Abstract
Feline immunodeficiency virus (FIV)-positive and FIV-negative cats (n = 4/group) received 2 × 106 CFU Mycobacterium tuberculosis ΔlysA ΔpanCD intramuscularly. Vaccination elicited antibody responses, albeit at lower levels in FIV-positive cats than in FIV-negative cats. Delayed-type hypersensitivity responses were minimal in both groups. No adverse reactions were found.
Bovine tuberculosis (bTB), caused by Mycobacterium bovis, seriously threatens wildlife populations in eastern and southern Africa (2). In 1998, more than 90% of African lions tested in the southern part of Kruger National Park were infected with bTB, and roughly 15% of the park's lion population has died from bTB in the last 5 years (L. M. de Klerk, personal communication). Concurrent feline immunodeficiency virus (FIV) infection may increase susceptibility to bTB, and it is suspected, as with other regions of Africa (3, 4), that more than 50% of the lions in the central part of Kruger National Park are FIV infected. The impact of bTB on human health, domestic livestock bordering parks, and ecotourism confounds the problem. Successful vaccination may be a reasonable option to consider for the management of bTB in lions.
M. bovis Bacillus Calmette-Guérin (BCG) vaccination has been used with varying success in several free-ranging species. A limited-replication Mycobacterium tuberculosis vaccine strain (mc26020, specifically designed for use in human immunodeficiency virus-infected humans) has improved safety over and similar efficacy to BCG in mouse (including immune-deficient strains), guinea pig, and nonhuman primate models (reviewed in reference 9). With mc26020, deletions in lysine (lysA) and pantothenate biosynthesis (panC and panD) genes result in severely limited replication, even in immune-deficient strains of mice receiving high (109 CFU) doses of the vaccine (8). A single dose in mice offered protection against an aerosol challenge with virulent M. tuberculosis, even at 7 months postvaccination, with extended survival periods and reduced tissue pathology in comparison to results for unvaccinated controls (8). Additionally, mc26020 has recently been evaluated for safety in simian immunodeficiency virus-infected neonatal macaques (M. H. Larsen, unpublished results). Our objective was to evaluate mc26020 in FIV-infected domestic cats as a safety screen prior to delivery to captive lions. Because the target population is free-ranging lions in Africa, many of which are FIV infected, evaluation of safety and immunogenicity of a candidate vaccine in FIV-positive (FIV+) domestic cats affords an additional assurance prior to studies with lions.
A total of eight specific-pathogen-free cats were purchased from Liberty Labs (Liberty, NY) and randomly divided into two groups of four cats each. Cats were housed and cared for in accordance with Association for the Assessment of Laboratory Animal Care standards and Institutional Animal Care and Use Committee guidelines. Cats were inoculated with unpassaged, primary feline lymphocytes infected with the pathogenic molecular clone FIV-NCSU1 by the intravenous route between 24 and 43 weeks of age. All inoculated cats seroconverted and were positive for provirus and plasma viremia as determined by PCR. Eight months after FIV challenge, all cats received 2 × 106 CFU mc26020 (∼10× the human dose for BCG) in 0.5 ml phosphate-buffered saline (PBS) by intramuscular injection (time point, 0 weeks postvaccination).
Eight weeks after vaccination, cats received 0.1 ml (100 μg) of M. bovis purified protein derivative (PPD), 0.1 ml (40 μg) of Mycobacterium avium PPD, and 0.1 ml PBS intradermally at separate clipped sites in the midthoracic region as a measure of delayed-type hypersensitivity. Skin thickness was measured with calipers at 24, 48, and 72 h after injection. Balanced PPDs were obtained from the Brucella and Mycobacterial Reagents section of the National Veterinary Services Laboratory, Ames, IA.
Electrophoresis and immunoblotting were performed at 0, 4, 8, and 12 weeks postvaccination using previously described procedures (1) with the following modifications. Antigens for immunoblotting were either a whole-cell sonicate (WCS) of M. bovis strain 95-1315 or recombinant MPB83 (kindly provided by Jim McNair, Agri-Food & Biosciences Institute, Belfast, United Kingdom). The WCS was prepared as described previously (10). Antigen was electrophoresed through preparative 12% (wt/vol) polyacrylamide gels and transferred to nitrocellulose. These membranes were placed in blocking solution consisting of PBS with 0.1% Tween 20 (PBST; Sigma-Aldrich, St. Louis, MO) and 2% (wt/vol) bovine serum albumin (PBST-BSA) and then into a 20-slot Mini-protean II multiscreen device (Bio-Rad Laboratories, Richmond, CA) with individual sera, diluted 1:400 in PBST-BSA, added to independent slots. After 2 h of incubation with gentle rocking, blots were washed five times with PBST and incubated with peroxidase-conjugated protein A (Sigma-Aldrich) diluted 1:80,000 in PBST-BSA for 1.5 h. Blots were again washed four times with PBST and developed for chemiluminescence in SuperSignal detection reagent (Pierce Chemical Company, Rockford, IL).
