Abstract
We developed a europium nanoparticle-based immunoassay (ENIA) for the sensitive detection of anthrax protective antigen (PA). The ENIA exhibited a linear dose-dependent pattern within the detection range of 0.01 to 100 ng/ml and was approximately 100-fold more sensitive than enzyme-linked immunosorbent assay (ELISA). False-positive results were not observed with serum samples from healthy adults, mouse plasma without PA, or plasma samples collected from mice injected with anthrax lethal factor or edema factor alone. For the detection of plasma samples spiked with PA, the detection sensitivities for ENIA and ELISA were 100% (11/11 samples) and 36.4% (4/11 samples), respectively. The assay exhibited a linear but qualitative correlation between the PA injected and the PA detected in murine blood (r = 0.97731; P < 0.0001). Anthrax PA was also detected in the circulation of mice infected with spores from a toxigenic Sterne-like strain of Bacillus anthracis, but only in the later stages of infection. These results indicate that the universal labeling technology based on europium nanoparticles and its application may provide a rapid and sensitive testing platform for clinical diagnosis and laboratory research.
Threats of bioterrorism have prompted renewed research interest in the development of rapid and sensitive assays for the detection of major CDC category A agents, such as Bacillus anthracis, which is of particular concern for bioterrorism, in light of the 2001 U.S. anthrax attack that led to 11 cases of inhalation anthrax and 5 deaths (10).
B. anthracis, the causative agent of anthrax, is a gram-positive bacterium that carries two virulence-related plasmids, pXO1 and pXO2 (32). The anthrax toxin is encoded by pXO1 and consists of three components: protective antigen (PA), lethal factor (LF), and edema factor (EF) (11). The PA component can rapidly bind to cellular receptors ANTXR1 or ANTXR2 and form a heptamer after the 83-kDa PA protein is cleaved by a host protease to yield a 63-kDa fragment. The PA heptamer subsequently combines with LF to form anthrax lethal toxin, which enters the cell through endocytosis and causes toxemia (1). Anthrax toxemia is the main cause of host system failure and death (17, 29). Most current approaches to anthrax therapy rely on antitoxin agents administered during the early stages of infection. Therefore, sensitive and rapid assays for the detection of B. anthracis toxin are urgently needed to facilitate an early and accurate diagnosis and successful treatment postexposure. Unfortunately, current enzyme-linked immunosorbent assays (ELISAs) for the detection of anthrax PA and LF are able to achieve sensitivity levels of only ∼1 to 20 ng/ml (15, 18).
Over the past decade, nanotechnology-based techniques have been developed for use in medical testing and clinical diagnosis because of their high degrees of sensitivity and specificity and their ability to operate without enzymes (24). For example, gold nanoparticle (NP)-based bio-bar-code amplification (BCA) assays have been reported to be highly sensitive (19, 20, 28) and capable of detecting proteins such as prostate-specific antigen (PSA) at levels of as low as 30 aM (20) and human immunodeficiency virus type 1 p24 antigen at a lower limit of 0.1 pg/ml (28). However, the BCA assay involves multiple steps, including NP-based silver enhancement and a microarray detection method, and further simplification of the BCA technique would be needed to generate a simple, rapid assay system that could be applied in resource-poor settings. Our studies showed that a suitable replacement for gold NPs that can be used to simplify the detection method without having a significant impact on detection sensitivity is highly fluorescent europium (Eu+) NPs. The use of Eu+ NPs further permits the assay to be adapted to an ELISA format that is already in place in testing laboratories because the antibody-antigen sandwich complex bound to Eu+ NPs coated with streptavidin (SA) can be directly measured with a fluorescence reader. Herein we report the development and evaluation of a Eu+ NP-based immunoassay (ENIA) with a lower detection limit of 10 pg/ml for the sensitive detection of B. anthracis PA as a proof of concept for such a testing platform. This ultrasensitive NP-based assay for the detection of anthrax toxin could provide a useful new tool for clinical diagnosis and laboratory research.
MATERIALS AND METHODS
Samples.
Control sera were obtained from healthy adults in a laboratory setting. BALB/cJ mice were injected intravenously (200 μl/vein) with different amounts of wild-type PA, LF, or EF or premixed combinations of these toxins (all of which were prepared in phosphate-buffered saline [PBS]). In select experiments, an uncleavable PA mutant (PA-U7) which has been shown to have a slower rate of clearance from the circulation was utilized (18). At 2 or 6 h after injection, the mice were terminally bled via cardiac puncture with heparin-coated needles and tubes, and the plasma was assessed for PA levels. In other experiments, plasma from untreated mice was spiked with known concentrations of toxins. Finally, DBA/2J mice were infected with 103 or 107 spores (500 μl subcutaneously) and were terminally bled in duplicate at 6, 8, 24, 32, 48, 72, 96, 191, and 237 h postinfection for the assessment of toxin levels. All experiments involving animals were performed under protocols approved by the Animal Care and Use Committee of the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH). It should be noted that all the samples were coded and tested in a blinded fashion.
Antigen, spore, antibody, and Eu+ NPs.
Purified anthrax PA, LF, and EF proteins and rabbit anti-PA polyclonal antibody 5819 were prepared as described previously (18, 21, 30). Spores were prepared from the B. anthracis Ames 35 strain (22). This strain is a nonencapsulated toxigenic strain. The bacteria were grown on nutrient sporulation agar (25) at 37°C for 2 days to allow sporulation to occur. The spores were then removed from the plates by washing the plates with sterile water and were further purified by centrifugation through a gradient of Renogradin (Bracco Diagnostics), as described previously (8).
