Abstract
Pneumovax 23 consists of a mixture of highly purified capsular polysaccharides (Ps) from 23 of the most prevalent serotypes of Streptococcus pneumoniae. Testing of vaccine immunogenicity has been historically performed on the enzyme-linked immunosorbent assay (ELISA) platform, validated to measure immunoglobulin G (IgG) antibodies to all 23 serotypes included in Pneumovax 23. In order to significantly improve the throughput of this form of testing, we have developed and validated a direct binding electrochemiluminescence (ECL)-based multiplex assay that can measure the antibody response in human serum to eight serotypes within a single microtiter well. The pneumococcal (Pn) ECL assay is based on the Meso Scale Discovery (MSD) technology which utilizes a Sulfo-Tag-labeled anti-human IgG antibody that emits light upon electrochemical stimulation. The Pn ECL assay exhibits a wide dynamic range and provides the ability to read concentrations down to the minimum reported concentration in the Merck ELISA (0.1 μg/ml). Cross-reactivity assessment using type-specific monoclonal antibodies showed no cross talk between antigen spots within a well. By use of the WHO Pn sample reference panel, the results obtained with the Pn ECL assay were compared to the results obtained with the international Pn ELISA. The results for the Pn ECL assay satisfied the WHO-recommended acceptance criterion for concordance for all seven serotypes with published Pn ELISA values, and the overall correlation (r value) across the seven serotypes was 0.994. The MSD methodology has great potential to be extremely useful for simultaneously quantitating IgG responses to several Pn serotypes while conserving serum volumes and laboratory testing time.
In the United States, there are currently two licensed vaccines available for the prevention of Streptococcus pneumoniae disease. These are Pneumovax 23 (Merck, Whitehouse Station, NJ), a liquid vaccine which consists of a mixture of highly purified capsular polysaccharides (CPs) from 23 of the most prevalent or invasive serotypes of Streptococcus pneumoniae, and Prevnar (Wyeth, Philadelphia, PA), a seven-valent pneumococcal Ps (PnPS) conjugate vaccine approved for use in infants and children (2). These vaccines have had a substantial impact in reducing invasive Pn disease and have significantly improved human health (25, 28).
At Merck & Co., the immunogenicity of Pneumovax 23 in early clinical trials was determined using a radioimmunoassay to measure antibodies to the PnPs. However, due to the practicality of supporting large-scale testing, the enzyme-linked immunosorbent assay (ELISA) became the preferred method for estimating antibody concentrations. In this respect, Merck has developed and validated individual ELISAs for all 23 serotypes in the vaccine (16). However, ELISAs are both time- and labor-intensive and use considerable volumes of serum, which is often limited. For these reasons, and in order to improve assay throughput for the testing of a large number of samples across a broad array of antigens, we have recently developed and characterized a direct binding electrochemiluminescence (ECL)-based multiplex assay for the simultaneous quantitation of immunoglobulin G (IgG) responses to selected Pn serotypes in Pneumovax 23.
ECL-based techniques provide an alternative to conventional colorimetric methods (1, 4, 9), allowing high sensitivity, good reproducibility, and generally low levels of interference from components in complex matrices such as serum or plasma (1, 9, 26). The Pn ECL assay that we have developed is based on the Meso Scale Discovery (MSD) technology which employs disposable multispot microtiter plates (multiarray plates; MSD, Gaithersburg, MD) that include integrated screen-printed carbon ink electrodes on the bottom of the wells. In contrast to other multiplex platforms, such as Luminex, the ECL technology is advantageous in that it does not require conjugation of the PnPs to the solid phase, since Ps directly bind to the carbon surface, minimizing the potential impact on Ps antigenicity. In this sense, the ECL platform more closely resembles the World Health Organization (WHO) reference ELISA (17).
A design-of-experiment (DOE) statistical approach was used to determine the optimal settings and conditions for the major controllable factors in the assay prior to validation. The optimized Pn IgG ECL assay exhibits a wide dynamic range and provides the ability to read concentrations down to the minimum reported concentration in the Merck ELISA (0.1 μg/ml). The assay was validated based on guidelines provided by the U.S. Food and Drug Administration (FDA; 5) and the International Conference on Harmonization on the Validation of Analytical Methods (14). The following assay characteristics were assessed as part of the validation testing: the quantifiable range of the standard curve, assay precision, assay specificity, the ruggedness of the assay to alternative analysts and reagent lots, and the effect of test sample dilution on response. Also, as part of the validation study, Pn antibody concentrations generated using the Pn ECL assay were compared to those generated by the WHO reference ELISA.
In this study, we describe the optimization, validation, and performance of the Merck Pn ECL-based assay for measuring antibodies to eight Pn serotypes (serotypes 3, 4, 6B, 9V, 14, 18C, 19F, and 23F) and their correlation with those of the WHO international Pn ELISA (17, 27). This new platform appears highly useful for measuring immune responses to PnPs-containing vaccines.
MATERIALS AND METHODS
CPs.
CPs is a Pn cell wall Ps (CPs used in this study obtained from the Statens Serum Institut, Copenhagen, Denmark). It is common to all Pn serotypes and is used for preadsorbing human serum samples before quantitation of serotype-specific Pn CPs antibodies in order to reduce reactivity to the CPs that cannot be differentiated from reactivity to the Pn serotype-specific CPs.
PnPs.
All PnPs powders for serotypes 3, 4, 6B, 9V, 14, 18C, 19F, 23F, 25, and 72 were manufactured and received from Merck Manufacturing Division, West Point, PA. Each PnPs was reconstituted in sterilized pyrogen-free water. The final concentration for each PnPs following reconstitution was 1 mg/ml. The serotype 25 and 72 PnPs (PnPs25 and PnPs72) are utilized in the Pn ECL assay for serum preadsorption in order to improve assay specificity.
Standard sera.
The U.S. FDA Pn reference standard, lot 89SF-2 (Lederle-Praxis Biologicals) was prepared from 17 individual serum samples from adults with high antibody titers following vaccination with Pnu-Imune (a 23-valent Pn vaccine; Lederle), Menomune (a meningococcal Ps vaccine; Connaught), and ProHIBIT (a Haemophilus influenzae conjugate vaccine; Connaught).
Pn WHO QC panel (Goldblatt sera).
The Pn quality control (QC) sera distributed by the National Institute for Biological Standards and Control (NIBSC; code 04-238, version 3), consists of 12 calibration serum samples with known, assigned antibody concentrations in the international ELISA (17, 21). The Center for Biologics Evaluation and Research (CBER; part of the FDA) and the WHO developed this panel from adults vaccinated with a 23-valent PnPs vaccine, and a collaborative study was organized by D. Goldblatt at the Institute for Child Health, London, United Kingdom, to determine the antibody titer of this reference material (21).
Giebink sera.
Serum obtained from G. S. Giebink, University of Minnesota, is human immune serum from an adult following vaccination with Pneumovax 23. The Giebink serum is the Pn ECL assay control tested at three (10-fold serial) dilutions on each plate within an assay run (1:1,000, 1:10,000, and 1:100,000 dilutions).
