Abstract
Studies by Western blot analyses have shown that antibodies in the sera of host species infected by Pythium insidiosum recognized several prominent proteins expressed by this fungus-like pathogen. Although these studies have utilized sera from infected patients and relevant local strains of P. insidiosum, the results are difficult to compare because of the lack of method standardization. In an effort to resolve this issue, we have utilized standardized methodologies to evaluate six P. insidiosum strains from Asia and the Americas and 15 serum samples from cattle, cats, dogs, horses, and humans with pythiosis from the same geographical regions. Our data show that the antibodies present in these sera recognize a wide variety of unique P. insidiosum immunogenic proteins. Although some of the prominent proteins in this study have been previously reported, several others have yet to be described. For instance, a ∼28-kDa-molecular-mass antigen was detected by the antibodies in all serum samples evaluated. However, this antigen was strongly expressed by only one of the strains evaluated. A diffuse ∼51-kDa protein was not detected by the antibodies in the human sera; but it was recognized by the antibodies in the sera of cattle, cats, dogs, and horses. This antigen was expressed by only two of the strains investigated. Several other similar examples were also observed. The variation of the P. insidiosum protein profile identified by the antibodies in the sera evaluated indicates that some geographically diverged P. insidosum strains expressed some unique immunogens in vitro and that during natural infection (in vivo) P. insidiosum might express a broader number of antigens variably detected by individuals within the same species but especially across species.
Pythiosis is a life-threatening infection of humans (10, 23, 26) and lower animals (2, 4, 14, 16), including birds (15). The disease is caused by Pythium insidiosum, a protist stramenopilan oomycete that develops sparsely septated hyphal elements which mimic the coenocytic hyphae of the zygomycetous fungi (4, 10). Thus, in the past pythiosis has been misdiagnosed as a fungal infection in both humans (10, 14) and animals (9). Once considered an exotic disease, new reports indicate that pythiosis is more common than was previously expected in humans, horses, and dogs in the United States and elsewhere (1, 2, 4-6, 9, 11-14, 17, 22, 25). Despite new diagnostic methods, however, pythiosis is still difficult to diagnose because most clinical laboratory workers are not familiar with this protistan oomycete (10, 17, 26). Moreover, physicians have only recently learned of this life-threatening infection, especially in the areas where the disease is endemic (9, 10, 26).
Serological assays (1, 2, 4-6, 9, 11-14) and other immunological studies (3, 8, 9) have been instrumental in the diagnosis of pythiosis. The first serological assay used for the diagnosis of the disease was developed in 1924 by Witkamp (27). In 1981, Miller and Campbell (14) introduced immunodiffusion and complement fixation assays on the basis of the findings of the study of Witkamp (27). Later on, several other investigators introduced more specific antigens tested in new serological tests for the diagnosis of pythiosis (9). Although several immunogens have been detected by the antibodies in the sera of P. insidiosum-infected hosts, those antigens have yet to be characterized and techniques for their detection have yet to be properly standardized (2, 5, 8, 12, 16, 22, 25). For instance, Western blot (WB) analysis showed that the sera of bovines, felines, canines, equines, and humans with the disease detect several antigenic proteins (2, 5, 9, 12, 16, 19, 22). However, the findings from those studies could not be compared due to the lack of standardization.
In an effort to begin characterizing the antigens detected by the immunoglobulins in infected hosts, we have used standardized WB techniques to evaluate the immunogens of six geographically diverged P. insidiosum strains expressed in vitro with sera from multiple host species. Variations in the protein profiles even among individuals within the same species were observed. The antibodies in the sera of the species tested identified numerous immunogens in the six P. insidiosum strains used in this study. Some of these immunogens were unique to at least two of the strains analyzed and were consistently detected by the antibodies in all sera. The detection of prominent immunogenic proteins, such as the 28-kDa-molecular-mass antigen and other proteins in some of the strains evaluated, is interesting, and these proteins warrant further studies.
MATERIALS AND METHODS
Sera and strains.
Fifteen serum samples were collected from various host species, as follows: three cats with subcutaneous pythiosis on different anatomical areas, three cattle with subcutaneous pythiosis on their limbs, three dogs (two dogs with intestinal phthiosis and one dog with subcutaneous pythiosis), three horses with cutaneous pythiosis on different anatomical areas, and three humans (two patients from Thailand with arterial pythiosis and one patient from the United States with orbital disease). The sera were kept at −80°C until they were used (Table 1). The diagnosis of pythiosis in the selected host species was confirmed by either culture or serology (enzyme-linked immunosorbent assay [11], WB [12], detection of anti-P. insidiosum fluorescent antibodies in tissue sections [16]) or by a combination of these methods. In addition, the following sera were used as controls: one serum sample each from an apparently healthy human, dog, cat, and horse; three serum samples from humans with candidiasis (serum sample 033227), aspergillosis (serum sample 026515), and histoplasmosis (serum sample 033351); and one serum sample from a horse with sporothrichosis, one serum sample from a dog with Aspergillus infection, and one serum sample from a cat with cryptococcosis from previous studies (11, 12). Six geographically diverse P. insidiosum strains were analyzed. Details about the strains can be found in Table 2.
TABLE 1.