Monoclonal anti-PA antibody W1 was isolated from a phage display library generated from immunized chimpanzees (4). This antibody was shown to bind to a conformational epitope formed by domain 4 (amino acids 614 to 735) of PA, which is responsible for cellular receptor binding (14, 31). The affinity (Kd) of W1 binding to PA was 4 × 10−11 to 5 × 10−11 mol/liter, which is 20- to 100-fold higher than the affinity of PA binding for the cellular receptor (4). Biotinylated goat anti-rabbit antibody was purchased from Pierce (Rockford, IL). Fluoro-Max polystyrene NPs (diameter, 107 nm) containing europium(III) β-diketone chelates and carboxyl groups on the surface for covalent bioconjugation were obtained from Seradyn Inc. (Indianapolis, IN). The preparation and the characterization of Eu+ NPs coated with SA have been described previously (6). Each NP contains about 30,000 europium ions and approximately 700 SA molecules (5). The Eu+ NPs can produce intense long-lifetime fluorescent light identical to that observed with the dissociation-enhanced lanthanide fluoroimmunoassay (DELFIA) method (7).
ELISA for detection of PA.
Fifty microliters of W1 (1.0 μg/ml) in 0.1 M phosphate buffer-0.15 M NaCl, pH 7.2 (coating solution), was incubated in Nunc Immuno Maxisorp strips (no. 437915; Fisher Scientific, Pittsburgh, PA) overnight at 4°C. The strips were then washed three times with 1× PBS-0.5% Tween 20 (PBS-T) in a DELFIA plate washer (Perkin-Elmer) and blocked with 300 μl of 1× PBS-1% casein (blocking buffer) at 37°C with shaking at 900 rpm for 1 h to remove unbound antibody and to ensure the blocking of nonspecific binding sites. For the assays, 100 μl of a series of purified PA dilutions in 1× PBS-0.25% Tween 20-0.5% casein (assay buffer) or appropriately diluted serum samples (initially diluted from 1:10) and appropriately diluted secondary rabbit anti-PA antibody were added to the strips; and the strips were incubated at 37°C with shaking at 900 rpm for 1 h. After the strips were washed five times with PBS-T, 100 μl of appropriately diluted biotinylated goat anti-rabbit antibody was added and the mixture was incubated for 30 min with shaking at 37°C. After the plate was washed with PBS-T, it was incubated with diluted peroxidase-SA conjugate (Pierce) for 30 min with shaking at 37°C. The ELISA reactions were developed with o-phenylenediamine tablets (Pierce) for 30 min at room temperature and were stopped by the addition of 100 μl of 5 M H2SO4. The signal was then measured and quantified with a microtiter plate reader (Molecular Devices, Sunnyvale, CA). The cutoff value was established by using the sum of the means of the absorbance of six negative controls plus 3 standard deviations (SDs). Samples with signal-to-cutoff (S/CO) ratios equal to or greater than 1.00 were considered positive for anthrax PA.
ENIA for detection of PA.
Coating of the microtiter plate, capture of the target (PA), and binding to the secondary anti-PA antibody as well as the biotinylated goat anti-rabbit antibody were performed under the same conditions used for the ELISA described above. After the formation of the antibody-antigen-antibody sandwich complex, 100 μl of assay buffer containing 107 SA-coated Eu+ NPs was added, and the mixture was incubated at 37°C with shaking at 900 rpm for 30 min, followed by further washing and incubation with 25 ng/ml of biotinylated anti-SA (Vector Laboratories, Inc., Burlingame, CA) and 1:500 diluted Eu+-labeled SA molecules (Perkin-Elmer). Finally, the strips were washed five times with PBS-T and 100 μl of DELFIA chelating enhancement solution (Perkin-Elmer) was added. The DELFIA solution is an acidic chelating detergent solution that can dissociate Eu+ from the solid phase bound to antibodies to form a homogenous and highly fluorescent Eu+ micellar chelate solution. Fluorescence was measured with a Victor 1420 fluorometer (Perkin-Elmer) by using a time-resolved mode (λ excitation, 340 nm; λ emission, 615 nm; delay time, 400 μs; counting window, 400 μs). The cutoff value was the average signal intensity of the negative controls plus 3 times the SD. Samples with S/CO values equal to or greater than 1.00 were considered positive for anthrax PA.
RESULTS
Sensitivity of ENIA compared to results of ELISA for detection of anthrax PA.
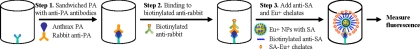
The ENIA scheme is depicted in Fig. 1. We adapted the immunosorbent assay format to capture target to form an antibody-antigen-antibody sandwich complex (steps 1 and 2). The immune complex is then coupled to SA-coated Eu+ NPs and Eu+ molecules through the biotin-SA interaction (step 3). After extensive washing between steps to remove unbound or nonspecifically bound conjugates, the fluorescence is measured with a fluorometer. The capture reagents that we used were purified monoclonal anti-PA antibody 14B7 and an engineered anti-PA single-chain variable fragment (scFv), W1. Both the 14B7 antibody and the engineered W1 scFv were shown to recognize an epitope in the receptor-binding domain of PA and to be able to bind to PA83 and PA63 in an ELISA and by Western blotting, although W1 shows a higher affinity than antibody 14B7 (4, 12, 13, 18). We found that W1 gave a slightly lower detection limit and a smaller variation than antibody 14B7, likely due to its higher affinity (data not shown). Therefore, the data presented here were derived by using W1 rather than 14B7.