MSD assay method overview.
MSD technology is based on ECL detection which utilizes a Sulfo-Tag label that emits light upon electrochemical stimulation. The mechanism for generation of ECL from ruthenium tris(bipyridine) complexes at an oxidizing electrode in the presence of tripropylamine read buffer has been previously documented (4). With a dedicated ECL plate reader, an electrical current is placed across the plate-associated electrodes, resulting in a series of electrically induced reactions leading to a luminescent signal. The multispot configuration used in development and validation was 10 spots/well in a 96-well plate format. Each well was coated with 5 ng PnPs per spot (unless specified otherwise for optimization studies) of the following serotypes: 3, 4, 6B, 9V, 14, 18C, 19F, and 23F. Each well also contained two bovine serum albumin (BSA) spots, which were used to assess the background reactivity of the assay (i.e., the response associated with serum and labeled secondary antibody in the absence of PnPs). Assay standard (89SF-2), controls, and test sera were diluted at appropriate dilutions in phosphate-buffered saline containing 0.05% Tween 20 (PBS-T), 1% BSA, 5 μg/ml CPs, 10 μg/ml Pn25, and 10 μg/ml Pn72 and incubated overnight at 4°C (2 to 8°C) or at ambient temperature for 45 min. Each antigen-coated plate was incubated at ambient temperature for 1 h on a shaker platform with blocking agent. Plates were washed with 0.05% PBS-T, and 25 μl per well of the preadsorbed and diluted test sera was added and incubated for 45 min at ambient temperature on a shaker platform. Plates were washed with 0.05% PBS-T, and MSD Sulfo-Tag-labeled goat anti-human IgG secondary antibody was added to each well and incubated 1 h at ambient temperature on a shaker platform. Plates were washed with 0.05% PBS-T, and 150 μl of MSD Read Buffer-T 4X (with surfactant) diluted 1:4 in water was added to each well. The plates were read using a MSD sector imager model no. 2400 or 6000.
Assay optimization experiments.
A DOE statistical approach was used to determine the optimal settings and conditions for the major controllable factors in the assay prior to validation. The following five major assay conditions were jointly assessed for optimization of the Pn MSD assay: blocking reagent (5% BSA, 1% human serum albumin [HSA], and SuperBlock buffer [Pierce]), antigen concentration (2.5 ng/spot and 7.5 ng/spot), incubation time for secondary antibody (30 min and 2 h), incubation time for samples (30 min and 2 h), and secondary antibody concentration (0.5 μg/ml and 2.0 μg/ml). Twelve samples consisting of matching pairs of pre- and postimmunization sera from adults in a previously completed Pneumovax 23 clinical trial were tested at 1:50, 1:250, and 1:1,250 dilutions on each of 24 plates, with each plate representing a unique experimental condition (e.g., 1% HSA, 2.5 ng/spot, 2-h incubation of secondary antibody, 30-min sample incubation, and 2.0-μg/ml secondary antibody concentration). A second optimization experiment using DOE methodology was performed to evaluate alternative formulations of the serum diluent in an attempt to further reduce the background reactivity in the assay without diminishing the detection of antigen-specific response. The following four components of the serum diluent were evaluated: Tween 20, PBS, Triton X-100, and EDTA. Two levels of each component were tested in a full-factorial fashion (all 16 [24] combinations of the components and component levels were tested), providing a total of 16 different formulations for evaluation. Each diluent formulation also included 1% BSA, 5 μg/ml CPs, 10 μg/ml Pn25, and 10 μg/ml Pn72. Each formulation was utilized to test a sample panel consisting of the reference serum 89SF-2, six matching pairs of pre- and postvaccination samples from adults from a previously completed Pneumovax 23 clinical trial, and three pediatric samples (from children 7 to 8 months old) purchased from an outside vendor.
Assay validation experiments.
To characterize the Pn ECL assay, an extensive validation study was undertaken. The multispot configuration used in validation was 10 spots/well in a 96-well plate format. A total of 20 runs (16 runs in part 1 and 4 runs in part 2) were performed, spanning two analysts, two lots of Sulfo-Tag-labeled secondary antibody (representing two labeling events), two lots of custom antigen-coated plates, and two lots of MSD blocker A (5% BSA). Within part 1, the runs were performed in a full-factorial fashion such that all 16 possible combinations of analyst, secondary antibody lot, plate lot, and BSA lot were tested. Twenty-five matched pre- and postvaccination adult samples (50 total) and 24 pediatric samples were selected for the validation study. Table 1 details the sample panels used in the validation.
TABLE 1.
Description of sample panels used in the validation of the Pn ECL assay
| Panel | Sample type (parameter) | Description/age group | No. of samples |
|---|---|---|---|
| A | Adult (ruggedness and precision) | Matched pre- and postvaccination adult samples from 50 subjects (≥50 yr of age) randomly selected from a Merck clinical protocol with 23-valent Pneumovax 23 | 25 (prevaccination), 25 (postvaccination) |
| B | Adult (dilutability) | Post-vaccination adult samples randomly selected from a Merck clinical protocol with 23-valent Pneumovax 23 (≥50 yr of age) | 24 (postvaccination) |
| C | Adult (specificity) | Post-vaccination adult samples randomly selected from a Merck clinical protocol with 23-valent Pneumovax 23 (≥50 yr of age) | 6 (postvaccination)/run |
| D | Pediatric (dilutability) | Human serum from vaccinated 7-8-mo-old infants (after dose 3 with Prevnar, not confirmed), obtained from an outside vendor | 12 |
| E | Pediatric (specificity) | Human serum from vaccinated 7-8-mo-old infants (after dose 3 with Prevnar, not confirmed), obtained from an outside vendor | 6/run |
| F | Pneumococcal WHO QC panel, serum set NIBSC code 04-238 | Human immune sera from adult individuals following vaccination with Pneumovax 23 | 12 (adult human sera) |
Assay validation parameters and statistical methods. (i) Test sample calibration and standard curve modeling.
Test sample concentrations were determined by referencing the ECL responses of the samples against a standard curve generated from the serially diluted 89SF-2 reference serum. A constrained (minimum parameter, >0) four-parameter logistic regression function was fit to the standard curve. The four-parameter equation has the form
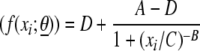 |
where xi denotes the concentration of the ith dilution of the reference serum, θ denotes the parameter vector [A, B, C, D], and A, B, C, and D are the four parameters. The A and D parameters correspond to the upper and lower asymptotes for the function, respectively. The B and C parameters correspond to the slope and the concentration corresponding to the 50% response, respectively. The fit was carried out using the NLIN procedure in SAS by identifying the parameter values that minimize the weighted residual sum of squares. The weighting function utilized in the iterative fit was chosen according to the variability exhibited by the response and is given by the function (f(xi;θ̂))−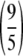 , with θ̂ being the vector of parameter estimates.
, with θ̂ being the vector of parameter estimates.
(ii) Quantifiable range.
