Abstract
The sensitivity for detection of Histoplasma antigen is lower in serum than in urine. In other antigen assays, treatment of serum at 104°C in the presence of EDTA was required for detection of antigenemia. Sensitivity and specificity for detection of Histoplasma antigenemia were examined with or without EDTA heat treatment of the serum using the MVista Histoplasma antigen enzyme immunoassay. A total of 94.6% of serum specimens from patients with AIDS and histoplasmosis that were negative untreated were positive after EDTA-heat treatment. Two-thirds of the negative serum specimens from patients with probable histoplasmosis, based upon clinical suspicion and Histoplasma antigenuria, were positive after heat treatment. Specificity was 99.0% in controls, including healthy subjects and patients in whom histoplasmosis or blastomycosis, were excluded. Precision and reproducibility were good and excellent, respectively. These findings demonstrate improvement in sensitivity without reduction in specificity, precision, or reproducibility after heat-EDTA treatment.
The sensitivity for detection of Histoplasma antigenemia is lower than for antigenuria. For example, in the quantitative MVista Histoplasma antigen enzyme immunoassay (EIA), among patients with AIDS and progressive disseminated histoplasmosis (PDH), antigenuria was detected in 95 to 100% compared to 92 to 95% for antigenemia (1, 3). Previously, we noted improvement in the sensitivity for detection of antigenuria after ultrafiltration (2). In the Platelia Aspergillus EIA, pretreatment of serum at 104°C in the presence of EDTA is essential for detection of antigenemia. The presumed mechanisms for improvement in sensitivity include dissociation of antigen-antibody complexes and denaturation of the freed antibody.
Testing for both antigenemia and antigenuria offers several advantages over testing for antigenuria alone. First, in some cases antigenuria may be undetectable, but antigenemia may be present. Second, urine may not be available in patients with renal failure. Third, antigenuria levels early in the infection often are above the reportable range of the MVista Histoplasma antigen EIA (1, 3). Clearance of antigenemia may provide a better marker for response to therapy in such cases. Fourth, antigenuria is more likely to be affected by hydration status, and consequently urine volume and concentration, than is antigenemia, making it a more accurate marker for fungal burden. The objective of this investigation was to evaluate the effect of preheating serum to 104°C in the presence of EDTA on detection of Histoplasma antigenemia.
MATERIALS AND METHODS
Clinical samples.
Serum and urine specimens were obtained from AIDS patients with PDH treated with amphotericin B, followed by itraconazole (4), or with itraconazole alone (5). The criteria for diagnosis included clinical findings of histoplasmosis supported by laboratory confirmation: positive culture, histopathology, or Histoplasma antigen. Positive cultures or histopathology was the basis for diagnosis in 89% and antigenuria in 11%. Serum and urine specimens had been frozen at −20°C since 1996 to 1998 in a study conducted by the Mycoses Study Group (4) and since 1991 for an AIDS Clinical Trials Group study (5). For this analysis, specimens obtained before or during antifungal therapy that were negative or <0.6 ng/ml in the quantitative MVista Histoplasma antigen EIA were evaluated with or without pretreatment at 104°C in EDTA. Additional serum specimens from patients with probable histoplasmosis, based upon detection of Histoplasma antigenuria in the MVista EIA or positive serologic findings, were tested. Other clinical or laboratory information was not available from these patients.
Clinical controls included nine patients with probable blastomycosis, based on repeatedly positive urine specimens in the Blastomyces antigen assay, and patients in whom histoplasmosis was excluded based upon clinical and laboratory findings in a study approved by the institutional review board at Clarian Health Partners, Indianapolis, IN. Control specimens from healthy subjects were purchased (Houchin Blood Bank, Bakersfield, CA; SeraCare, Milford, MA).
MVista Histoplasma antigen assay.
The MVista EIA was performed as previously described (1). The results were quantitated in ng/ml by extrapolation from a human source material antigen calibration curve matched to primary reference galactomannan standards. Specimens with optical density values that exceeded the cutoff for the assay but that are less than the 0.6-ng/ml standard were reported as positive (<0.6 ng/ml) and those with results exceeding the 39-ng/ml standard are reported as positive (>39 ng/ml). Testing was performed at MiraVista Diagnostics, Indianapolis, IN.
Pretreatment of serum at 104°C with EDTA.