FIG. 1.
Scheme of ENIA for detection of anthrax toxin (PA). ENIA uses monoclonal anti-PA antibody-coated microtiter wells to capture the anthrax PA in the samples. The PA protein is then sandwiched with secondary rabbit anti-PA antibody and biotinylated goat anti-rabbit antibody. The Eu+ NPs modified with SA recognize and bind to this biotinylated antigen-antibody complex. The addition of biotinylated anti-SA antibody and Eu+-labeled SA molecules enhances the signal intensity of the binding event. After extensive washing of the complex, the fluorescence signal released from the sandwiched complex is then recorded and quantified with a Vector fluorometer.
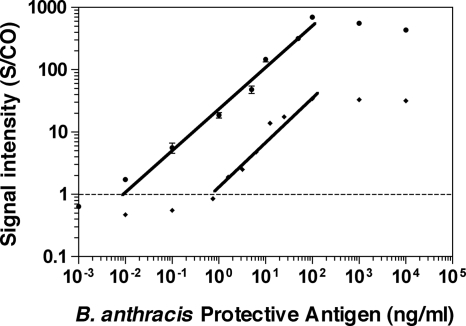
Before subject samples were analyzed, we used ENIA and a conventional chromogenic in-house ELISA to establish calibration curves for the purified anthrax PA protein (Fig. 2). The ENIA exhibited an analytical target concentration range of 4 orders of magnitude (10 pg/ml to 100 ng/ml), with a lower limit of detection of 10 pg/ml. A good correlation between the anthrax PA concentration and the signal intensity (S/CO) was demonstrated (r [Pearson correlation coefficient] = 0.9357; R2 = 0.8756; P < 0.0001) (Prism software, version 4.0; GraphPad Software, Inc., San Diego, CA) and characterized a linear dose-dependent model (Fig. 2). However, at high target concentrations (>1 μg/ml), the assay responses were nonlinear and eventually leveled off. The detection range of the ELISA was 1 to 100 ng/ml (Fig. 2). These results demonstrate that under these conditions ENIA offers an approximately 100-fold improvement in the lower limit of detection over that of the traditional colorimetric ELISA.
FIG. 2.
Increased sensitivity of detection of anthrax PA of ENIA compared with that of an in-house ELISA. Purified anthrax PA at concentrations ranging from 1 pg/ml to 1 μg/ml in serial dilutions in PBS served as the targets. The normalized relative signal intensities are represented as the ratios of the samples over the cutoff value of the negative control (S/CO). Closed circles, results of ENIA; closed squares, results of ELISA. The error bars represents the standard deviations of at least three independent repeated experiments for each assay. The correlation (r value) between the S/CO ratios by the PA ENIA and the concentrations of anthrax PA was 0.9357 (R2 = 0.8756; P < 0.0001).
Sensitivity and specificity of ENIA for detection of anthrax PA in serum samples from healthy adults and spiked mouse plasma samples.
To determine the specificity of the assay, we first tested 30 serum samples from healthy adults and found that the average S/CO values were considerably less than 1.00 (0.71 ± 0.2); no false-positive results were observed. Next, mouse plasma spiked with purified PA protein was used to evaluate the sensitivity of the assay. As shown in Table 1, our assay could detect PA protein in samples spiked with tens of picograms of PA per milliliter, such as samples 32 and 54 in panel II, which were spiked with 20 and 70 pg/ml of PA, respectively. We must note that the results were obtained for samples diluted 1:10. So the final concentrations of PA in the test wells for samples 32 and 54 were 2 and 7 pg/ml respectively. These results further confirmed that our assay could detect about 10 pg/ml of PA diluted in PBS or animal plasma. However, the PA in sample 5 in panel I, spiked with 40 pg/ml of PA, was not detected by ENIA, indicating that the reproducibility of ENIA for the detection of trace amounts of toxin remains to be further improved. Furthermore, the quantitation of PA by ENIA yielded results that were similar to the actual amounts spiked into the samples, especially for samples with PA at levels of ng/ml. However, for the samples with a very high concentration (>1 μg/ml) or a very low concentration (<1 ng/ml) of PA, the quantified results were less reliable and were not exactly equal to the amount that was spiked. For example, samples 1 and 9 in panel I were spiked with 100 and 1 μg/ml of PA, respectively, but the quantities detected by ENIA were 27 and 0.02 μg/ml, respectively. Sample 32 in panel II, spiked with 0.02 ng/ml of PA, was found to contain about 1.40 ng/ml of PA. A similar phenomenon was observed in Fig. 2, in which the signal intensity reached the platform when the concentration of PA was 1 or 10 μg/ml. These results indicated that our current assay is only qualitative or semiquantitative rather than absolutely quantitative. In addition, we found that PA was detected in the samples spiked with PA-EF (samples 45 and 46 in panel II) or PA-LF (samples 47, 48, and 54 in panel II). These results indicate that the presence of LF or EF does not cause false-negative or false-positive results during the detection of PA. In addition, all samples without PA protein or spiked with LF or EF alone were negative for PA by our assay, indicating that the ENIA was very specific for the detection of PA. Finally, on the basis of the data for panel II (Table 1), the sensitivities of ENIA and ELISA for PA detection were 100% (11/11 samples) and 36.4% (4/11 samples), respectively. The specificity of both tests was 100% (10/10 samples).