For multiantigen print immunoassay (MAPIA) at 0, 4, 8, and 12 weeks postvaccination, seven recombinant proteins of M. bovis were used (Rv numbers are in brackets): ESAT-6 [Rv3875] and CFP-10 [Rv3874] (Statens Serum Institut, Copenhagen, Denmark); MPB59 [Rv1886c], MPB64 [Rv1980c], MPB70 [Rv2875], and MPB83 [Rv2873] (Veterinary Sciences Division, Stormont, Belfast, United Kingdom); and Acr1 [Rv3391] (Standard Diagnostics, Seoul, South Korea). Two polyprotein fusions (CFP-10/ESAT-6 and Acr1/MPB83; Statens Serum Institut, Copenhagen, Denmark) and one native antigen (M. bovis culture filtrate T/91/1378; Veterinary Sciences Division, Stormont, Belfast, United Kingdom) were also used. MAPIA was performed as described previously (6), using horseradish peroxidase-conjugated protein A (Sigma-Aldrich) diluted 1:1,000 and 3,3′,5,5′-tetramethyl benzidine (Kirkegaard and Perry Laboratories, Inc., Gaithersburg, MD).
Adverse local or systemic reactions, such as edema, ulceration, erythema, fever, or malaise, were not detected in any of the animals throughout the 10-day observation period postvaccination. Evaluation of serum chemistry profiles and complete blood cell counts revealed decreased (P < 0.05) white blood cell counts and increased (P < 0.05) red blood cell counts and hematocrits for FIV+ cats compared to results for FIV-negative (FIV−) cats at each time point (i.e., prevaccination and 4, 8, and 12 weeks postvaccination). Demonstrable effects of vaccination on complete blood cell counts and chemistry profiles were not evident. Data were analyzed as a completely randomized design using the Statview software program (version 5.0; SAS Institute Inc., Cary, NC). Full necropsies (except the brain and spinal cord) were performed on all eight cats. No gross lesions were observed in the FIV− cats, and lesions in the FIV+ cats were consistent with chronic FIV infection.
Delayed-type hypersensitivity reactions to M. avium and M. bovis PPD were minimal, regardless of FIV status. Responses for FIV+ cats did not differ (P > 0.05) from those for FIV− cats. Mean (standard error) increases in skin thickness (in millimeters) in response to M. bovis PPD compared to results with PBS were as follows: 0.4 (0.2) at 24 h, 1.3 (0.3) at 48 h, and 1.4 (0.4) at 72 h (i.e., compiled responses for FIV+ and FIV− cats; n = 8). Responses to M. avium did not differ (P > 0.05) from responses to M. bovis PPD. Serum antibody responses to mycobacterial antigens were boosted by intradermal injection of PPDs for skin testing (Fig. 1 and 2), as demonstrated previously with other species (5, 7, 10).
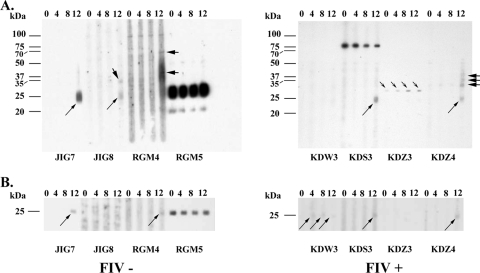
FIG. 1.
Preparative immunoblots of Mycobacterium bovis whole-cell sonicate antigen (A) or recombinant MPB83 (B) probed with sera from FIV− or FIV+ mc26020-vaccinated cats. Animal identification and FIV status are provided on the bottom margins, and molecular mass markers are provided in the left margin. Responses to ∼25-kDa, ∼32-kDa, and ∼35- to 37-kDa antigens are indicated by long, short, and heavy arrows, respectively (except with cat RGM5, which had a preexisting strong response to the 25-kDa antigen).