The procedure was modified after that used in the Platelia Aspergillus EIA (7). A total of 200 μl of EDTA was added to 600 μl of serum, vortex mixed, and placed in a heat block (Fisher Scientific) at 104°C for 6 min. The modifications included doubling the volume of EDTA and serum to provide sufficient supernatant for robotic pipetting and use of a heat block rather than a water bath. After that, the specimen was centrifuged, and the supernatant was removed for testing in the Histoplasma antigen EIA.
Statistical analysis.
The respective proportion of patients with positive results was compared by using the Fisher exact test. The reproducibility was analyzed by linear regression.
RESULTS
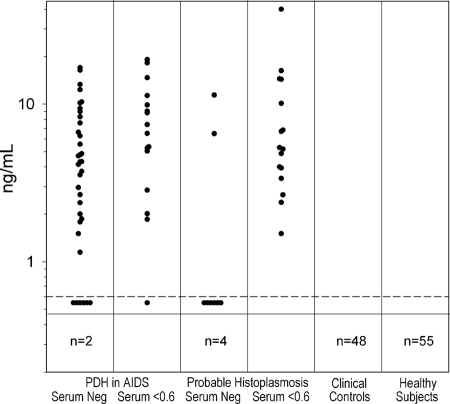
Among the AIDS patients with PDH and undetectable antigenemia, 35 of 37 (94.6%) were positive after treatment at 104°C in EDTA and at levels of >2 ng/ml in 25 (67.6%) (Fig. 1). Of those with positive results below 0.6 ng/ml, the results increased by at least 2 ng/ml in 13 of 16 (81.3%). Among cases of probable histoplasmosis, based upon the presence of Histoplasma antigenuria, of 12 with negative results in serum, 8 (66.7%) were positive after EDTA-heat treatment, and of 16 with antigenemia at <0.6 ng/ml, antigenemia increased at least 2 ng/ml in 13 (81.3%).
FIG. 1.
Antigenemia and histoplasmosis cases and controls after EDTA-heat treatment. Each point represents the result for an individual patient. The proven and probable histoplasmosis groups are subdivided into those with negative results in serum and low positive results of <0.6 ng/ml. The results above the horizontal line are positive. The number in the box below the horizontal line represents negative results.
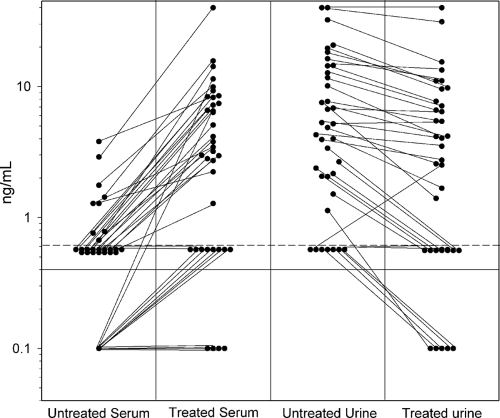
The effect of EDTA-heat treatment was compared on the serum and urine from 37 probable histoplasmosis cases based upon detection of antigenuria. As shown in Fig. 2, antigenemia became detectable or increased at least 2 ng/ml in 28 of 37 (75.7%), while antigenuria increased at least 2 ng/ml in no specimens and decreased in 32 (86.5%), presumably related to dilution of the specimen by addition of 200 μl of EDTA to 600 μl of urine. EDTA-heat treatment produced a coagulum in all of the serum specimens but in none of the urine specimens. The mean increase was 5.59 ng/ml (range, 0.5 to 19.14 ng/ml).
FIG. 2.
Effect of EDTA-heat treatment on detection of antigenemia and antigenuria in patients with probable histoplasmosis. The solid horizontal line represents the cutoff for positivity. Untreated and EDTA-heat treated results are connected by the diagonal line for individual patients.
In two patients with probable histoplasmosis, based upon serologic findings in the absence of antigenuria, EDTA-heat treatment permitted detection of antigenemia. The first was a child with acute pulmonary histoplasmosis. The Histoplasma antigen was negative in urine and serum, but the immunodiffusion test was positive, demonstrating an M precipitin band. Subsequently, the Histoplasma complement fixation test also was positive at a titer of 1:64. Antigenemia was detectable at 2.1 ng/ml after EDTA-heat treatment. The second was an adult who was receiving infliximab for inflammatory arthritis and presented with pneumonia and probable disseminated disease. The Histoplasma complement fixation test was positive at a titer of 1:256. The Histoplasma antigen was negative in urine and serum, but antigenemia was detected at 1.0 ng/ml after EDTA-heat treatment. In both cases, antigenuria also was detectable at <0.6 ng/ml after ultrafiltration of the specimen (2).