TABLE 1.
Detection of anthrax PA in mouse plasma spiked with different doses of PA, LF, and EF by ENIA and ELISAa
| Panel and sample no. | Toxin concn spiked (ng/ml)
|
PA concn detected (ng/ml)
|
|||
|---|---|---|---|---|---|
| PA | LF | EF | ENIA | ELISA | |
| Panel I | |||||
| 1 | 100,000.000 | 27,000.00 | NDb | ||
| 9 | 1,000.000 | 20.00 | ND | ||
| 2 | 400.000 | 425.00 | ND | ||
| 4 | 40.000 | 18.00 | ND | ||
| 6 | 4.000 | 3.30 | ND | ||
| 3 | 0.400 | 0.30 | ND | ||
| 5 | 0.040 | 0.00 | ND | ||
| 7 | 0.004 | 0.00 | ND | ||
| 8 | 0.000 | 0.00 | ND | ||
| Panel II | |||||
| 5 | 1,720.00 | 3,400.00 | 2,500.00 | ||
| 10 | 172.00 | 125.00 | 37.00 | ||
| 24 | 17.20 | 11.00 | 0.00 | ||
| 25 | 1.72 | 0.60 | 0.00 | ||
| 28 | 0.17 | 0.60 | 0.00 | ||
| 32 | 0.02 | 1.40 | 0.00 | ||
| 47 | 170.00 | 200.00 | 10.00 | 0.01 | |
| 48 | 1.70 | 2.00 | 1.50 | 0.00 | |
| 54 | 0.07 | 0.08 | 1.00 | 0.00 | |
| 45 | 170.00 | 165.00 | 41.00 | 0.01 | |
| 46 | 1.70 | 1.65 | 1.20 | 0.00 | |
| 34 | 2,000.00 | 0.00 | 0.00 | ||
| 39 | 200.00 | 0.00 | 0.00 | ||
| 40 | 20.00 | 0.00 | 0.00 | ||
| 19 | 2.00 | 0.00 | 0.00 | ||
| 14 | 0.20 | 0.00 | 0.00 | ||
| 41 | 0.02 | 0.00 | 0.00 | ||
| 42 | 1,650.00 | 0.00 | 0.00 | ||
| 43 | 165.00 | 0.00 | 0.00 | ||
| 2 | 0.16 | 0.00 | 0.00 | ||
| 53 | 0.02 | 0.00 | 0.00 | ||
Plasma from untreated mice was spiked with known concentrations of toxins and PA levels were assessed by two methods. The samples were diluted from 1:10 to 1:1,000.
ND, not done.
Evaluation of sensitivity and specificity of ENIA with samples from toxin-injected animals.
In order to further evaluate the sensitivity and the specificity of ENIA, plasma samples from mice injected with anthrax toxin were tested. We first measured the concentration of uncleavable PA-U7 (which retains the epitope for capture antibody W1) in the blood of mice injected with a range of doses (0 to 100 μg) (Table 2). It was apparent that the animals injected with high doses of PA exhibited high concentrations of PA in their circulation. The assay exhibited a linear, but qualitative, correlation between the PA injected and the PA detected in murine blood (r = 0.9773l, P < 0.0001) (Prism software, version 4.0; GraphPad Software, Inc.). We next tested an additional 48 mouse plasma samples collected from two different experiments in which wild-type PA was injected into mice (Tables 3 and 4). Table 3 lists the results for the animals injected with 50 μg of PA, LF, or EF or 50 μg PA plus 50 μg LF. At 2 to 6 h after the injection, all 11 plasma samples from animals injected with PA or PA plus LF were still positive for PA, while another 12 samples collected from mice injected with LF or EF alone were PA negative. We also found that the PA concentration did not show a significant difference among animals injected with PA alone or with PA plus LF, although the PA concentration at 2 h after injection was slightly higher than that at 6 h after injection in both groups. It should be noted that the results obtained by ENIA and ELISA were highly similar. This finding was further confirmed by the data shown in Table 4. For the animals injected with 10 or 50 μg of PA and different amounts (5, 25, or 50 μg) of LF, the concentrations of PA detected in the blood were almost equal (0.81 to 1.09 μg/ml for mice receiving 10 μg of PA and 1.11 to 1.41 μg/ml for mice receiving 50 μg of PA). In addition, all seven animals injected with different doses of LF alone were PA negative. Our studies also confirmed the previous finding that uncleavable PA-U7 has a slower clearance rate than wild-type PA, as we found that the mouse injected with 50 μg of PA-U7 (Table 2, mouse 8) had dramatically higher levels of PA after 2 h than mice injected with same amount of wild-type PA (Tables 3 and 4) (18). These results further confirm the sensitivity and the specificity of our ENIA.
TABLE 2.
Detection by ENIA of PA in plasma from mice injected with different doses of toxina
| Sample no. | PA dose injected (μg) | PA concn detected (μg/ml) |
|---|---|---|
| 2 | 100 | 26.80 ± 5.9 |
| 8 | 50 | 19.00 ± 3.4 |
| 6 | 10 | 1.60 ± 0.8 |
| 5 | 5 | 0.24 ± 0.1 |
| 4 | 1 | 0.08 ± 0.01 |
| 7 | 0 | 0.00 |
Mice (n = 1 per dose) were injected with uncleavable PA (PA-U7) and were bled 2 h after injection. PA levels were then assessed by ENIA. The samples were diluted from 1:10 to 1:1,000.