The quantifiable range is the concentration range of the standard over which the coefficient of variation (relative standard deviation [RSD]) of a calibrated concentration is less than a fixed percent. The lower and upper limits of quantitation (LOQs) were determined according to the method specified by O'Connell et al. (18). Additionally, to ensure that acceptable precision (RSD, ≤25%) was achieved throughout the determined quantifiable range, precision profiles based on the calibrated concentrations of the test samples were constructed (7).
(iii) Ruggedness.
The ruggedness of the assay to various conditions, namely, analysts, lots of labeled total IgG secondary antibody, BSA, and antigen-coated custom plates, was assessed based on the performance of the samples that were tested across each of the 16 runs in part 1 of the validation. Analysis of variance was used to estimate the differences between the levels of the ruggedness factors. Panel A was used for the ruggedness assessment (Table 1).
(iv) Precision.
Intra-assay variability is the within-run variation that represents the repeatability of the assay under the same conditions, whereas interassay variability represents the between-run variation attributable to different days, analysts, and reagents, etc. The total assay precision is the sum of both the intra- and interassay variabilities. Interassay precision was estimated by testing the same set of adult test samples (n = 50) across multiple assay runs, and intra-assay precision was estimated by testing the same Giebink control on each of the plates within each run. The intra- and interassay variability estimates were obtained by variance component analysis. Panel A was used for the precision assessment (Table 1).
(v) Dilutability (parallelism).
Dilutability is an attribute of a biological assay which demonstrates that when a test sample is diluted through a series, equivalent dilution-corrected concentrations can be obtained across that series. The minimum required test sample dilution for the Pn ECL assay is 1:1,000. Adult and pediatric samples (panels B and D described in Table 1) were tested at dilutions of 1:1,000, 1:10,000, and 1:100,000. Dilutability of the assay between the 1:1,000 and 1:10,000 dilutions was estimated by the average difference (n-fold) in concentration between samples tested at the 1:10,000 dilution and samples tested at the 1:1,000 dilution based on the subset of samples having quantifiable values at both the 1:1,000 and 1:10,000 dilutions. Dilutability of the assay between the 1:10,000 and 1:100,000 dilutions was similarly evaluated.
(vi) Analytical specificity.
Specificity is the ability of an analytical method to measure only the component it purports to measure unequivocally in the presence of components that may be expected to be present in the sample. The assay specificity was determined by competitive inhibition. A total of 24 samples from adults (6 samples/run, panel C; Table 1), the reference 89SF-2 serum, and 24 pediatric samples (6 samples/run, panel E; Table 1) were preincubated individually with 0 or 1 μg of PnPs of each of the eight serotypes spotted on the MSD plate for 1 h. For a control, the sera were also preincubated with an irrelevant Ps (Haemophilus influenzae type b [Hib] polyribitol phosphate). The preadsorbed sera were then tested in the serology assay in accordance with the protocol described above. ECL units were measured for all samples and concentrations were interpolated from a standard curve. The percentage of inhibition was calculated by using the formula 1 − [(competed sample concentration − X)/(uncompeted sample concentration − X)], where X denotes the lowest determinable concentration for the serotype under study.
(vii) Quantifying the effect of the preadsorbent on 89SF-2.
The standard curve reference serum for this assay is the Pn international reference standard 89SF-2. IgG concentrations have been assigned to each of the type-specific antibodies in 89SF-2 by Quataert et al. (22, 23). Quataert values were assigned without preadsorption of the standard with either 22F or Pn25 and Pn72. Since, in the Merck Pn ECL assay, the 89SF-2 standard is preadsorbed with the same preadsorbents as the test sample, that is, heterologous serotypes Pn25 and Pn72 in addition to CPs, validation testing included an assessment of the impact of the CPs, Pn25, and Pn72 preadsorbents on the Pn antibody concentrations of 89SF-2 relative to preadsorption with CPs alone. For this purpose, two standard curves were generated within each validation run; one standard was preadsorbed with CPs alone and the other standard was preadsorbed with the Merck preadsorbent (CPs, PnPs25, and PnPs72).
(viii) Accuracy (comparison to published results of WHO ELISA).
Accuracy is a measure of the closeness of agreement of a test result obtained by an analytical method to its theoretical true value or the accepted reference/standard value. The accuracy of the Pn ECL assay was assessed by comparing the results from the Pn ECL assay to published results obtained using the international WHO ELISA for the Pn WHO QC panel comprised of the 12 Goldblatt sera. Per a WHO working group, for a Pn IgG ELISA to be considered acceptably accurate, 9 of the 12 WHO QC sera need to be within ±40% of their assigned concentrations (21, 27). The 12 WHO QC sera were run across the first 16 runs of the validation at dilutions of 1:1,000 and 1:100,000. Observed concentrations for each of the 12 samples were averaged across the 16 runs for each serotype. These geometric mean concentrations (GMCs) were also adjusted using potencies from Table 3 (generated during assay validation) to account for the effect of the additional preadsorbents on the reference standard.
TABLE 3.
Relative potency estimates of international reference standard lot 89SF-2 for eight Pn serotypes run with and without preadsorption of heterologous Ps25 and Ps72a
| Serotype | Slope ratio estimate (95% CI) | Relative potency estimate (95% CI) |
|---|---|---|
| 3 | 1.00 (0.99, 1.01) | 1.00 (0.98, 1.03) |
| 4 | 1.01 (1.00, 1.02) | 1.54 (1.49, 1.59) |
| 6B | 1.01 (1.00, 1.01) | 1.07 (1.05, 1.10) |
| 9V | 1.00 (1.00, 1.01) | 1.14 (1.10, 1.17) |
| 14 | 1.01 (1.00, 1.01) | 1.02 (1.00, 1.05) |
| 18C | 1.01 (1.00, 1.02) | 1.09 (1.07, 1.12) |
| 19F | 1.02 (1.01, 1.03) | 1.17 (1.14, 1.19) |
| 23F | 1.01 (1.01, 1.02) | 1.04 (1.01, 1.07) |
Estimates are based on the ratio of test values (CPs only) to reference standard values (CPs, Pn25, and Pn72).
RESULTS
Pn ECL assay development and optimization.
Eight PnPs serotypes were immobilized on the surface of 10-plex 96-well carbon plates via absorption binding by the manufacturer (MSD). With a preliminary concentration of antigen and secondary antibody targeted (data not shown), we evaluated the effect of the most critical assay incubation times and reagents on assay sensitivity, background reactivity, dynamic range, and precision. The goal was to identify the conditions that provide (i) the ability to read concentrations down to the minimum reported concentration in the Merck ELISA (0.1 μg/ml), (ii) a wide dynamic range for the standard curve, and (iii) reduction of nonspecific background reactivity. Table 2 highlights the assay conditions evaluated, as well as the conditions recommended for further development. When we assessed the reactivity of the background spot associated with each well (i.e., serum plus labeled secondary antibody in the absence of PnPs), the data indicated that the optimal blocking buffer is BSA (purchased as MSD blocker A, catalog no. R93AA-1), as it significantly reduced background reactivity. Background ECL signals when using HSA or SuperBlock buffer for plate coating were approximately 1.8-fold higher than ECL signals obtained when using BSA. There were no differences among the blocking buffers for the PnPs-coated wells. In the comparison between the 2.5-ng/spot and 7.5-ng/spot PnPs antigen conditions, there was no significant effect on test sample response, and therefore, we chose 5 ng/spot for all types. For each of the eight serotypes, the ECL assay provided for the ability to read concentrations down to the minimum reported concentration in the Merck ELISA (0.1 μg/ml) and for a dynamic range exceeding 100-fold.