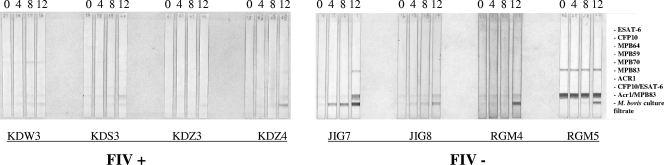
FIG. 2.
Antibody responses to M. bovis antigens detected by MAPIA with sera from FIV+ and FIV−, mc26020-vaccinated cats. Antigens immobilized on each strip are indicated on the right. These include seven recombinant proteins of M. bovis (ESAT-6 [Rv3875], CFP-10 [Rv3874], MPB64 [Rv1980c], MPB59 [Rv1886c], MPB70 [Rv2875], MPB83 [Rv2873], and Acr1 [Rv3391]), two polyprotein fusions (CFP-10/ESAT-6 and Acr1/MPB83), and one native antigen (M. bovis culture filtrate T/91/1378). Numbers of weeks after vaccination are shown at the top. Cat identification is at the bottom. Bands on the strips indicate the presence of detectable antibodies to corresponding antigens.
As determined by immunoblotting with M. bovis WCS, responses to ∼25-kDa, 35-kDa, 37-kDa, and 70-kDa antigens were detected for 2/4, 1/4, 1/4, and 1/4 FIV− cats, respectively (Fig. 1A). Each of these responses was elicited after injection of PPD for the skin test. One FIV− cat (RGM5) showed a preexisting response to ∼20-kDa and 25-kDa antigens that persisted throughout the study. This cat also had a preexisting response to MPB83, as detected by immunoblotting (Fig. 1B) and MAPIA (Fig. 2), that persisted throughout the study, and the response did not change following vaccination. Another cat (RGM4) had a preexisting response to a ∼100-kDa antigen that persisted throughout the study. Preexisting antibody responses likely indicate prior exposure to ubiquitous nontuberculous mycobacteria that cross-react with M. bovis antigens as detected for wildlife and domestic livestock (K. P. Lyashchenko and W. R. Waters, unpublished observations). Responses to MPB83 are elicited by certain nontuberculous mycobacteria, such as Mycobacterium kansasii (11), often confounding interpretation of M. bovis-specific tests. The cat (JIG7) whose serum responded to the 25-kDa antigen of the M. bovis WCS also showed a response to MPB83 as detected by immunoblotting and MAPIA. By MAPIA, serum from JIG7 also reacted to M. bovis culture filtrate beginning 4 weeks after vaccination, with the response increasing in intensity on subsequent sampling. Serum from all four FIV− cats had weak to moderate responses to M. bovis culture filtrate and the 16-kDa/MPB83 fusion protein.
In general, the intensity of antibody responses for FIV+ cats was less than that observed for FIV− cats (Fig. 1 and 2). With that said, 3/4 FIV+ cats showed weak responses to MPB83 after skin testing (Fig. 1 and 2). One FIV+ cat (KDW3) had a weak response to MPB83 beginning at 4 weeks after vaccination (Fig. 1B). As with FIV− cats, sera from all four FIV+ cats showed responses to M. bovis culture filtrate as detected by MAPIA (Fig. 2). By immunoblotting with M. bovis WCS, responses to ∼25-kDa, 32-kDa, 35-kDa and 37-kDa antigens were detected for 2/4, 2/4, 1/4, and 1/4 FIV+ cats, respectively. The response to the 32-kDa antigen for KDZ3 was detectable at the time of vaccination yet increased in intensity at each time point thereafter. Serum from one FIV+ cat, KDS3, reacted intensely to an ∼75-kDa antigen at each time point, including prior to vaccination. As with domestic livestock and wildlife (7), antigen recognition patterns varied between animals demonstrating the diversity of the antibody response elicited. Regardless, detection of vaccine-elicited antibody demonstrates immunogenicity despite FIV infection.
Together, these findings demonstrate the following: (i) vaccination with mc26020 elicits an antibody response in all vaccinated cats, regardless of FIV status, (ii) intradermal injection of PPDs boosts the antibody response, (iii) FIV infection dampens the antibody response, and most importantly, (iv) vaccination with an attenuated live M. tuberculosis vaccine (mc26020) at 10× the human dose is well tolerated by domestic cats regardless of FIV infection status. These findings provide the assurance necessary to proceed further with studies with captive lions.
Acknowledgments
This study was funded through a Conservation Action Network grant from the Memphis Zoo and Aquarium.