TABLE 3.
Detection of anthrax PA in mice injected with PA, LF, and EFa
| Toxin(s) | Bleeding time (h) | No. of animals | PA concn in blood (μg/ml) measured by:
|
|
|---|---|---|---|---|
| ENIA | ELISA | |||
| PA | 2 | 3 | 1.60 ± 0.40b | 2.23 ± 0.31 |
| PA | 6 | 2 | 1.14 ± 1.51 | 0.94 ± 1.08 |
| PA + LF | 2 | 3 | 1.23 ± 0.15 | 1.33 ± 0.08 |
| PA + LF | 6 | 3 | 0.70 ± 0.10 | 1.10 ± 0.15 |
| LF | 2 | 3 | 0.00 | 0.00 |
| LF | 6 | 3 | 0.00 | 0.00 |
| EF | 2 | 3 | 0.00 | 0.00 |
| EF | 6 | 3 | 0.00 | 0.00 |
BALB/cJ mice were injected intravenously (200 μl/vein) with 50 μg of wild-type PA, LF, or EF or with premixed combinations of these toxins (all prepared in PBS) and were bled 2 or 6 h after injection. The PA levels in plasma were assessed by the ENIA and ELISA methods. The samples were diluted from 1:10 to 1:1,000.
The data are means ± SDs.
TABLE 4.
Detection of anthrax PA in mice injected with different doses of PA and LF by ENIAa
| Toxin dose injected (μg)
|
No. of animals | PA concn measured in blood (μg/ml) | |
|---|---|---|---|
| PA | LF | ||
| 50 | 50 | 2 | 1.37 ± 0.42b |
| 50 | 25 | 2 | 1.41 ± 0.09 |
| 50 | 5 | 2 | 1.11 ± 0.21 |
| 10 | 50 | 4 | 0.83 ± 0.36 |
| 10 | 25 | 4 | 0.81 ± 0.28 |
| 10 | 5 | 2 | 1.09 ± 0.05 |
| 10 | 0 | 2 | 0.14 ± 0.04 |
| 0 | 50 | 2 | 0.00 |
| 0 | 25 | 2 | 0.00 |
| 0 | 5 | 3 | 0.00 |
BALB/cJ mice (n = 2 to 4) were injected with different amounts of wild-type PA or LF or with premixed combinations of these toxins (all prepared in PBS) and were bled 2 h after injection. PA levels in plasma were assessed by ENIA. The samples were diluted from 1:10 to 1:1,000.
The data are means ± SDs.
Detection of PA in blood samples from B. anthracis-infected mice.
To investigate the ability of ENIA to detect toxin produced during infection, DBA/2J mice were infected with a nonlethal dose (103) or a lethal dose (107) of the nonencapsulated toxigenic Ames 35 strain (22). As shown in Table 5, 13 animals infected with the lower dose showed no signs of illness over 6 days, and toxins were never detected in bleeds from these animals during the 6-day period. Animals infected with the higher spore doses showed substantial edema by 24 h and were ill by 48 h. PA was consistently detected 24 h after infection with this dose, when signs of malaise had manifested, but not at 6 or 8 h after infection, when the animals still appeared to be healthy (Table 5). These results demonstrate that the current ENIA can detect PA in the circulation at later stages of infection with B. anthracis.
TABLE 5.
Detection of anthrax PA in mice infected with B. anthracis spores by ENIAa
| Dosage (no. of spores/mouse) | Bleed time (h) | No. of animals | Illness grade | PA concn in blood (ng/ml) |
|---|---|---|---|---|
| 103 | 24-237 | 13 | − | 0.00 |
| 107 | 6 | 2 | − | 0.00 |
| 107 | 8 | 2 | − | 0.00 |
| 107 | 24 | 2 | +b | 68 ± 75 |
| 107 | 42-48 | 2 | +++ | 408 ± 275 |
| 0 | 24 | 2 | − | 0.00 |
DBA/2J mice were exposed to different doses of B. anthracis spores and were bled at different times after infection. PA levels in plasma were assessed by ENIA. The samples were diluted from 1:10 to 1:1,000.
Edema.
DISCUSSION
Sandwich ELISAs for the detection of anthrax PA with one monoclonal antibody and two polyclonal antibodies have been reported previously (15, 18). In these systems, the capture antibody was mouse monoclonal antibody M18 or 14B7. These antibodies have specificities similar to the specificity of monoclonal antibody W1 used in our study and are single-epitope capture reagents that target PA domain 4 (4, 12, 16). Interestingly, these assays, developed independently, were able to achieve the same lower detection limit of ∼1 ng/ml for the purified PA protein (15, 18). However, when Eu+ NPs were used to replace the traditional colorimetric development reagents in the conventional ELISA, the lower limit of detection was dramatically improved by almost 100-fold. Similar results have also been observed for the detection of the B. anthracis LF protein and the Yersinia pestis LcrV and F1 proteins, as well as human immunodeficiency virus type 1 p24 antigen (unpublished data). For example, our preliminary data indicate that the current assay conditions can detect 10 pg/ml of anthrax LF by using monoclonal anti-LF antibody and 20 ng/ml of anthrax EF by using PA63 as the capture reagent (data not shown). These results further support the importance of detection chemistries and new labeling technologies in improving assay performance and clearly indicate the potential of NP-based testing methods to enhance the sensitivity of immunoassays and to allow the ultrasensitive detection of pathogens.