Host sera with pythiosis used in this study and pathological features and geographical origin of each host
| Host serum sample | Pathological feature | Geographical origin |
|---|---|---|
| Cat A | Subcutaneous infection of limbs | Georgia |
| Cat B | Subcutaneous nasal infection | North Carolinaa |
| Cat C | Subcutaneous infection of neck and a limb | Florida |
| Cattle A | Multiple ulcerated lesions on the limbs | Venezuelab |
| Cattle B | Multiple fistulas on limbs | Venezuelab |
| Cattle C | Multiple fistulas on limbs | Venezuelab |
| Dog A | Subcutaneous infection on a limb | Georgia |
| Dog B | Intestinal | North Carolina |
| Dog C | Intestinal | Florida |
| Horse A | Large abdominal ulcerate tissue | Costa Rica |
| Horse B | Subcutaneous infection on limbs | Texas |
| Horse C | Subcutaneous infection on limbs | Alabama |
| Human A | Orbital infection | Tennesseea |
| Human B | Carotid artery | Thailandc |
| Human C | Femoral artery | Thailand |
TABLE 2.
Pythium insidiosum strains used in this study, the host from which the isolate was recovered, the pathological features of the host with the infection, the geographical origins of the strains, and additional information on their accessibility in other collections
| Identifier used in this study | Host | Pathology | Geographical origin | Strain(s) in other collectionsa |
|---|---|---|---|---|
| MTPI-19 (type strain) | Horse | Abdominal ulcer | Costa Rica | ATCC 68643 = CBS 574.85 |
| MTPI-04 | Horse | Ulcerated limbs | United States | ND |
| MTPI-12 | Human | Arterial pythiosis | Thailand | ND |
| MTPI-14 | Human | Lymph nodes | Thailand | ND |
| MTPI-21 | Horse | Limb | New Guinea | ATCC 28251 |
| MTPI-24 | Horse | Limb | Australia | ATCC 64218 |
ATCC, American Type Culture Collection; CBS, Centraalbureau voor Schimmelcultures; MTPI, Medical Technology Pythium insidiosum; ND, not deposited in other collections.
Antigen production.
The P. insidiosum strains described above were subcultured at 37°C on 2% Sabouraud dextrose agar every 2 weeks and were then transferred to cornmeal agar slants and incubated at 37°C for 24 h. After incubation, ∼3-mm2 blocks with hyphal elements of P. insidiosum were cut and used to inoculate a 1.0-liter flask containing 500 ml of 2% Sabouraud dextrose broth. The broth cultures were incubated at 37°C while being rotated at 150 rpm for 5 days. After incubation, the cells in the cultures were killed with merthiolate (0.02%, wt/vol) and were then filtered to separate the hyphal cell mass. The hyphal cell mass was then transferred to a mortar and ground in the presence of liquid nitrogen. The resulting powder was resuspended in 5 ml of sterile distilled water, and the antigenic preparation was used on the same day in all experiments. The protein concentration was estimated by a microassay procedure (Bio-Rad Laboratories, Hercules, CA).
SDS-polyacrylamide gel electrophoresis (PAGE).
Ten-microliter samples containing ∼20 μg of P. insidiosum protein were added to an equal volume of buffer consisting of 125 mM Tris-HCl (pH 6.8), 6% sodium dodecyl sulfate (SDS), 20% glycerol, 10% 2-mercaptoethanol, and 0.05% bromophenol blue; and the samples were boiled for 10 min. The samples were then centrifuged at 14,000 × g and subjected to electrophoresis on a 12% (wt/vol) polyacrylamide resolving gel and a 4% (wt/vol) stacking gel, as described by Laemmli (7). The gels were then stained with Coomassie brilliant blue (Bio-Rad Laboratories), and the molecular masses were estimated by use of a standard preparation (Bio-Rad Laboratories).
Immunoblotting.
The immunogens separated in SDS-polyacrylamide gels were electrophoretically transferred from a one-dimensional polyacrylamide gel to Immun-Blot polyvinylidene difluoride membranes (0.2 μm; Bio-Rad Laboratories) according to the methods described by Towbin et al. (24). After overnight transfer, the membranes were blocked for 2 h at 37°C with 5% gelatin from the skin of a cold-water fish (Sigma, St. Louis, MO) in phosphate-buffered saline (PBS)-10% Tween, pH 7.2 (8.5 g NaCl, 1.07 g Na2HPO4 anhydrous, 0.39 g NaH2PO4·H2O, 1 liter distilled water, 10% [wt/vol] Tween 20). The sera analyzed (Table 1) were diluted 1:500 in 5% gelatin blocking solution and were then incubated at 37°C for 90 min with shaking before they were washed once with PBS-10% Tween, twice with 0.01 M PBS, pH 7.2, and once with 0.1 M Tris, pH 9.2. Antibody binding to the membrane antigens was detected in triplicate with goat anti-human immunoglobulin G (IgG; H+L)-horseradish peroxidase (HRP; Bio-Rad Laboratories); goat anti-cat, anti-cattle, anti-dog, or anti-horse IgG (H+L)-HRP (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA); or HRP-mouse anti-human IgG1, IgG2, IgG3, and IgG4 monoclonal antibodies (Zymed Laboratories Inc. San Francisco, CA) at a 1:1,000 dilution (vol/vol) in the gelatin blocking solution after 1 h of incubation at room temperature in a rotating shaker. The membranes were washed as described above and then once with PBS-Tween and twice with 0.01 M PBS, pH 7.2; the antibody reaction was developed with an Immun-Blot assay kit (Bio-Rad Laboratories); and the reacted membranes were digitally scanned at high resolution.