TABLE 2.
Parameters tested in the Pn ECL assay
| Exptl condition or diluent component | Values or agents tested | Recommended value or agenta |
|---|---|---|
| Exptl condition | ||
| Antigen concn (ng/spot) | 2.5, 7.5 | 5 |
| Plate blocking agent | BSA, HSA, SuperBlock buffer | BSA |
| Sample incubation time | 30 min, 2 h | 45 min |
| Secondary antibody concn (μg/ml) | 0.5, 2.0 | 2.0 |
| Secondary antibody incubation time | 30 min, 2 h | 1 h |
| Diluent componentb | ||
| PBS (M NaCl) | 0.3, 0.15 | 0.15 |
| Tween 20 (%) | 0.5, 0.05 | 0.05 |
| Triton X-100 (%) | 0, 0.05 | 0 |
| EDTA | 0%, 5 mM | 0% |
For the experimental antigen concentrations, sample incubation times, and secondary antibody incubation times, there was no marked advantage among the factor levels evaluated in the experiment, and therefore, an intermediate level was recommended for subsequent assay development.
For the diluent component, assay conditions were tested on a separate DOE with the goal of reducing reactivity in the assay background wells (i.e., serum plus labeled secondary antibody in the absence of PnPs coating) associated with each well.
Additional experiments were performed in an attempt to further reduce the background reactivity in the assay without diminishing the specific response. For this purpose, alternative levels of four components of the serum diluent (Tween 20, PBS, Triton X-100, and EDTA) were evaluated (Table 2). Two levels of each component were tested in a full-factorial fashion providing for a total of 16 different formulations for evaluation. Each diluent formulation also included 1% BSA, 5 μg/ml CPs, 10 μg/ml Pn25, and 10 μg/ml Pn72. Each formulation was utilized to test a sample panel consisting of the reference serum 89SF-2, human serum from vaccinated infants (unconfirmed Prevnar immunization), and matching pairs of pre- and postvaccination adult samples randomly selected from a clinical protocol with 23-valent Pneumovax 23. The reactivity in the assay background wells (i.e., serum plus labeled secondary antibody in the absence of PnPs coating) associated with each well was assessed across the various serum diluent conditions. Overall, the serum diluent consisting of 0.05% Tween 20 and PBS at 0.15 M NaCl in addition to 1% BSA, 5 μg/ml CPs, 10 μg/ml Pn25, and 10 μg/ml Pn72 resulted in background titers about 1.25-fold lower than those generated under most of the other conditions evaluated, while maintaining a >95% specificity when tested on the reference serum 89SF-2.
Assay spot cross-reactivity assessment.
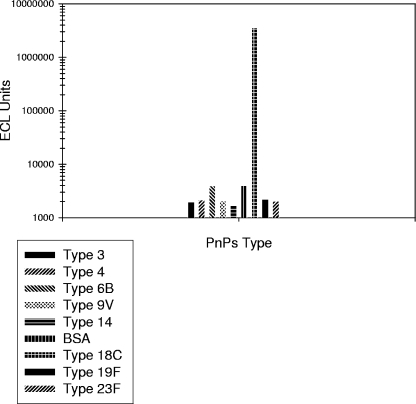
Monoclonal antibodies (MAbs) specific to PnPs serotypes 4, 6B, 14, and 18C (provided by Moon Nahm, University of Alabama) were tested to assess cross-reactivity among the antigen-coated spots on the multiplex plates. Results showed no cross talk among spots within a well, as the signal exhibited by the spot of the corresponding MAb type was at least 100-fold higher than the signal exhibited by the heterologous spots. Figure 1 illustrates the cross-reactivity data for the MAb specific to 18C. Data also confirm that the antigenicity of the coated Ps is preserved upon binding to the plates, since the Ps-specific MAbs were able to successfully bind to their corresponding antigen.
FIG. 1.
Representative example of the Pn ECL cross-reactivity assessment for serotype 18C via the 10-plex MSD technology.
Standard curve potency adjustment.
For each serotype, the measure of the effect of the Merck preadsorbent (Pn25, Pn72, and CPs) on 89SF-2 beyond that due to preadsorption with CPs alone is given by the relative potency estimates provided in Table 3. The corresponding slope ratios are also provided in Table 3 and indicate that the standard curve generated using the Merck preadsorbent dilutes in parallel to the standard curve using CPs alone as the preadsorbent. The estimated potencies of the standard preadsorbed with CPs alone relative to those of the standard preadsorbed with the Merck preadsorbents ranged from 1.00 for serotype 3 to 1.54 for serotype 4. The relative potency estimates in Table 3, obtained for each of the eight serotypes, will be used to adjust the 89SF-2 standard reference concentration to account for the effect of the additional preadsorbents utilized in the Pn ECL assay.
Operating characteristics of the Pn ECL IgG antibody assay determined through validation.
The set of experimental runs performed in the validation of the Pn ECL IgG assay is described in the Materials and Methods section. The results of the validation, presented below, were used to define the performance characteristics of the Pn ECL IgG assay. The validation testing was performed across a 20-day period and was conducted by two analysts using multiple reagent lots.
Standard curve quantifiable range.
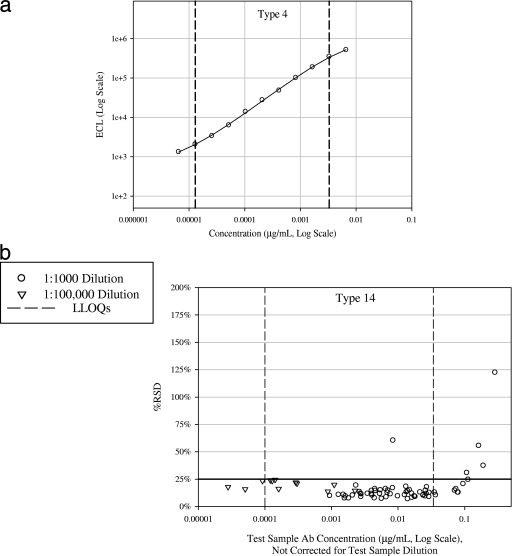
Within each validation run, the 89SF-2 standard was tested at 11 twofold serial dilutions starting at 1:400. The standard curve was fit according to the procedures noted in the Materials and Methods section. The lower and upper LOQs were defined as the lowest and highest concentrations between which test samples could be measured with acceptable precision (RSD, ≤25%). For each serotype, the lower LOQ extended to <0.0001 μg/ml (i.e., 0.1 μg/ml for a sample tested at the 1:1,000 minimum required test sample dilution), and the upper LOQ extended to at least 100-fold the lower limit of quantitation. Thus, standard curves for all eight serotypes exhibit a wide dynamic range (>100-fold) and provide the ability to read concentrations down to the minimum reported concentration in the Merck ELISA (0.1 μg/ml). Table 4 lists the estimated assay limits of detection (LOD) and LOQs by serotype obtained from the validation. During routine testing, samples with concentrations above or below the LOQs would be retested at higher or lower dilutions as necessary. The fitted standard curve and the test sample precision profile are illustrated in Fig. 2a and b, respectively, for serotype 14.