Labeling technologies commonly used in immunoassays include radioactivity, enzyme activity, and chemicals (chemiluminescence and fluorescence). The use of radioisotopic labeling is being discontinued in many laboratories for several reasons, including the safety hazards that they pose. One major disadvantage of the enzyme-based colorimetric ELISA is its relatively low detection sensitivity. In the past decade, lanthanide chelates have successfully been used in immunoassays, such as the DELFIA technology. The DELFIA technology is based on the dissociation of lanthanide ions from chelates conjugated to detecting molecules such as SA. This technique yields a high sensitivity with a stable signal, a high signal-to-noise ratio, and a nonenzymatic, flexible platform with a short incubation time. Fortunately, the highly fluorescent chelates used in the DELFIA technique can also be used in fluorescent lanthanide chelate NPs. For example, Harma et al. reported that an Eu+ NP-based immunoassay can detect as little as 1.6 pg/ml of PSA in microtiter wells in a rapid assay (5). This assay is 100-fold more sensitive than a similar assay that uses conventional Eu+-labeled SA (5) and could detect even 0.38 pg/ml of PSA when the analyte was directly biotinylated (6). Soukka et al. reported an extremely low limit of detection of 0.04 pg/ml for PSA with the Eu+ NP label technology in a two-step immunoassay and a 3-h assay time (27). The improved sensitivity is due to the high specific activity of the SA-coated NPs, its low nonspecific binding, and the high affinity of the NPs toward the analyte. This is not surprising, given the large surface area of NPs, the capacity to coat a large number of molecules and the huge content of Eu+ ions in a single NP, and in particular, the unique characteristic that the time-resolved lanthanide chelates do not self-quench even when they are used at high millimolar concentrations (5). In addition, Huhtinen et al. reported that with the use of SA-coated NPs and biotinylated antibody, the steric hindrance caused by the antibody-coated NPs in immunometric sandwich assays can be resolved when the analyte is structurally complex (9). These unique characteristics indicate that Eu+ NPs, especially SA-coated NPs, may be more useful than Eu+ chelates in the development of assays with high amplification ratios and extremely high sensitivities.
In our study, we also used biotinylated anti-SA antibody and SA-coated Eu+ chelates, followed by the addition of chelating enhancement solution to further enhance the signal intensity, since each NP contains more than 700 SA molecules and more than 2,800 biotin binding sites (5). We found that the enhancement step could further improve the signal intensity and could increase the sensitivity of the assay by twofold. The major advantage of our current assay format is that all the reagents are universal and can easily be adapted for the detection of different targets by replacing the coating and detection ligands. Furthermore, since the anti-PA IgG antibody is an important marker for the evaluation of anthrax vaccines, anti-PA assays with improved sensitivities would be useful in vaccine development and evaluation. Current assays are able to reach sensitivity limits of 1.5 to 3 μg/ml of anti-PA IgG antibody (2, 3, 23). We believe that the detection limits for the anti-PA IgG antibody could be further decreased by employing the ENIA. Although our current assay involves several incubation steps, it could be further developed as a two-step assay by using NP-antibody bioconjugate (26). The entire assay could be completed within 30 min. Therefore, the ENIA format could be suitable for rapid and point-of-care use.
As a proof-of-concept study, our preliminary results indicate that the ENIA described here has the potential to significantly improve the sensitivity of detection of anthrax toxin. The current ENIA also detected purified anthrax toxin and toxin-injected animal samples. The assay could also detect anthrax toxin after infection with B. anthracis spores, but only after the animals were sick and not at earlier stages of disease, before symptoms were apparent. Similar results have been reported by Mabry et al. (15). They used an engineered ELISA to measure the amounts of PA in blood samples from guinea pigs and rabbits exposed to a lethal dose of anthrax spores and found that PA could be detected only in the late stages of infection, especially within 12 h of death for the infected guinea pigs or 48 h for the infected rabbits (15). These findings are not surprising, as the anthrax toxin that is released into the circulation in the early stages of disease is likely to continuously bind to the available tissue receptors until receptor saturation is achieved. Only after saturation will the toxins accumulate in the blood and provide measurable levels in the blood, and the animals are usually sick by this stage.
More studies are needed to develop this nanotechnology-based testing method as a clinical diagnostic assay. Ideally, the early detection of the toxin in infected samples needs to be improved. Improvement of the sensitivity of the assay, coupled with knowledge of the kinetics of infection, may allow the detection of toxin in different sample types, such as infected tissues or cells which bind large amounts of toxin early in infection. In addition, to increase the sensitivity of detection of PA with mutations that may not be detectable with a single monoclonal antibody, a polyclonal anti-PA antibody or a mixture of monoclonal anti-PA antibodies against different PA-reactive epitopes may be used.
Due to the high specificity of SA-coated NPs, the high affinity between biotin and SA, and this universal labeling technology, the assay that we have developed may be suitable for the detection of biotinylated molecules and could be further developed as a rapid and universal testing platform for clinical diagnosis or laboratory research and for resource-limited settings upon further optimization and simplification.
Acknowledgments
We acknowledge the Biodefense Advanced Research and Development Agency, DHHS, for funding and/or support. This work was also supported in part by the DIR, NIAID, NIH.