Among nine patients with blastomycosis with negative results in untreated serum, eight (88.9%) became positive after EDTA-heat treatment, increasing by a mean of 6.52 ng/ml (range, 0.5 to 9.45 ng/ml). Among 48 patients without histoplasmosis based upon review of medical records and negative antigen results in urine, antigenemia was detected in 1 (2.1%) after EDTA-heat treatment, at 2.1 ng/ml. That patient had an underlying connective tissue disorder and an undiagnosed febrile illness, but the immunodiffusion was negative. The complement fixation test was uninterpretable. Four of the controls had a positive serologic test for histoplasmosis (M precipitin in one, complement fixation titer of 1:8 in another, and M precipitins and complement fixation titers of 1:16 and 1:128 in two), but none had clinical findings for histoplasmosis: the diagnoses in these four controls were pulmonary fibrosis with a remote history of histoplasmosis, chronic sinusitis, asthma, and atypical mycobacterial lung disease in one patient each. The immunodiffusion test was performed in all 48 patients, and the complement fixation in 24, and each were positive in three patients. Other diagnoses in the hospital controls included nonfungal pneumonia in eight, sarcoidosis in six, lung transplantation in four, mycobacterial infection in three, and a variety of other infectious or noninfectious conditions in one or two patients each among the remaining 27 patients. Furthermore, results were negative in 55 of 55 (100%) healthy subjects before and after EDTA-heat treatment. Thus, antigenemia was negative in 102 of 103 (99.0% [95% confidence interval, 94.66 to 99.82]) controls without histoplasmosis or blastomycosis.
Intra- and interassay precision were examined by testing five aliquots of a moderate (5 to 10 ng/ml) and low (<0.6 ng/ml) positive serum pool with or without EDTA-heat treatment on three consecutive days. For the low-antigenemia pool, the mean level for the combined results over the 3 days was 1.261 ± 0.34 ng/ml (coefficient of variation [CV] 0.272) for the untreated specimen compared to 8.786 ± 0.820 ng/ml (CV 0.083) after treatment, Table 1. For the moderate antigenemia pool, the mean for the 3 days was 17.123 ± 2.123 ng/ml (CV = 0.124) compared to 37.189 ± 7.973 ng/ml (CV = 0.214) after treatment. The five aliquots of normal serum were negative with or without treatment on all 3 days of testing.
TABLE 1.
Precision and reproducibility for low and moderate level antigenemia
| Pool | Untreated
|
EDTA-heat treated
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 1-3 | Day 1 | Day 2 | Day 3 | Day 1-3 | ||
| <0.6 ng/ml | |||||||||
| Mean | 0.896 | 1.318 | 1.570 | 1.261 | 7.992 | 8.670 | 9.698 | 8.786 | |
| SD | 0.131 | 0.152 | 0.284 | 0.343 | 0.263 | 0.489 | 0.484 | 0.820 | |
| CV | 0.135 | 0.115 | 0.18 | 0.272 | 0.033 | 0.056 | 0.05 | 0.083 | |
| 5-10 ng/ml | |||||||||
| Mean | 15.026 | 19.296 | 17.048 | 17.123 | 31.06 | 45.142 | 35.368 | 37.189 | |
| SD | 1.765 | 0.901 | 0.689 | 2.123 | 6.26 | 5.546 | 4.070 | 7.973 | |
| CV | 0.118 | 0.047 | 0.04 | 0.124 | 0.20 | 0.129 | 0.115 | 0.214 | |
Reproducibility was examined by testing 30 positive and 25 negative specimens on two occasions. The results were reproducibly positive or negative in all 55 specimens. Furthermore, the antigen concentration of positive specimens agreed closely when tested on two separate occasions (R2 = 0.9431) (data not shown).