We thank Jim McNair for kindly providing recombinant antigens from M. bovis and Thierry Olivry for performing the intradermal tuberculin tests. In addition, we acknowledge Jenny Sun and Shelly Zimmerman for excellent technical support.
Footnotes
Published ahead of print on 14 January 2009.
REFERENCES
- 1.Bannantine, J., and J. R. Stabel. 2000. HspX is present within Mycobacterium paratuberculosis-infected macrophages and is recognized by sera from some infected cattle. Vet. Microbiol. 76343-358. [DOI] [PubMed] [Google Scholar]
- 2.De Vos, V., R. G. Bengis, N. P. Kriek, A. Michel, D. F. Keet, J. P. Raath, and H. F. Huchzermeyer. 2001. The epidemiology of tuberculosis in free-ranging African buffalo (Syncerus caffer) in the Kruger National Park, South Africa. Onderstepoort J. Vet. Res. 68119-130. [PubMed] [Google Scholar]
- 3.Driciru, M., L. Siefert, K. C. Prager, E. Dubovi, R. Sande, F. Princee, T. Friday, and L. Munson. 2006. A serosurvey of viral infections in lions (Panthera leo), from Queen Elizabeth National Park, Uganda. J. Wildl. Dis. 42667-671. [DOI] [PubMed] [Google Scholar]
- 4.Hofmann-Lehmann, R., D. Fehr, M. Grob, M. Elgizoli, C. Packer, J. S. Martenson, S. J. O'Brien, and H. Lutz. 1996. Prevalence of antibodies to feline parvovirus, calicivirus, herpesvirus, coronavirus, and immunodeficiency virus and of feline leukemia virus antigen and the interrelationship of these viral infections in free-ranging lions in east Africa. Clin. Diagn. Lab. Immunol. 3554-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lyashchenko, K., A. O. Whelan, R. Greenwald, J. M. Pollock, P. Andersen, R. G. Hewinson, and H. M. Vordermeier. 2004. Association of tuberculin-boosted antibody responses with pathology and cell-mediated immunity in cattle vaccinated with Mycobacterium bovis BCG and infected with M. bovis. Infect. Immun. 722462-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lyashchenko, K. P., M. Singh, R. Colangeli, and M. L. Gennaro. 2000. A multi-antigen print immunoassay for the serological diagnosis of infectious diseases. J. Immunol. Methods 24291-100. [DOI] [PubMed] [Google Scholar]
- 7.Lyashchenko, K. P., R. Greenwald, J. Esfandiari, M. A. Chambers, J. Vicente, C. Gortazar, N. Santos, M. Correia-Neves, B. M. Buddle, R. Jackson, D. J. O'Brien, S. Schmitt, M. V. Palmer, R. J. Delahay, and W. R. Waters. 2008. Animal-side serologic assay for rapid detection of Mycobacterium bovis infection in multiple species of free-ranging wildlife. Vet. Microbiol. 132283-292. [DOI] [PubMed] [Google Scholar]
- 8.Sambandamurthy, V. K., and W. R. Jacobs, Jr. 2005. Live attenuated mutants of Mycobacterium tuberculosis as candidate vaccines against tuberculosis. Microbes Infect. 7955-961. [DOI] [PubMed] [Google Scholar]
- 9.Sambandamurthy, V. K., X. Wang, B. Chen, R. G. Russell, S. Derrick, F. M. Collins, S. L. Morris, and W. R. Jacobs, Jr. 2002. A pantothenate auxotroph of Mycobacterium tuberculosis is highly attenuated and protects mice against tuberculosis. Nat. Med. 81171-1174. [DOI] [PubMed] [Google Scholar]
- 10.Waters, W. R., M. V. Palmer, J. P. Bannantine, R. Greenwald, J. Esfandiari, P. Andersen, J. McNair, J. M. Pollock, and K. P. Lyashchenko. 2005. Antibody responses in reindeer (Rangifer tarandus) infected with Mycobacterium bovis. Clin. Diagn. Lab. Immunol. 12727-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waters, W. R., M. V. Palmer, T. C. Thacker, J. B. Payeur, N. B. Harris, F. C. Minion, R. Greenwald, J. Esfandiari, P. Andersen, J. McNair, J. M. Pollock, and K. P. Lyashchenko. 2006. Immune responses to defined antigens of Mycobacterium bovis in cattle experimentally infected with Mycobacterium kansasii. Clin. Vaccine Immunol. 13611-619. [DOI] [PMC free article] [PubMed] [Google Scholar]