We acknowledge Drusilla Burns, Krishna Devadas, and Hira Nakhasi for review of the manuscript. We thank Devorah Crown and Sharmina Miller-Randolph for help with the animal experiments.
The findings and conclusions in this article have not been formally disseminated by the FDA and should not be construed to represent any agency determination or policy.
Footnotes
Published ahead of print on 7 January 2009.
REFERENCES
- 1.Abrami, L., S. Liu, P. Cosson, S. H. Leppla, and F. G. van der Goot. 2003. Anthrax toxin triggers endocytosis of its receptor via a lipid raft-mediated clathrin-dependent process. J. Cell Biol. 160321-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biagini, R. E., D. L. Sammons, J. P. Smith, B. A. MacKenzie, C. A. Striley, V. Semenova, E. Steward-Clark, K. Stamey, A. E. Freeman, C. P. Quinn, and J. E. Snawder. 2004. Comparison of a multiplexed fluorescent covalent microsphere immunoassay and an enzyme-linked immunosorbent assay for measurement of human immunoglobulin G antibodies to anthrax toxins. Clin. Diagn. Lab. Immunol. 1150-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biagini, R. E., D. L. Sammons, J. P. Smith, B. A. MacKenzie, C. A. Striley, J. E. Snawder, S. A. Robertson, and C. P. Quinn. 2006. Rapid, sensitive, and specific lateral-flow immunochromatographic device to measure anti-anthrax protective antigen immunoglobulin G in serum and whole blood. Clin. Vaccine Immunol. 13541-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, Z., M. Moayeri, Y. H. Zhou, S. Leppla, S. Emerson, A. Sebrell, F. Yu, J. Svitel, P. Schuck, M. St. Claire, and R. Purcell. 2006. Efficient neutralization of anthrax toxin by chimpanzee monoclonal antibodies against protective antigen. J. Infect. Dis. 193625-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harma, H., T. Soukka, S. Lonnberg, J. Paukkunen, P. Tarkkinen, and T. Lovgren. 2000. Zeptomole detection sensitivity of prostate-specific antigen in a rapid microtitre plate assay using time-resolved fluorescence. Luminescence 15351-355. [DOI] [PubMed] [Google Scholar]
- 6.Harma, H., T. Soukka, and T. Lovgren. 2001. Europium nanoparticles and time-resolved fluorescence for ultrasensitive detection of prostate-specific antigen. Clin. Chem. 47561-568. [PubMed] [Google Scholar]
- 7.Hemmila, I., S. Dakubu, V. M. Mukkala, H. Siitari, and T. Lovgren. 1984. Europium as a label in time-resolved immunofluorometric assays. Anal. Biochem. 137335-343. [DOI] [PubMed] [Google Scholar]
- 8.Hu, H., Q. Sa, T. M. Koehler, A. I. Aronson, and D. Zhou. 2006. Inactivation of Bacillus anthracis spores in murine primary macrophages. Cell. Microbiol. 81634-1642. [DOI] [PubMed] [Google Scholar]
- 9.Huhtinen, P., T. Soukka, T. Lovgren, and H. Harma. 2004. Immunoassay of total prostate-specific antigen using europium(III) nanoparticle labels and streptavidin-biotin technology. J. Immunol. Methods 294111-122. [DOI] [PubMed] [Google Scholar]
- 10.Jernigan, J. A., D. S. Stephens, D. A. Ashford, C. Omenaca, M. S. Topiel, M. Galbraith, M. Tapper, T. L. Fisk, S. Zaki, T. Popovic, R. F. Meyer, C. P. Quinn, S. A. Harper, S. K. Fridkin, J. J. Sejvar, C. W. Shepard, M. McConnell, J. Guarner, W. J. Shieh, J. M. Malecki, J. L. Gerberding, J. M. Hughes, and B. A. Perkins. 2001. Bioterrorism-related inhalational anthrax: the first 10 cases reported in the United States. Emerg. Infect. Dis. 7933-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leppla, S. 2006. Bacillus anthracis toxins, p. 323-347. In J. E. Alouf and M. R. Popoff (ed.), The comprehensive sourcebook of bacterial protein toxins. Academic Press, Burlington, MA.