TABLE 4.
Assay limits of detection and limits of quantitation by serotype
| Serotype | Without dilution correction
|
With 1:1,000 dilution correction
|
||
|---|---|---|---|---|
| LOD | LOQs | LOD | LOQs | |
| 3 | 0.000012 | 0.000012, 0.0030 | 0.012 | 0.012, 2.95 |
| 4 | 0.000008 | 0.000013, 0.0033 | 0.008 | 0.013, 3.30 |
| 6B | 0.000050 | 0.000077, 0.0197 | 0.050 | 0.077, 19.71 |
| 9V | 0.000016 | 0.000030, 0.0076 | 0.016 | 0.030, 7.57 |
| 14 | 0.000066 | 0.000100, 0.0340 | 0.066 | 0.100, 34.02 |
| 18C | 0.000010 | 0.000020, 0.0051 | 0.010 | 0.020, 5.11 |
| 19F | 0.000037 | 0.000054, 0.0139 | 0.037 | 0.054, 13.86 |
| 23F | 0.000019 | 0.000038, 0.0098 | 0.019 | 0.038, 9.78 |
FIG. 2.
(a) Pn ECL reference curve for serotype 14. Dashed lines represent lower and upper LOQs. Serotype 14 was randomly chosen for illustration purposes. (b) Pn ECL precision profile for serotype 14 for samples tested at the 1:1,000 (n = 62) and 1:100,000 (n = 12) dilutions. Ab, antibody.
Assay ruggedness.
For each serotype, the assay was considered acceptably rugged to different analysts, BSA lots, plate lots, and secondary antibody labeling events (differences among factor levels were within 1.15-fold for samples tested at the 1:1,000 dilution and within 1.4-fold for samples tested at the 1:100,000 dilution).
Dilutability (parallelism).
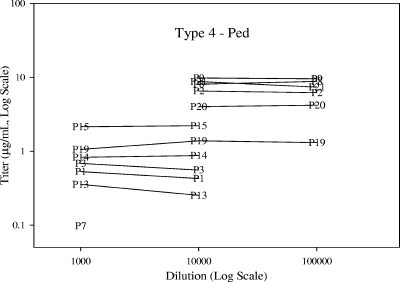
Dilution effect estimates and accompanying tests of statistical significance are provided by serotype and sample set in Table 5. No meaningful differences in dilutability were observed between the pediatric and adult samples. For all eight serotypes, the assay was considered to be acceptably dilutable between dilutions of 1:1,000 and 1:100,000, meeting the preacceptance criterion that the dilution bias per 10-fold dilution needed to be less than twofold. In fact, the majority of dilution effect estimates between the 1:1,000 and 1:10,000 dilutions and between the 1:10,000 and 1:100,000 dilutions were within ±10%. Figure 3 illustrates a representative example of assay dilutability.
TABLE 5.
Dilution effect estimates and accompanying tests of statistical significancea
| Serotype | Panel | 1:1,000 to 1:10,000
|
1:10,000 to 1:100,000
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of subjects | Dilution effectb (%) | 95% CI (%) | Paired t test P value | No. of subjects | Dilution effect (%) | 95% CI (%) | Paired t test P value | ||
| 3 | B | 3 | 14.2 | 3.6, 25.8 | 0.028 | 19 | 1.3 | −10.1, 14.3 | 0.820 |
| D | 9 | −17.8 | −32.9, 0.7 | 0.056 | |||||
| 4 | B | 8 | 8.9 | −4.1, 23.7 | 0.158 | 22 | −3.5 | −12.0, 5.9 | 0.436 |
| D | 6 | −6.7 | −26.3, 18.3 | 0.488 | 6 | −2.9 | −11.6, 6.7 | 0.461 | |
| 6B | B | 15 | 4.3 | −3.7, 13.0 | 0.280 | 15 | 3.0 | −3.1, 9.5 | 0.316 |
| D | 6 | −3.6 | −23.6, 21.7 | 0.706 | |||||
| 9V | B | 9 | 5.2 | −3.8, 14.9 | 0.226 | 20 | −3.5 | −13.1, 7.2 | 0.488 |
| D | 8 | 3.6 | −9.1, 18.1 | 0.540 | 5 | −17.8 | −42.4, 17.3 | 0.200 | |
| 14 | B | 15 | 7.7 | 2.5, 13.2 | 0.006 | 18 | 2.7 | −3.9, 9.9 | 0.408 |
| D | 10 | −1.7 | −11.4, 9.0 | 0.716 | 2 | −26.2 | −90.5, 470.8 | 0.310 | |
| 18C | B | 4 | 12.2 | −6.0, 34.0 | 0.131 | 23 | 9.3 | 2.7, 16.4 | 0.007 |
| D | 11 | 9.3 | −5.7, 26.7 | 0.211 | 7 | −4.1 | −15.4, 8.8 | 0.452 | |
| 19F | B | 16 | 13.6 | 6.4, 21.2 | <0.001 | 21 | 1.8 | −6.1, 10.4 | 0.646 |
| D | 10 | 2.9 | −16.0, 26.2 | 0.756 | 3 | −6.4 | −34.8, 34.5 | 0.517 | |
| 23F | B | 13 | 10.5 | 3.9, 17.5 | 0.004 | 18 | 2.0 | −9.0, 14.3 | 0.719 |
| D | 9 | −8.6 | −16.8, 0.4 | 0.059 | |||||
Panels B (adults) and D (pediatrics) are described in Table 1. 95% CI, 95% confidence interval.
Dilution effects describe the effect per 10-fold dilution.
FIG. 3.
Representative example of Pn ECL dilutability assessment. Ped, pediatric. P1 to P21 indicate the pediatric samples.
Precision.
Overall, the variability observed for samples tested at the 1:100,000 dilutions was greater than that observed for samples tested at the 1:1,000 and 1:10,000 dilutions. For test samples diluted 1:1,000 and control samples diluted 1:1,000 and 1:10,000, the total assay precision (RSD) was less than 20% for each serotype. The total precision of samples diluted 1:100,000 ranged between 22.6% and 41.7% across the eight serotypes.
Specificity.
