin. Vaccine Immunol. 13722-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lyashchenko, K. P., R. Greenwald, J. Esfandiari, M. A. Chambers, J. Vicente, C. Gortazar, N. Santos, M. Correia-Neves, B. M. Buddle, R. Jackson, D. J. O'Brien, S. Schmitt, M. V. Palmer, R. J. Delahay, and W. R. Waters. 2008. Animal-side serologic assay for rapid detection of Mycobacterium bovis infection in multiple species of free-ranging wildlife. Vet. Microbiol. 132283-292. [DOI] [PubMed] [Google Scholar]
- 14.Nol, P., M. V. Palmer, W. R. Waters, F. E. Aldwell, B. M. Buddle, J. M. Triantis, L. M. Linke, G. E. Phillips, T. C. Thacker. J. C. Rhyan, M. R. Dunbar, and M. D. Salman. 2008. Efficacy of oral and parenteral Mycobacterium bovis bacille Calmette-Guerin vaccination against experimental bovine tuberculosis in white-tailed deer (Odocoileus virginianus): a feasibility study. J. Wildl. Dis. 44247-259. [DOI] [PubMed] [Google Scholar]
- 15.O'Brien, D. J., S. M. Schmitt, J. S. Fierke, S. A. Hogle, S. R. Winterstein, T. M. Cooley, W. E. Moritz, K. L. Diegel, S. D. Fitzgerald, D. E. Berry, and J. B. Kaneene. 2002. Epidemiology of Mycobacterium bovis in free-ranging white-tailed deer, Michigan, USA, 1995-2000. Prev. Vet. Med. 5447-63. [DOI] [PubMed] [Google Scholar]
- 16.Palmer, M. V., T. C. Thacker, and W. R. Waters. 2007. Vaccination of white-tailed deer (Odocoileus virginianus) with Mycobacterium bovis bacille Calmette-Guerin. Vaccine 256589-6597. [DOI] [PubMed] [Google Scholar]
- 17.Rosseels, V., S. Marché, V. Roupie, M. Govaerts, J. Godfroid, K. Walravens, and K. Huygen. 2006. Members of the 30- to 32-kilodalton mycolyl transferase family (Ag85) from culture filtrate of Mycobacterium avium subsp. paratuberculosis are immunodominant Th1-type antigens recognized early upon infection in mice and cattle. Infect. Immun. 74202-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmitt, S. M., S. D. Fitzgerald, T. M. Cooley, C. S. Bruning-Fann, L. SullIvan, D. Berry, T. Carlson, R. B. Minnis, J. B. Payeur, and J. Sikarskie. 1997. Bovine tuberculosis in free-ranging white-tailed deer from Michigan. J. Wildl. Dis. 33749-758. [DOI] [PubMed] [Google Scholar]
- 19.Vordermeier, H. M., M. A. Chambers, P. J. Cockle, A. O. Whelan, J. Simmons, and R. G. Hewinson. 2002. Correlation of ESAT-6-specific gamma interferon production with pathology in cattle following Mycobacterium bovis BCG vaccination against experimental bovine tuberculosis. Infect. Immun. 703026-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waters, W. R., J. M. Miller, M. V. Palmer, J. R. Stabel, D. E. Jones, K. A. Koistinen, E. M. Steadham, M. J. Hamilton, W. C. Davis, and J. P. Bannantine. 2003. Early induction of humoral and cellular immune responses during experimental Mycobacterium avium subsp. paratuberculosis infection of calves. Infect. Immun. 715130-5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waters, W. R., M. V. Palmer, and D. L. Whipple. 2002. Mycobacterium bovis-infected white-tailed deer (Odocoileus virginianus): detection of immunoglobulin specific to crude mycobacterial antigens by ELISA. J. Vet. Diagn. Investig. 14470-475. [DOI] [PubMed] [Google Scholar]
- 22.Waters, W. R., M. V. Palmer, D. L. Whipple, R. E. Slaughter, and S. L. Jones. 2004. Immune responses of white-tailed deer (Odocoileus virginianus) to Mycobacterium bovis BCG vaccination. J. Wildl. Dis. 4068-78. [DOI] [PubMed] [Google Scholar]
- 23.Waters, W. R., M. V. Palmer, J. P. Bannantine, D. Whipple, R. Greenwald, J. Esfandiari, P. Andersen, J. McNair, J. M. Pollock, and K. P. Lyashchenko. 2004. Antigen recognition by serum antibodies in white-tailed deer (Odocoileus virginianus) experimentally infected with Mycobacterium bovis. Clin. Diagn. Lab. Immunol. 11849-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waters, W. R., M. V. Palmer, J. P. Bannantine, R. Greenwald, J. Esfandiari, P. Andersen, J. McNair, J. M. Pollock, and K. P. Lyashchenko. 2005. Antibody responses in reindeer (Rangifer tarandus) infected with Mycobacterium bovis. Clin. Diagn. Lab. Immunol. 12727-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waters, W. R., M. V. Palmer, T. C. Thacker, J. P. Bannantine, H. M. Vordermeier, R. G. Hewinson, R. Greenwald, J. Esfandiari, J. McNair, J. M. Pollock, P. Andersen, and K. P. Lyashchenko. 2006. Early antibody responses to experimental Mycobacterium bovis infection of cattle. Clin. Vaccine Immunol. 13648-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Welsh, M. D., R. T. Cunningham, S. M. Corbett, R. M. Girvin, J. McNair, R. A. Skuce, D. G. Bryson., and J. M. Pollock. 2005. Influence of pathological progression on the balance between cellular and humoral immune responses in bovine tuberculosis. Immunology 114101-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wiker, H. G., and M. Harboe. 1992. The antigen 85 complex: a major secretion product of Mycobacterium tuberculosis. Microbiol. Rev. 56648-661. [DOI] [PMC free article] [PubMed] [Google Scholar]