DISCUSSION
EDTA-heat treatment improved the sensitivity for detection of antigenemia in patients with proven or probable histoplasmosis. Among AIDS patients with undetectable antigenemia, at baseline or during the first 3 months of antifungal therapy, antigenemia was detectable in 95% of cases after EDTA-heat treatment. Intra- and interassay precision were similar with or without EDTA-heat treatment. Furthermore, positive results were highly reproducible, both qualitatively and quantitatively. Specificity was excellent in controls without histoplasmosis or blastomycosis. Demonstration of antigenemia in specimens that were negative without EDTA-heat treatment may improve the sensitivity of antigen detection for diagnosis of histoplasmosis, as illustrated in two patients in whom antigenuria was undetectable but serologic tests were positive. A cross-reactive galactomannan is present in the urine of patients with blastomycosis and a few other endemic mycoses (1). EDTA-heat treatment did not eliminate cross-reactivity, as galactomannan is not affected by EDTA-heat. Antigen clearance is often used as one factor in making decisions about stopping antifungal treatment in patients with PDH (6). These studies show that antigenemia in the form of antigen-antibody complexes often persists after clearance of noncomplexed antigenemia. Further studies are needed to determine whether a positive result after EDTA-heat treatment is associated with an increased risk for relapse.
The precise mechanism whereby EDTA-heat treatment facilitates antigenemia detection has not been established. Presumably, anti-Histoplasma antibodies formed immune complexes with circulating antigen, reducing the antigen's availability to attach to antibody coated plates. Heating at 104°C in the presence of EDTA dissociated immune complexes and denatured the freed antibody, thereby allowing the antigen to bind to the antibody coated plates. Whether denaturation of other serum proteins contributes to the observed effect remains unknown.
In summary, these findings demonstrate the need for EDTA-heat treatment to accurately measure Histoplasma antigenemia.
Acknowledgments
S.S., P.C., M.D., A.L., J.W., and L.J.W. are employees of MiraVista Diagnostics, the company performing the MVista Histoplasma antigen EIA. The study was funded by MiraBella Technologies, the company that produces the MVista Histoplasma antigen EIA.
Footnotes
Published ahead of print on 14 January 2009.
REFERENCES
- 1.Connolly, P. A., M. M. Durkin, A. M. LeMonte, E. J. Hackett, and L. J. Wheat. 2007. Detection of Histoplasma antigen by a quantitative enzyme immunoassay. Clin. Vaccine Immunol. 141587-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Egan, L., P. A. Connolly, D. Fuller, T. E. Davis, J. Witt III, K. S. Knox, C. A. Hage, and L. J. Wheat. 2008. Detection of Histoplasma capsulatum antigenuria by ultrafiltration of samples with false-negative results. Clin. Vaccine Immunol. 15726-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gutierrez, M. E., A. Canton, P. Connolly, R. Zarnowski, and L. J. Wheat. 2008. Detection of Histoplasma capsulatum antigen in Panamanian patients with disseminated histoplasmosis and AIDS. Clin. Vaccine Immunol. 15681-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson, P. C., L. J. Wheat, G. A. Cloud, M. Goldman, D. Lancaster, D. M. Bamberger, W. G. Powderly, R. Hafner, C. A. Kauffman, and W. E. Dismukes. 2002. Safety and efficacy of liposomal amphotericin B compared with conventional amphotericin B for induction therapy of histoplasmosis in patients with AIDS. Ann. Intern. Med. 137105-109. [DOI] [PubMed] [Google Scholar]
- 5.Wheat, J., R. Hafner, A. H. Korzun, M. T. Limjoco, P. Spencer, R. A. Larsen, F. M. Hecht, W. Powderly, et al. 1995. Itraconazole treatment of disseminated histoplasmosis in patients with the acquired immunodeficiency syndrome. Am. J. Med. 98336-342. [DOI] [PubMed] [Google Scholar]
- 6.Wheat, L. J., A. G. Freifeld, M. B. Kleiman, J. W. Baddley, D. S. McKinsey, J. E. Loyd, and C. A. Kauffman. 2007. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 45807-825. [DOI] [PubMed] [Google Scholar]
- 7.Wheat, L. J., and T. J. Walsh. 2008. Diagnosis of invasive aspergillosis by galactomannan antigenemia detection using an enzyme immunoassay. Eur. J. Clin. Microbiol. Infect. Dis. 27245-251. [DOI] [PubMed] [Google Scholar]