RESULTS
Protein profile by SDS-PAGE of Pythium insidiosum strains.
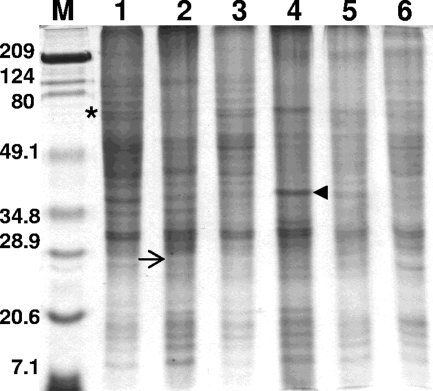
The protein profiles of the six geographically divergent P. insidiosum strains used in this study were very similar. Several proteins ranging in molecular mass from ≥209 to 9 kDa were consistently found in the Coomassie brilliant blue-stained SDS-polyacrylamide gels (Fig. 1). The molecular masses of the proteins expressed by the six P. insidiosum strains tested usually ranged from ≥209 to ∼74 kDa, ∼52 to ∼45 kDa, ∼40 to ∼30 kDa, and ∼21 to ∼9 kDa. High-molecular-mass proteins with molecular masses ranging from 209 to 124 kDa, the proteins of ∼74 to ∼52 kDa, as well as those with molecular masses ranging from ∼28 to ∼21 kDa were moderately expressed. Some strains weakly expressed an array of proteins with different molecular masses that were consistently absent from the others strains tested. All strains overexpressed a double band at ∼30 and ∼32 kDa. The overexpressed ∼38-kDa protein on Thai strain MTPI-14 was not found on any of the other strains examined (Fig. 1, arrowhead).
FIG. 1.
SDS-PAGE profiles of the proteins from six Pythium insidiosum strains in this study. The gels were stained with Coomassie brilliant blue. Lanes: 1, MTPI-19; 2, MTPI-04; 3, MTPI-12; 4, MTPI-14; 5, MTPI-21; and 6, MTPI-24. Asterisk, the ∼74-kDa-molecular-mass antigen, solid arrowhead, one of the antigens in strain MTPI-14 prominently expressed in vitro; arrow, putative location of the immunodominant ∼28-kDa-molecular-mass antigen found on almost all WB membranes (Fig. 2 to 4). Note that the 28-kDa immunodominant antigen is faintly expressed on the SDS-polyacrylamide gels. Lane M, molecular mass marker (the numbers on the left are in kilodaltons).
Immunoblotting (WB).
The antibodies in the control sera, including sera from host species with various diseases, did not detect the antigens of P. insidiosum (data not shown). The antibodies in the sera from various species with pythiosis detected multiple P. insidiosum immunogens with molecular masses that ranged from ≥209 to ∼20 kDa in all strains, but the intensities of detection differed (Fig. 2 to 4). The majority of immunodominant antigens were high-molecular-mass immunogens that ranged from ∼209 to ∼49 kDa and ∼35 to ∼28 kDa. High-molecular-mass antigens (≥209 kDa) were also detected by some of the serum samples evaluated. Some immunogens were more strongly detected by the antibodies in one host species than by the antibodies in others. Our data showed that, with the exception of strains MTPI-21 (from New Guinea; Fig. 2 and 3, lanes 5) and MTPI-24 (from Australia; Fig. 2 and 3, lanes 6), the antibodies in the sera from patients from the Americas strongly detected the immunogens expressed by the Costa Rican and U.S. strains. In contrast, the antibodies in sera from human Thai patients detected the P. insidiosum immunogens in the two Thai, the New Guinean, and the Australian strains used in this study with greater intensities (Fig. 4, lanes 3 to 6). A summary of the prominent antigens in all sera and strains detected by WB is shown in Table 3.
FIG. 2.
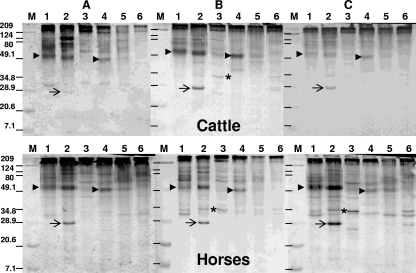
Western blot analysis of the three serum samples collected from cattle with pythiosis (A to C, upper panels) and the three serum samples collected from horses with pythiosis (A to C, lower panels). Lanes: 1, MTPI-19; 2, MTPI-04; 3, MTPI-12; 4, MTPI-14; 5, MTPI-21; and 6, MTPI-24. Arrows, the ∼28-kDa antigen on strain MTPI-04; asterisks, the ∼34-kDa antigen; arrowheads, the ∼51- and 49-kDa-molecular-mass immunodominant antigens detected by the antibodies of cattle and horses; lanes M, molecular mass markers (the numbers on the left are in kilodaltons); dashes in lanes M in panels B and C, the estimated locations of the molecular mass markers on those membranes.
FIG. 4.