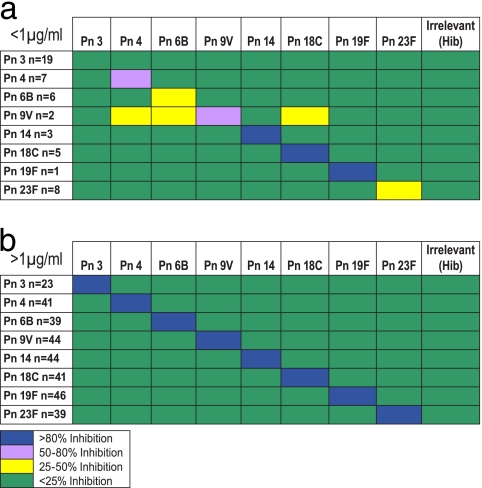
Preincubation of sera with an irrelevant Ps, Hib polyribitol phosphate, resulted in less than 20% inhibition of binding to the solid phase (measured as a reduction in concentration). Furthermore, inhibition of binding by preincubation with heterologous serotypes resulted in <15% inhibition. For seven of the eight serotypes, preincubation of sera with homologous serotypes resulted in ≥87% inhibition (68% inhibition for type 3) across the entire set of samples analyzed (≥45 pediatric and adult samples). For the 89SF-2 reference serum, homologous competition for all eight serotypes was 97% or greater. In general, for samples having endogenous concentrations below 1 μg/ml, a lesser degree of inhibition was generally seen during homologous competition, with the smallest degrees of inhibition being those for serotypes 3 and 6B and the largest degrees of inhibition being those for serotypes 14 and 18C (>90% competition for samples below 1 μg/ml) (Fig. 4).
FIG. 4.
Cross-specificity of homologous and heterologous PnPs for samples having endogenous concentrations below 1 μg/ml (a) and above 1 μg/ml (b).
Concordance with WHO data.
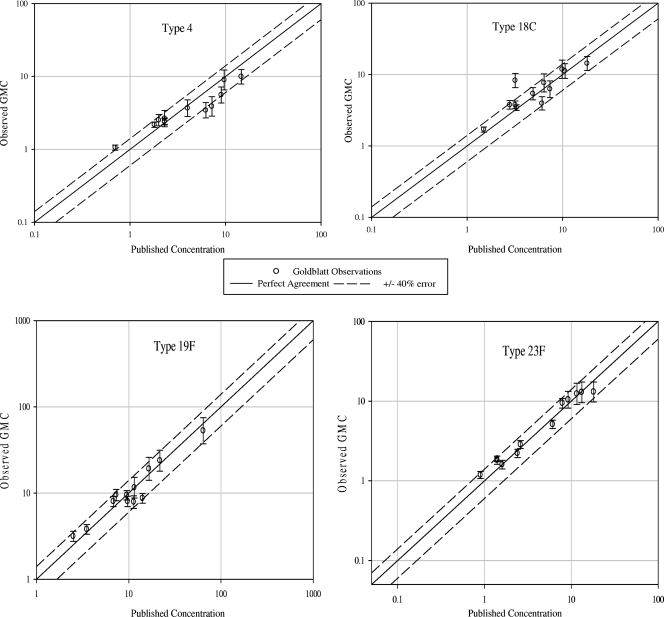
For each of the seven serotypes evaluated (there are no published concentrations for serotype 3), at least 9 of the 12 WHO QC sample GMCs fell within ±40% of the published concentrations (Table 6), thereby meeting the preestablished WHO criteria for concordance. The overall correlation (r value) of the Merck Pn ECL assay results to the published ELISA results for the seven serotypes was 0.994. Individual correlations for each serotype are listed in Table 6. Figure 5 displays the observed Pn ECL assay GMCs plotted against the published concentrations (ELISA) for serotypes 4, 18C, 19F, and 23F (serotypes randomly chosen for illustration purposes).
TABLE 6.
Observed WHO QC panel within 40% of the WHO ELISA published concentrations and individual correlations for each serotype
| Serotype | No. (%) of WHO QC panel samples within 40% of international ELISA value | Correlation (r value) of Pn ECL assay results to ELISA results for the WHO QC panel |
|---|---|---|
| 4 | 9 (75) | 0.942 |
| 6B | 10 (83) | 0.997 |
| 9V | 12 (100) | 0.943 |
| 14 | 11 (92) | 0.995 |
| 18C | 11 (92) | 0.885 |
| 19F | 12 (100) | 0.982 |
| 23F | 12 (100) | 0.958 |
FIG. 5.
Representative plots of the Merck concentrations (averaged across the runs) versus the reported concentrations for the WHO QC panel. Results for the WHO QC panel calibration are reported for only seven serotypes (available at http://www.vaccine.uab.edu/qc3.pdf).
DISCUSSION
Streptococcus pneumoniae is a major human pathogen causing pneumonia, bacteremia, meningitis, and otitis media (6, 8, 11, 12). Antibody responses to the serotype-specific CPs of this pathogen are known to be protective against disease (3). In order to determine these antibody responses in vaccinated patients, Merck Research Laboratories has developed the Pn ECL assays. The assays utilize a 96-well plate and direct binding format similar to that used in ELISAs, facilitating bridging of the two platforms. Additionally, the assays use CPs, PnPs25, and PnPs72 for preadsorption of samples, standards, and controls in order to improve the specificity of the sample reactivity to the Pn serotypes in Pn vaccines, and this diluent is also used for the 89SF-2 reference standard (16). To maintain consistency with other Pn antibody assays that utilize 89SF-2 as the reference standard and limit preadsorption of the standard to CPs alone, an adjustment to the concentrations assigned to the 89SF-2 reference standard by Quataert is utilized in the Pn ECL assay to account for the effect of the added preadsorbents.
Since immunogenicity assays are used by vaccine manufacturers and regulatory agencies to assess and define vaccine performance, these assays must be well characterized and statistically supported. Through the application of DOE methodology during the Pn ECL assay optimization phase, a functional set of assay conditions that satisfied the experimental objectives of reading concentrations down to a desired level, obtaining a wide dynamic range for the standard curve, and reducing nonspecific background reactivity was identified. The DOE approach provides an extra level of confidence in the appropriateness of the assay conditions selected (10). The selected set of assay working conditions resulted in reproducible standard curves with good sensitivity and a >100-fold dynamic range. Also as part of assay development and optimization, multiple lots and brands of the polyclonal secondary antibody, goat anti-human IgG, were assessed for binding specificity, as recommended in the WHO training manual for Pn serology (17, 17a). Lots that were found to bind to all human IgG subclasses (IgG1, IgG2, IgG3, and IgG4) equivalently and to have minimal cross-reactivity (less than 4%) with IgM (data not shown) were chosen for use in the assay. This is especially important because the properties of polyclonal antisera can differ from lot to lot. Also during the assay development phase, assay characterization was performed to assess spot cross-reactivity by using MAbs. Based on the spot reactivity data, the individual Ps remain within their respective spots within a well. The data also confirm that the antigenicity of the coated Ps is preserved upon binding to the plates as evidenced by the corresponding MAb's ability to bind only the corresponding Ps.