- 12.Little, S. F., S. H. Leppla, and E. Cora. 1988. Production and characterization of monoclonal antibodies to the protective antigen component of Bacillus anthracis toxin. Infect. Immun. 561807-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Little, S. F., J. M. Novak, J. R. Lowe, S. H. Leppla, Y. Singh, K. R. Klimpel, B. C. Lidgerding, and A. M. Friedlander. 1996. Characterization of lethal factor binding and cell receptor binding domains of protective antigen of Bacillus anthracis using monoclonal antibodies. Microbiology 142(Pt 3)707-715. [DOI] [PubMed] [Google Scholar]
- 14.Liu, S., and S. H. Leppla. 2003. Cell surface tumor endothelium marker 8 cytoplasmic tail-independent anthrax toxin binding, proteolytic processing, oligomer formation, and internalization. J. Biol. Chem. 2785227-5234. [DOI] [PubMed] [Google Scholar]
- 15.Mabry, R., K. Brasky, R. Geiger, R. Carrion, Jr., G. B. Hubbard, S. Leppla, J. L. Patterson, G. Georgiou, and B. L. Iverson. 2006. Detection of anthrax toxin in the serum of animals infected with Bacillus anthracis by using engineered immunoassays. Clin. Vaccine Immunol. 13671-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mabry, R., M. Rani, R. Geiger, G. B. Hubbard, R. Carrion, Jr., K. Brasky, J. L. Patterson, G. Georgiou, and B. L. Iverson. 2005. Passive protection against anthrax by using a high-affinity antitoxin antibody fragment lacking an Fc region. Infect. Immun. 738362-8368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moayeri, M., and S. H. Leppla. 2004. The roles of anthrax toxin in pathogenesis. Curr. Opin. Microbiol. 719-24. [DOI] [PubMed] [Google Scholar]
- 18.Moayeri, M., J. F. Wiggins, and S. H. Leppla. 2007. Anthrax protective antigen cleavage and clearance from the blood of mice and rats. Infect. Immun. 755175-5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nam, J. M., S. I. Stoeva, and C. A. Mirkin. 2004. Bio-bar-code-based DNA detection with PCR-like sensitivity. J. Am. Chem. Soc. 1265932-5933. [DOI] [PubMed] [Google Scholar]
- 20.Nam, J. M., C. S. Thaxton, and C. A. Mirkin. 2003. Nanoparticle-based bio-bar codes for the ultrasensitive detection of proteins. Science 3011884-1886. [DOI] [PubMed] [Google Scholar]
- 21.Park, S., and S. H. Leppla. 2000. Optimized production and purification of Bacillus anthracis lethal factor. Protein Expr. Purif. 18293-302. [DOI] [PubMed] [Google Scholar]
- 22.Pomerantsev, A. P., R. Sitaraman, C. R. Galloway, V. Kivovich, and S. H. Leppla. 2006. Genome engineering in Bacillus anthracis using Cre recombinase. Infect. Immun. 74682-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quinn, C. P., V. A. Semenova, C. M. Elie, S. Romero-Steiner, C. Greene, H. Li, K. Stamey, E. Steward-Clark, D. S. Schmidt, E. Mothershed, J. Pruckler, S. Schwartz, R. F. Benson, L. O. Helsel, P. F. Holder, S. E. Johnson, M. Kellum, T. Messmer, W. L. Thacker, L. Besser, B. D. Plikaytis, T. H. Taylor, Jr., A. E. Freeman, K. J. Wallace, P. Dull, J. Sejvar, E. Bruce, R. Moreno, A. Schuchat, J. R. Lingappa, S. K. Martin, J. Walls, M. Bronsdon, G. M. Carlone, M. Bajani-Ari, D. A. Ashford, D. S. Stephens, and B. A. Perkins. 2002. Specific, sensitive, and quantitative enzyme-linked immunosorbent assay for human immunoglobulin G antibodies to anthrax toxin protective antigen. Emerg. Infect. Dis. 81103-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosi, N. L., and C. A. Mirkin. 2005. Nanostructures in biodiagnostics. Chem. Rev. 1051547-1562. [DOI] [PubMed] [Google Scholar]
- 25.Schaffer, P., H. Ionesco, A. Ryter, and G. Balassa. 1963. Sporulation of Bacillus subtilis: genetic and physiological study. Colloq. Int. CNRS 124553-563. [Google Scholar]
- 26.Soukka, T., K. Antonen, H. Harma, A. M. Pelkkikangas, P. Huhtinen, and T. Lovgren. 2003. Highly sensitive immunoassay of free prostate-specific antigen in serum using europium(III) nanoparticle label technology. Clin. Chim. Acta 32845-58. [DOI] [PubMed] [Google Scholar]
- 27.Soukka, T., J. Paukkunen, H. Harma, S. Lonnberg, H. Lindroos, and T. Lovgren. 2001. Supersensitive time-resolved immunofluorometric assay of free prostate-specific antigen with nanoparticle label technology. Clin. Chem. 471269-1278. [PubMed] [Google Scholar]
- 28.Tang, S., J. Zhao, J. J. Storhoff, P. J. Norris, R. F. Little, R. Yarchoan, S. L. Stramer, T. Patno, M. Domanus, A. Dhar, C. A. Mirkin, and I. K. Hewlett. 2007. Nanoparticle-based biobarcode amplification assay (BCA) for sensitive and early detection of human immunodeficiency type 1 capsid (p24) antigen. J. Acquir. Immune. Defic. Syndr. 46231-237. [DOI] [PubMed] [Google Scholar]
- 29.Turk, B. E. 2007. Manipulation of host signalling pathways by anthrax toxins. Biochem. J. 402405-417. [DOI] [PubMed] [Google Scholar]
- 30.Varughese, M., A. Chi, A. V. Teixeira, P. J. Nicholls, J. M. Keith, and S. H. Leppla. 1998. Internalization of a Bacillus anthracis protective antigen-c-Myc fusion protein mediated by cell surface anti-c-Myc antibodies. Mol. Med. 487-95. [PMC free article] [PubMed] [Google Scholar]
- 31.Varughese, M., A. V. Teixeira, S. Liu, and S. H. Leppla. 1999. Identification of a receptor-binding region within domain 4 of the protective antigen component of anthrax toxin. Infect. Immun. 671860-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williamson, E. D., I. Hodgson, N. J. Walker, A. W. Topping, M. G. Duchars, J. M. Mott, J. Estep, C. Lebutt, H. C. Flick-Smith, H. E. Jones, H. Li, and C. P. Quinn. 2005. Immunogenicity of recombinant protective antigen and efficacy against aerosol challenge with anthrax. Infect. Immun. 735978-5987. [DOI] [PMC free article] [PubMed] [Google Scholar]