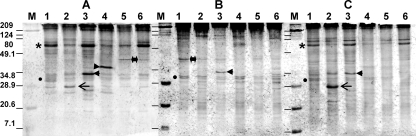
WB analysis of the three serum samples collected from two Thai humans with pythiosis (A and B) and a U.S. boy with pythiosis (C). Lanes: 1, MTPI-19; 2, MTPI-04; 3, MTPI-12; 4, MTPI-14; 5, MTPI-21; and 6, MTPI-24. Right-pointing arrowhead, the ∼41-kDa-molecular-mass prominent antigen in one of the human Thai patients (panel A, lane 4); left-pointing arrowhead, the ∼34-kDa antigen (panel A, lane 3); asterisks, estimated location of the double ∼74-kDa prominent proteins; double-headed arrow, location of the ∼46-kDa antigen; solid circle, location of the ∼30- and ∼32-kDa double band; arrows, the ∼28-kDa-molecular-mass immunodominant antigen; lanes M, molecular mass markers (the numbers on the left are in kilodaltons); dashes in lanes M in panels B and C, estimated locations of the molecular markers on those membranes.
FIG. 3.
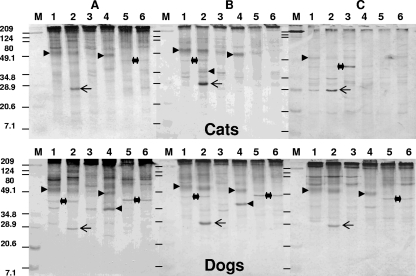
Western blot analysis of the three serum samples collected from U.S. cats with pythiosis (A to C, upper panels) and the three serum samples collected from dogs with pythiosis (A to C, lower panels). Lanes: 1, MTPI-19; 2, MTPI-04; 3, MTPI-12; 4, MTPI-14; 5, MTPI-21; and 6, MTPI-24. Left-pointing arrowheads, the prominent ∼41-kDa-molecular-mass antigen in one of the cats (B, upper panel) and the three dogs (A to C, lower panels); right-pointing arrowheads, the ∼51- and 49-kDa-molecular-mass immunodominant antigens also detected by the antibodies of calves and horses, as shown in Fig. 2; double-headed arrows and asterisks, the location of the ∼46-kDa P. insidiosum antigen detected by the antibodies of the dogs and one of the cats on some of the strains (dogs, panels A to C; cat, panel C); arrows, the ∼28-kDa-molecular-mass immunodominant antigen; lanes M, molecular mass marker (the numbers on the left are in kilodaltons); dashes in lanes M in panels B and C, the estimated locations of the molecular markers on those membranes.
TABLE 3.
Prominent P. insidiosum immunogens found by WB analysis in each of the 15 serum samples from individuals with pythiosis evaluated in this study
| Host serum sample | Immunogen (kDa)a
|
|||||
|---|---|---|---|---|---|---|
| MTPI-19 | MTPI-04 | MTPI-12 | MTPI-14 | MTPI-21 | MTPI-24 | |
| Bovine A | 51, 30 | 124, 80, 51 | 209 | 209, 49 | 209 | 209 |
| Bovine B | 51 | 51, 28 | 34 | 49 | Very weak bands | 209 |
| Bovine C | 51 | 51, 28 | 209 | 49 | Very weak bands | 209 |
| Horse A | 51 | 51, 28 | Very weak bands | 49 | Very weak bands | Very weak bands |
| Horse B | 74, 51, 32 | 124, 51, 34, 28 | 209 | 49, 34 | Very weak bands | Very weak bands |
| Horse C | 74, 51, 34, 32 | 51, 34, 32, 28 | Very weak bands | 49 | 49, 32 | 34, 32 |
| Cat A | 74, 51 | 124, 80, 74, 51, 28 | Very weak bands | 80, 74, 49 | Very weak bands | Very weak bands |
| Cat B | 51, 34, 32 | 51, 34, 32, 28 | 34 | 49 | Very weak bands | Very weak bands |
| Cat C | 51, 46, 34, 32, 28 | 46, 28 | 46 | 46, 20 | Very weak bands | Very weak bands |
| Dog A | 74, 51, 46, 34 | 124, 78, 51, 46, 28 | 41 | 78, 60, 49, 41, 27 | 74, 46 | 74, 46 |
| Dog B | 51 | 80, 51, 46, 34, 28 | Very weak bands | 49, 41 | 46 | 46 |
| Dog C | 80, 51 | 80, 74, 51, 28 | 209, 80, 74, 46 | 209, 49, 41 | 209, 46 | 209, 80, 74, 46 |
| Human A | 74, 34, 32, 30 | 76, 74, 34, 32, 28, 27 | 209, 74, 34, 30, 28, 26, 25 | 209, 74, 46 | 209, 74, 46, 34, 32 | 209, 74, 46, 34, 32 |
| Human B | 46, 32, 30 | 80, 46, 32, 30, 27, 21 | 80, 34, 32, 30 | 78, 32, 30 | 32, 30 | 32, 30, 27 |
| Human C | 80, 76, 74, 41, 34, 32, 30 | 80, 76, 74, 41, 34, 32, 30, 28, 23 | 209, 76, 74, 41, 34, 32, 30 | 76, 74, 27 | 76, 74 | 76, 74 |
The strongest bands are underlined and in boldface.