The benefits of multiplex technology over ELISA methodology for the measurement of various antigens simultaneously have been previously described for Pn assays (19, 20, and 24). The investigators of such studies cited speed, smaller sample volumes (particularly desirable for infants), equivalent or better sensitivity, increased dynamic range, and the ability to multiplex as benefits of chemiluminescence- or Luminex-based assays over ELISA. Comparing with the ELISA, the Pn ECL assay provides a ≥25-fold reduction in required serum volumes for the reference standard and test samples and a 200-fold reduction in the amount of Ps required. Furthermore, the broader dynamic range of the assay has the potential to minimize sample retesting for high-concentration samples. Additional benefits of the ECL format include the speed of the ECL analysis, which is approximately 2 min per 96-well plate for eight serotypes, and the lack of integrated fluids that may result in clogging issues typically experienced by bead-based systems. When comparing to the Luminex technology, a major disadvantage of Luminex is the requirement of conjugation chemistry to covalently bind the PnPs to the microspheres. The conjugation procedure involves chemical alteration to the Ps structure which may damage conformation epitopes. Several chemistries for conjugation of PnPs to Luminex microspheres have been reported; however, coupling procedures may be difficult to standardize (19, 24). An advantage of the Luminex technology over the ECL assay is the ability to perform multiplexed analysis of up to 100 unique analytes in a single sample.
In the Merck ELISA, the LOQ for each serotype has been reported as <0.1 μg/ml, allowing the minimum required ELISA serum dilution of 1:50 (16). In the Pn ECL assay, for each serotype evaluated, we were able to increase the initial dilution for test samples and for the 89SF-2 reference standard ≥4-fold higher than that used in the validated Pn ELISAs yet retain the ability to quantitate down to 0.1 μg/ml, which is below the minimal levels of protection for infants as described by Henckaerts et al. and Jódar et al. for conjugate vaccines (0.2 and 0.35 μg/ml, respectively) (13, 15). The increase in the initial test sample dilution is also beneficial in that it reduces the potential for interference of the test sample matrix. An eightfold increase in sensitivity in the ECL platform compared to the sensitivity of the colorimetric ELISA has been reported by others (9). The assay was shown to have no dilution effect between samples tested at the 1:100,000 and 1:1,000 dilutions and excellent precision at the 1:1,000 dilution (RSD, <20%). The higher variability observed when some serotypes were tested at the 1:100,000 dilution is not considered a critical deficiency for the assay, as the 1:100,000 dilution is used only for samples at very high concentrations (i.e., samples whose concentration exceeds the maximum concentration that can be obtained at the 1:1,000 dilution), and this represents a minority of results generated by the assay. The elevated variability at the 1:100,000 dilution observed during assay validation has been subsequently reduced by the use of a larger initial sample volume when preparing the 1:100,000 dilution (20 μl instead of 5 μl).
The specificity of the assay was evaluated by competitive inhibition with homologous and heterologous Ps. The Pn ECL assay is resistant to heterologous inhibition, as cross-specificity with other heterologous Ps as well as an irrelevant Ps (Hib polyribitol phosphate) was in general <20%. Inhibition of binding by the homologous competitor for the seven Prevnar serotypes was ≥87%, and this value for serotype 3 was 67.6%. The level of inhibition for samples with very low antibody concentrations was serotype dependent. Poor performance in regard to specificity was evidenced only for serotypes 3 and 6B and was restricted to samples with low antibody concentrations (<1.0 μg/ml). Because types 3 and 6B are the most highly charged of the Ps, there may be a correlation between the charge of the PnPs and the level of homologous inhibition achieved in the liquid-phase competition. In regard to these inhibition experiments, it is noteworthy that the pediatric samples included in this study were not immunized with the type 3 Ps. It is known that serum from nonimmunized individuals may bind to any ionically charged PnPs serotype in the ELISA platform (23). The use of adsorbents with different cell wall and CPs components may not improve the specificity of the serotypes where the charge of the molecules attracts IgG nonspecifically. In a comparison of the specificities of the Pn ECL assay and the ELISA, results show that specificities are comparable across the two platforms (data not shown).
Before adopting an alternative Pn antibody assay, it is important to assess whether the analytical accuracy of the new assay is at least equivalent to the analytical accuracy of the reference assay. To this end, CBER and the WHO developed a set of sera from adults vaccinated with a 23-valent PnPs vaccine. The WHO QC panel has been used by several laboratories to compare the abilities of different assays to produce the assigned antibody concentrations within an acceptable tolerance. There was excellent concordance between the two assay formats, as evidenced by the number of serotypes that satisfied the criterion of having 9 out of 12 calibration sera within ±40% error. In general, the Merck assay reported lower concentrations, as would be expected due to the double preadsorbent in the serum diluent, which potentially results in a reduction of the binding of nonspecific antibodies. The MSD methodology has great potential to be extremely useful for simultaneously quantitating IgG responses to Pn serotypes in Pneumovax 23, as well as Pn conjugate vaccines administered to pediatric populations for which serum volume prohibits extensive serology testing. Because of the ease of optimization, this technology can also be expanded to benefit serologic analysis of other multivalent vaccine formulations.
Acknowledgments
We thank Jennifer Raab, Wan-Sang (May) Kwan, and Lani Indrawati for their contributions. In addition, we thank the Merck Manufacturing Division for the supply of the purified Ps, D. Goldblatt for the supply of the Pn WHO QC panel, and M. Nahm for the anti-Pn MAbs.
Footnotes
Published ahead of print on 21 January 2009.
REFERENCES
- 1.Blackburn, G. F., H. P. Shah, J. H. Kenten, J. Leland, R. A. Kamin, J. Link, J. Peterman, M. J. Powell, A. Shah, D. B. Talley, et al. 1991. Electrochemiluminescence detection for development of immunoassays and DNA probe assays for clinical diagnostics. Clin. Chem. 371534-1539. [PubMed] [Google Scholar]
- 2.CDC. 2000. Recommendations of the Advisory Committee on Immunization Practices (ACIP). Preventing pneumococcal disease among infants and young children. MMWR Recommend. Rep. 49(RR09)1-38. [PubMed] [Google Scholar]
- 3.Committee on Issues and Priorities for New Vaccine Development. 1986. New vaccine development establishing priorities, vol. II, appendix D-17, p. 357-375. National Academy Press, Washington, DC. [Google Scholar]
- 4.Deaver, D. 1995. A new non-isotopic detection system for immunoassays. Nature (London) 377758-760. [DOI] [PubMed] [Google Scholar]
- 5.FDA. 2001. Guidance for industry bioanalytical method validation. Food and Drug Administration, Rockville, MD.
- 6.Fedson, D. S., G. Scott, and J. A. Scott. 1999. The burden of pneumococcal disease among adults in developed and developing countries: what is and is not known. Vaccine 17(Suppl.)S11-S18. [DOI] [PubMed] [Google Scholar]
- 7.Findley, J. W. A., W. C. Smith, and J. W. Lee. 2000. Validation of immunoassay for bioanalysis: a pharmaceutical industry perspective. J. Pharm. Biomed. Anal. 211249-1273. [DOI] [PubMed] [Google Scholar]
- 8.Greenwood, B. 1999. The epidemiology of pneumococcal infection in children in the developing world. Philos. Trans. R. Soc. Lond. Ser. B 354777-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guglielmo-Viret, V., and P. Thullier. 2007. Comparison of an electrochemiluminescence assay in plate format over a colorimetric ELISA, for the detection of ricin B chain (RCA-B). J. Immunol. Methods 32870-78. [DOI] [PubMed] [Google Scholar]
- 10.Haaland, P. D. 1989. Experimental design in biotechnology, p. 37-59. Marcel Dekker, New York, NY.