For convenience, the data were compared according to the host sera analyzed. For instance, the WB results for sera from cattle and horses (large animals) are placed together in Fig. 2, the WB results for sera from cats and dogs (small animals) are placed together in Fig. 3, and the WB results for sera from humans are placed together in Fig. 4. The P. insidiosum immunogens detected in the six strains tested by the antibodies present in the sera from each of the five host species tested were as follows: the antibodies in the sera from three different Venezuelan cattle, two U.S. horses, and one Costa Rican horse with pythiosis strongly detected high-molecular-mass immunogens ranging from ≥209 to ∼49 kDa, especially in the strains from the Americas (strains MTPI-04 and MTPI-19), and detected these immunogens in one of the Thai strains tested (strain MTPI-14), but with less intensity (Fig. 2A to C). The antibodies in the bovine sera weakly detected the immunogens mentioned above in the strains from Thailand (MTPI-12), New Guinea (MTPI-21), and Australia (MTPI-24) (Fig. 2A to C, lanes 3, 5, and 6, respectively). A diffuse prominent ∼51-kDa antigen in strains MTPI-04, and MTPI-19 from the Americas and an antigen at ∼49 kDa in strain MTPI-14 from Thailand were also detected by the antibodies of both host species (Fig. 2A to C, lanes 1, 2, and 4, respectively, arrowhead, left side only). Several P. insidiosum immunogens with low molecular masses ranging from ∼40 to ∼25 kDa were also detected with less intensity by the antibodies in bovine serum samples A and B. The detection of these lower-molecular-mass antigens by bovine serum sample C was less evident (Fig. 2). The antibodies in the sera from the three horses readily detected the ∼35- to ∼25-kDa-molecular-mass antigens, especially those in Fig. 2B and C. In contrast, the antibodies in the bovine sera detected only some of these antigens. An antigen located at ∼124 kDa expressed only by strain MTPI-04 was strongly detected by the antibodies in the sera of cattle (Fig. 2A, lane 2). In addition, the ∼28-kDa and ∼34-kDa-molecular-mass immunogens in strains MTPI-04 and MTPI-12, which were not expressed by any of the other strains tested, were detected with different intensities by the antibodies in the sera from some cattle and horses (Fig. 2A to C, lanes 2 and 3, arrows and asterisks, respectively).
Compared to the antibodies in the sera from cattle and horses (Fig. 2), the antibodies in the sera from cats and dogs (Fig. 3) recognized the smallest number of immunogens, especially high-molecular-mass immunogens. For instance, the diffuse ∼51- and ∼49-kDa-molecular-mass antigens were consistently detected by the antibodies in these sera but not in strains MTPI-12 (Thailand), MTPI-21 (New Guinea), and MTPI-24 (Australia) (Fig. 3A to C, lanes 1, 2, and 4, respectively). An exception was the cat serum sample in Fig. 3C, which weakly detected this immunogen in strain MTPI-12 but not in strain MTPI-24. An ∼80-kDa immunogen was detected by the antibodies in the cat and dog sera (Fig. 3A and C, lanes 1, 2, and 3) but not by the antibodies in the other sera. The ∼74- and ∼78-kDa immunogens were strongly detected in all strains except strain MTPI-12 by the dog serum (Fig. 3A). One of these immunogens (∼74 kDa) was weakly detected in the same strains by the cat serum (Fig. 3A, lanes 1 to 3). The ∼28-kDa immunodominant antigen expressed only by strain MTPI-04 was also consistently detected by the antibodies in the sera from the cats and dogs (Fig. 3A to C, lanes 2, arrows). In addition, an ∼46-kDa immunogen was detected by the antibodies in the sera from the three dogs (Fig. 3A to C, lanes 1, 2, 5, and 6) and the three cats (Fig. 3A, lanes 1, 2, 4, and 5; Fig. 3B, lanes 1 and 2; Fig. 3C, lanes 1, 2, and 3). Various other low-molecular-mass immunogens, some of which were very prominent and that ranged in molecular mass from ∼46 to ∼25 kDa, including a ∼38-kDa antigen, were also detected by these sera (Fig. 3A to C, lanes 1 to 6, single arrowheads for both dogs and cats).
The antibodies in the sera from two humans with pythiosis from Thailand (Fig. 4A and B) identified some but not all of the most prominent antigens detected by the sera described above. The antibodies in the sera from the Thai patients (Fig. 4A) and the U.S. patient (Fig. 4C) tested consistently detected an ∼74-kDa-molecular-mass antigen composed of a double band and other high-molecular-mass immunogens in all strains tested, but this antigen was not detected by the antibodies in the human serum for which the results are shown in Fig. 4B. In addition, these sera detected other low-molecular-mass antigens, particularly the prominent ∼28-kDa antigen (Fig. 4A and C, lane 2), an ∼34-kDa antigen (Fig. 4A to C, lane 3), and the ∼38-kDa antigen (Fig. 4A, lane 4). In contrast, the antibodies in the serum from a Thai patient with pythiosis (Fig. 4B) was unable to detect most of the prominent antigens detected by the antibodies in the sera from the two other humans from Thailand and the human from the United States (Fig. 4A and C). In addition, the antibodies in the human serum in Fig. 4B readily detected several low-molecular-mass immunogens, including a double band at ∼30 to ∼32 kDa, and the antibodies in the sera from the other two humans (Fig. 4A and C) and the antibodies from some host species also identified this double band.
The anti-human IgG1, IgG2, and IgG4 monoclonal antibodies detected most of the high- and low-molecular-mass P. insidiosum immunogens identified by the polyclonal antibodies in the Thai human serum used in the gel shown in Fig. 4A (data not shown). The anti-human IgG2 and IgG4 antibodies readily detected the antigens detected by the polyclonal antibodies of host species in all strains except strain MTPI-19, whereas the anti-human IgG1 antibody only mildly detected similar P. insidiosum immunogens. The anti-human IgG3 monoclonal antibody did not react to the anti-P. insidiosum human antibodies coupled to P. insidiosum immunogens on the membranes investigated (data not shown).
DISCUSSION
The protein profiles on the SDS-polyacrylamide gels of the strains analyzed in our study showed similar patterns among geographically diverse isolates (Fig. 1). Strikingly, the protein profiles reported by others who used either Asian or American strains could not be compared to the profiles obtained in this study (2, 5, 18). For instance, Krajaejun et al. (5) and Supabandhu et al. (22), who used Asian strains to prepare their antigens, and Leal et al. (8), who used American isolates to prepare their antigens, reported the detection of different P. insidiosum protein profiles by SDS-PAGE. One possible explanation for this inconsistency might be the fact that they used different concentrations of SDS-polyacrylamide gels (8% or 10%) and different methodologies to culture and extract the P. insidiosum antigens. However, the separation of their P. insidiosum proteins according to their standard markers should not be completely different from that in other studies, but that is not the case. The protein profiles that we detected on 12% SDS-polyacrylamide gels showed that the Asian and the American strains analyzed displayed similar protein profiles, with only minor differences being detected between strains. An exception was Thai strain MTPI-14, which overexpressed a protein at 38 kDa that was not present in the other strains tested. Interestingly, Mendoza et al. (12), using several American strains and a methodology similar to that used in the present study, also showed that the strains had comparable protein profiles.
In the past decade, numerous investigators in Thailand and the Americas have been using local strains of P. insidiosum isolated from cases of pythiosis to investigate their antigenic profiles utilizing the antibodies in the sera of a limited number of host species in serological assays (1, 2, 4-6, 9, 11-14, 17, 18, 22, 25). From the findings of those studies, they concluded that P. insidiosum expresses several immunodominant antigens during infection (5, 8, 9, 12, 16, 22). The main problem so far has been the lack of standardization of the methodologies used to culture and extract the antigens as well as a lack of consistency between laboratories performing the serological assays used to generate the data (9). Krajaejun et al. (5) analyzed 16 Thai P. insidiosum strains and 12 human serum samples from Thai patients with different clinical manifestations of pythiosis and reported the presence of antibodies against the 74-kDa immunodominant antigen in human patients with the disease. They also described the presence in some of the sera analyzed of prominent lower-molecular-mass immunogens that were not properly recognized by the antibodies in the sera detecting the 74-kDa antigen. This and other examples suggest that during infection the immune system of the host species is being stimulated by different types of immunogens in geographically divergent P. insidosum strains, thus generating different WB profiles (2, 5, 12, 16, 22).
Our study found that the antibodies in the sera from the host species analyzed consistently detected immunogens of similar molecular masses among the strains evaluated in this study. Of interest was the detection of a diffuse ∼51-kDa antigen (strains MTPI-19 and MTPI04) and a ∼49-kDa antigen (strain MTPI-14) by antibodies in the sera from cattle, cats, dogs, and horses but not in the sera from humans with the disease. The antibodies in the animal sera detected these antigens on the strains from the Americas with different intensities, but the detection of these antigens on the Asian strains was less evident. Pérez et al. (16) also reported the presence of a similar diffuse antigen in studies with three Venezuelan cattle. However, comparable antigens in different WB studies with antibodies in the sera from other infected cats, cattle, dogs, or horses could not be found (8, 9, 18, 25), supporting our belief for the need for standardization across laboratories for the proper characterization of P. insidosum immunogens. We have made a unique observation that sera from cattle and horses infected with P. insidiosum reacted with high-molecular-mass immunogens expressed by P. insidiosum strains originally isolated from horses in the Americas, whereas strains originally isolated from humans from Asia and the Americas did not. However, the antibodies in the sera from human patients with pythiosis did not show greater reactivity with strains of P. insidiosum isolated from humans than with strains of P. insidiosum isolated from horses. This might suggest that (i) that cattle and horses may have repeated subclinical environmental exposures to different P. insidosum strains during their lifetimes, as reported by some (9, 11); (ii) the reaction is due to the stimulation of the immune system by local strains within the three different phylogenetically described groups (20, 21); and/or (iii) the immune systems of horses could recognize a broader range of P. insidiosum immunogens than those of other species may, as recently reported by Garcia et al. (3).