- 11.Hausdorff, W. P. 2002. Invasive pneumococcal disease in children: geographic and temporal variations in incidence and serotype distribution. Eur. J. Pediatr. 161(Suppl.)S135-S139. [DOI] [PubMed] [Google Scholar]
- 12.Hausdorff, W. P., J. Bryant, R. Cohen, R. Dagan, M. R. Jacobs, S. L. Kaplan, T. Kilpi, E. L. Lopez, E. O. Mason, Jr., S. I. Pelton, V. Syriopoulou, B. Wynne, and G. Yothers. 2002. Multinational study of pneumococcal serotypes causing acute otitis media in children. Pediatr. Infect. Dis. J. 211008-1016. [DOI] [PubMed] [Google Scholar]
- 13.Henckaerts, I., D. Goldblatt, L. Ashton, and J. Poolman. 2006. Critical differences between pneumococcal polysaccharide enzyme-linked immunosorbent assays with and without 22F inhibition at low antibody concentrations in pediatric sera. Clin. Vaccine Immunol. 13356-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.International Conference on Harmonization on the Validation of Analytical Methods. 1994. Validation of analytical procedures: text and methodology Q2(R1). ICH harmonized tripartite guideline. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, Geneva, Switzerland.
- 15.Jódar, L., J. Butler, G. Carlone, R. Dagan, D. Goldblatt, H. Käyhty, K. Klugman, B. Plikaytis, G. Siber, R. Kohberger, I. Chang, and T. Cherian. 2003. Serological criteria for evaluation and licensure of new pneumococcal conjugate vaccine formulations for use in infants. Vaccine 213265-3272. [DOI] [PubMed] [Google Scholar]
- 16.Marchese, R., N. Jain, J. Antonello, L. Mallette, K. Butterfield-Gerson, J. Raab, P. Burke, C. Schulman, A. Adgate, D. Sikkema, and N. Chirmule. 2006. ELISAs for measuring antibodies to pneumococcal polysaccharides for the PNEUMOVAX 23 vaccine: assay operating characteristics and correlation to the WHO international assay. Clin. Vaccine Immunol. 13905-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nahm, M., and D. Goldblatt. 26 November 2002, posting date. Training manual for enzyme linked immunosorbent assay for the quantitation of Streptococcus pneumoniae serotype specific IgG. WHO Pneumococcal Serology Reference Laboratories, London, United Kingdom. http://www.vaccine.uab.edu.
- 17a.Nahm, M., and D. Goldblatt. 26 November 2002, posting date. SOP5: selection of a new lot of enzyme-labeled secondary antibody specific for all human IgG subclasses. In Training manual for enzyme linked immunosorbent assay for the quantitation of Streptococcus pneumoniae serotype specific IgG. WHO Pneumococcal Serology Reference Laboratories, London, United Kingdom. http://www.vaccine.uab.edu.
- 18.O'Connell, M. A., B. A. Belanger, and P. D. Haaland. 1992. The four parameter logistic model for calibration and assay development. Am. Stat. Assoc. Proc. Biopharm. Section 1992180-185. [Google Scholar]
- 19.Pickering, J. W., T. B. Martis, R. W. Greer, M. C. Schroder, M. E. Astill, C. M. Litwin, S. W. Hildreth, and H. R. Hill. 2002. A multiplexed fluorescent microsphere immunoassay for antibodies to pneumococcal capsular polysaccharides. Am. J. Clin. Pathol. 117589-596. [DOI] [PubMed] [Google Scholar]
- 20.Pickering, J. W., J. D. Hoopes, M. C. Groll, H. K. Romero, D. Wall, H. Sant, M. E. Astill, and H. R. Hill. 2007. A 22-plex chemiluminescent microarray for pneumococcal antibodies. Am. J. Clin. Pathol. 12823-31. [DOI] [PubMed] [Google Scholar]
- 21.Plikaytis, B. D., D. Goldblatt, C. Frasch, C. Blondeau, M. J. Bybel, G. S. Giebink, I. Jonsdottir, H. Kayhty, H. B. Konradsen, D. V. Madore, M. H. Nahm, C. A. Schulman, P. F. Holder, T. Lezhava, C. M. Elie, and G. M. Carlone. 2000. An analytical model applied to a multicenter pneumococcal enzyme-linked immunosorbent assay study. J. Clin. Microbiol. 382043-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quataert, S. A., C. S. Kirch, L. J. Quackenbush Wiedl, D. C. Phipps, S. Strohmeyer, C. O. Cimino, J. Skuse, and D. V. Madore. 1995. Assignment of weight-based antibody units to a human antipneumococcal standard reference serum, lot 89-S. Clin. Diagn. Lab. Immunol. 2590-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quataert, S. A., K. Rittenhouse-Olson, C. S. Kirsch, B. Hu, S. Secor, N. Strong, and D. V. Madore. 2004. Assignment of weight-based antibody units for 13 serotypes to a human antipneumococcal standard reference serum lot 89-S(F). Clin. Diagn. Lab. Immunol. 111064-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schlottmann, S. A., N. Jain, N. Chirmule, and M. T. Esser. 2006. A novel chemistry for conjugating pneumococcal polysaccharides to luminex microspheres. J. Immunol. Methods 30975-85. [DOI] [PubMed] [Google Scholar]
- 25.Shapiro, E. D., and J. D. Clemens. 1984. A controlled evaluation of the protective efficacy of pneumococcal vaccine for patients at high risk of serious pneumococcal infections. Ann. Intern. Med. 101325-330. [DOI] [PubMed] [Google Scholar]
- 26.Swanson, S. J., S. J. Jacobs, D. Mytych, C. Shah, S. R. Indelicato, and R. W. Bordens. 1999. Applications for the new electrochemiluminescent (ECL) and biosensor technologies. Dev. Biol. Stand. 97135-147. [PubMed] [Google Scholar]
- 27.Wernette, C. M., C. E. Frasch, D. Madore, G. Carlone, D. Goldblatt, B. Plikaytis, W. Benjamin, S. A. Quataert, S. Hildreth, D. J. Sikkema, H. Käyhty, I. Jonsdottir, and M. H. Nahm. 2003. Enzyme-linked immunosorbent assay for quantitation of human antibodies to pneumococcal polysaccharides. Clin. Diagn. Lab. Immunol. 10514-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whitney, C. G., T. Pilishvili, M. M. Farley, W. Schaffner, A. S. Craig, R. Lynfield, A. C. Nyquist, K. A Gershman, M. Vazquez, N. M Bennett, A. Reingold, A. Thomas, M. P. Glode, E. R. Zell, J. H. Jorgensen, B. Beall, and A. Schuchat. 2006. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: a matched case-control study. Lancet 281495-1502. [DOI] [PubMed] [Google Scholar]