A similar situation was encountered with the ∼28-kDa immunodominant antigen detected only in strain MTPI-04. With the exception of the Thai patient whose serum weakly detected this antigen (Fig. 4B), this antigen was strongly detected by the antibodies of all sera. Interestingly, the antibodies in the serum of the Thai patient (Fig. 4A) weakly detected this ∼28-kDa immunogen in all P. insidiosum strains, although the band was the most prominent with strain MTPI-04. Camus et al. (2), Krajaejun et al. (5), and Supabandhu et al. (22) also reported the detection of an antigen with a similar molecular mass by antibodies in sera from several Thai human patients and U.S. cats with the disease. Moreover, we believe that the ∼28-kDa-molecular-mass immunodominant protein previously reported by Mendoza et al. (12) is probably the same ∼28-kDa antigen detected in this study. The fact that the ∼28-kDa P. insidiosum antigen was faintly expressed in the stained SDS-polyacrylamide gels suggests that this prominent antigen could play an important role during infection. The finding of antibodies in host species that detect a ∼28-kDa-molecular-mass antigen expressed predominantly by strain MTPI-04 is intriguing and deserves further investigation.
The anti-P. insidiosum antibodies from human sera (Fig. 4A and C) detected the ∼74-kDa protein reported by Krajaejun et al. (5). However, the serum from a Thai patient with arterial pythiosis (23) did not identify this antigen (Fig. 4B), a finding also reported for some of the sera used by Krajaejun et al. (5). The antibodies in the sera from humans and animals with pythiosis did detect the double ∼30- and ∼32-kDa-molecular-mass antigens that Mendoza et al. (12) previously reported to be immunodominant antigens in studies with sera from horses with the disease. These antigens were also expressed by all strains in the stained SDS-polyacrylamide gels (Fig. 1). The detection of these antigens has been long questioned by some (8, 22). However, our data showed that sera from a number of host species, including cats, dogs, horses, and humans, readily detect these twin antigens on the strains evaluated. In addition, other immunodominant proteins were also detected by the antibodies in the human and animal sera evaluated in this study. These included an ∼34-kDa-molecular-mass antigen that was detected by several serum samples on strain MTPI-12 strain but that was less evident on strain MPTI-04; the ∼38-kDa antigen on strain MTPI-14 detected by the antibodies in the sera from cats, dogs, and humans; and a ∼46-kDa antigen on the New Zealand and Australian strains that was mainly detected by the antibodies in the sera from dogs; as well as several other high-molecular-mass P. insidiosum immunogens (≥109 kDa) not previously reported.
The strong detection of the blotted P. insidiosum immunogens by anti-human IgG2 and IgG4 monoclonal antibodies but not by anti-human IgG1 monoclonal antibodies suggests that P. insidiosum presents antigens more likely through a Th2 response in the human patients evaluated (9, 26). The lack of a reaction with the anti-human IgG3 antibody could be related to the fact that the life span of this Ig is only a few hours. In summary, our data show that several prominent immunogenic proteins are detected by the antibodies in sera from geographically diverse host species. We propose the use of standardized techniques, such as the one used in the present study, to continue the characterization of the P. insidiosum immunogens described above and possibly other immunogens not reported in this study. The recognition of these immunodominant proteins and their future characterization could help provide an understanding of the immunological and immunotherapeutic features of P. insidiosum antigens and their potential use for the diagnosis and the management of pythiosis in mammals.
Acknowledgments
We thank Hermes Fernandez-Lopez for performing the IgG subtyping experiments and the many veterinary practitioners who provided some of the serum samples used in this study.
A.C. was supported by a research grant from the Faculty of Medicine, Chulalongkorn University, and L.M. was supported in part by the Biomedical Laboratory Diagnostics Program, Michigan State University.
Footnotes
Published ahead of print on 30 December 2008.
REFERENCES
- 1.Brown, C. C., and J. J. MacClure. 1988. Use of immunohistochemical methods for diagnosis of equine pythiosis. Am. J. Vet. Res. 111866-1868. [PubMed] [Google Scholar]
- 2.Camus, A. C., A. M. Grooters, and R. F. Aquilar. 2004. Granulomatous pneumonia caused by Pythium insidiosum in a central American jaguar, Pantera onca. J. Vet. Diagn. Investig. 16567-571. [DOI] [PubMed] [Google Scholar]
- 3.Garcia, R. B., A. Pastor, and L. Mendoza. 2007. Mapping of Pythium insidiosum hyphal antigens and ultrastructural features using TEM. Mycol. Res. 1111352-1360. [DOI] [PubMed] [Google Scholar]
- 4.Grooters, A. M. 2003. Pythiosis, lagenidiosis, and zygomycosis in small animals. Vet. Clin. N. Am. Small Anim. Pract. 33695-720. [DOI] [PubMed] [Google Scholar]
- 5.Krajaejun, T., M. Kunakorn, R. Pracharktam, P. Chongtrakool, B. Sathapatayavongs, A. Chaiprasert, N. Vanittanakom, A. Chindamporn, and P. Mootsikapun. 2006. Identification of a novel 74-kilodalton immunodominant antigen of Pythium insidiosum recognized by sera from human patients with pythiosis. J. Clin. Microbiol. 441674-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krajaejun, T., M. Kunakorn, S. Sopaporn, P. Chongtrakool, and R. Pracharktam. 2002. Development and evaluation of an in-house enzyme-linked immunosorbent assay for early diagnosis and monitoring of human pythiosis. Clin. Diagn. Lab. Immunol. 9378-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 227680-685. [DOI] [PubMed] [Google Scholar]
- 8.Leal, A. T., J. M. Santurio, A. B. M Leal, J. B. Catto, E. F. Flores, I. Lubeck, and S. H. Alves. 2005. Characterization of the specificity of the humoral response to Pythium insidiosum antigens. J. Mycol. Med. 1563-68. [Google Scholar]
- 9.Mendoza, L., and J. C. Newton. 2005. Immunology and immunotherapy of the infections caused by Pythium insidiosum. Med. Mycol. 43477-486. [DOI] [PubMed] [Google Scholar]
- 10.Mendoza, L., S. H. Prasla, and L. Ajello. 2003. Orbital pythiosis: a non-fungal disease mimicking orbital mycotic infections, with a retrospective review of the literature. Mycoses 4714-23. [DOI] [PubMed] [Google Scholar]
- 11.Mendoza, L., L. Kaufman, W. Mandy, and R. Glass. 1997. Serodiagnosis of human and animal pythiosis using an enzyme-linked immunosorbent assay. Clin. Diagn. Lab. Immunol. 4715-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mendoza, L., N. Nicholson, J. F. Prescott. 1992. Immunoblot analysis of the humoral immune response to Pythium insidiosum in horses with pythiosis. J. Clin. Microbiol. 302980-2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mendoza, L., L. Kaufman, and P. Standard. 1987. Antigenic relationship between the animal and human pathogen Pythium insidiosum and nonpathogenic Pythium species J. Clin. Microbiol. 252159-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller, R. I., and R. S. F. Campbell. 1982. Immunological studies on equine phycomycosis. Aust. Vet. J. 58227-231. [DOI] [PubMed] [Google Scholar]
- 15.Pasavento, P. A., B. Barr, S. M. Riggs, A.L. Eigenheer, R. Pamma, and R. L. Walker. 2008. Cutaneous pythiosis in a nestling white-faced ibis. Vet. Pathol. 45538-541. [DOI] [PubMed] [Google Scholar]
- 16.Pérez, R. C., J. J. Luis-León, J. L. Vivas, and L. Mendoza. 2005. Epizootic pythiosis in beef calves. Vet. Microbiol. 109121-128. [DOI] [PubMed] [Google Scholar]
- 17.Pracharktam, R., P. Changtrakool, B. Sathapatayavongs, P. Jayanetra, and L. Ajello. 1991. Immunodiffusion test for diagnosis and monitoring of human pythiosis insidiosi. J. Clin. Microbiol. 292661-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rakich, P. M., A. M. Grooters, and K. N. Tang. 2005. Gastrointestinal pythiosis in two cats J. Vet. Diagn. Investig. 17262-269. [DOI] [PubMed] [Google Scholar]
- 19.Santurio, J. M., A. T. Leal, A. B. M. Leal, R. Festugatto, I. Lubeck, E. S. V. Sallis, M. V. Copetti, S. H. Alves, and L. Ferreiro. 2003. Three types of immunotherapeutics against pythiosis insidiosi developed and evaluated. Vaccine 212535-2540. [DOI] [PubMed] [Google Scholar]
- 20.Schurko, A. M., L. Mendoza, C. A. Lévesque, N. L. Désaulniers, A. W. de Cock, and G. R. Klassen. 2003. A molecular phylogeny of Pythium insidiosum. Mycol. Res. 107537-544. [DOI] [PubMed] [Google Scholar]
- 21.Schurko, A. M., L. Mendoza, A. W. A. M. de Cock, and G. R. Klassen. 2003. Molecular genetic differences between strains of Pythium insidiosum, from Asia, Australia, and the Americas: evidence for geographic clusters. Mycologia 95200-2008. [PubMed] [Google Scholar]
- 22.Supabandhu, J., M. C. Fischer, L. Mendoza, and N. Vanittanakom. 2008. Isolation and identification of the human pathogen Pythium insidiosum from environmental samples collected in Thai agricultural areas. Med. Mycol. 4641-52. [DOI] [PubMed] [Google Scholar]
- 23.Thitithanyanont, A., L. Mendoza, A. Chuansumrit, R. Pracharktam, J. Laothamatas, B. Sathapatayavongs, S. Loleka, and L. Ajello. 1998. Use of an immunotherapeutic vaccine to treat a life-threatening human arteritic infection caused by Pythium insidiosum Clin. Infect. Dis. 271394-1400. [DOI] [PubMed] [Google Scholar]
- 24.Towbin, H., T. Stahelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 764350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vanittanakom, N., J. Supabandhu, C. Khamwa, J. Praparattanapan, S. Thirach, N. Prasertwitayakij, W. Louthrenoo, S. Chiewchanvit, and N. Tananuvat. 2004. Identification of emerging human-pathogenic Pythium insidiosum by serological and molecular assay-based methods. J. Clin. Microbiol. 423970-3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wanachiwanawin, W., M. Thianprasit, S. Fucharoen, A. Chaiprasert, N. Sudasna, N. Ayudha, N. Sirithanaratkul, and A. Piankijagum. 1993. Fatal arteritis due to Pythium insidiosum infection in patients with thalassaemia. Trans. R. Soc. Trop. Med. Hyg. 87296-298. [DOI] [PubMed] [Google Scholar]
- 27.Witkamp, J. 1924. Bijdrage tot de kennis van der hyphomycosis destruens. Ned. Ind. Bland. Diergeaeskd. Dierenteelt. 36229-345. [Google Scholar]