Abstract
Determination of the avidity of immunoglobulin G (IgG) directed against a specific marker has become an established diagnostic tool for identifying or excluding acute infections with pathogens. A novel assay format termed AVIcomp (avidity competition based on mass action) circumventing the conventional chaotropic format has been developed for determination of the avidity of marker-specific IgG in patient specimens. Its applications for cytomegalovirus (CMV) and Toxoplasma gondii are presented. Specific high-avidity IgG from the patient specimen is selectively blocked using a soluble antigen in a sample pretreatment reagent, and the amount of remaining specific low-avidity IgG is determined relative to that in an untreated control. The comparison of the conventional chaotropic format, represented by the Radim CMV IgG Avidity assay, and the newly developed AVIcomp method, as exemplified by the Architect CMV IgG Avidity assay, on blood drawn within 4 months after seroconversion revealed a sensitivity of 100% (97.3% by an alternative calculation) for the AVIcomp format versus 87.5% (75.7% by an alternative calculation) for the chaotropic avidity assay. The specificity on 312 CMV IgG reactive and CMV IgM nonreactive specimens from pregnant women was 100% for the AVIcomp assay and 99.7% for the conventional avidity assay. The Architect Toxo IgG Avidity assay showed an agreement of 97.2% with the bioMérieux Vidas Toxo IgG Avidity Assay employing chaotropic reagents. These performance data suggest that the AVIcomp format shows superior sensitivity and equivalent specificity for the determination of IgG avidity to assays based on the chaotropic method and that the AVIcomp format may also be applicable to other disease states.
Over recent years, numerous publications have shown that the avidity of marker-specific immunoglobulin G (IgG) is a suitable tool for distinguishing between acute and recurrent or past infection with a pathogen (7). Avidity tests have been developed for rubella virus (17), Toxoplasma gondii (5, 8, 18), cytomegalovirus (CMV) (1), varicella-zoster virus (11), human immunodeficiency virus (25), hepatitis viruses (22, 26, 27, 29), Epstein-Barr virus (28), and others. For immunocompetent, untreated individuals, the presence of low-avidity IgG directed against pathogens may indicate a recent infection, whereas the presence of high-avidity IgG excludes a primary infection (13, 14). During the early immune response, IgG antibodies are targeting a multiplicity of different epitopes of the pathogen with relatively low avidity. Clonal selection finally results in high-avidity antibodies directed mainly against a limited number of immunodominant epitopes (5).
For T. gondii infections, high-avidity IgG serves to rule out a recent infection as well; however, low-avidity results are not indicative of a recent or past infection (16). This is due to the fact that the antibody avidity maturation kinetics for T. gondii IgG in response to infection are slow in general and are further slowed in treated patients compared to untreated individuals (2). Accordingly, low-avidity antibodies can persist for more than 1 year and therefore cannot be used to diagnose an acute T. gondii infection (8).
Different methods for antibody avidity determination have been investigated in the past, but only one has become the basis for all current on-market assays for determination of the avidity of IgG directed against infectious agents (4, 6). This chaotropic assay format separates high- and low-avidity antibodies by a denaturing wash step, which elutes low-avidity antibodies from the solid phase (9, 10). The automation of this method represents a problem for immunoanalyzer instruments, since their fluidic systems would need to handle harsh denaturants. Crystallization of urea and reagent cross-contamination using urea-H2O2 (12) or ammonium thiocyanate (19) have been observed as major drawbacks. Other methods to determine avidity, such as Scatchard plots (23) and the Friguet method (4), have been used but were limited to determination of the affinity of purified proteins or monoclonal antibodies. Based on the limitations of these formats, a new and easier-to-automate assay format to measure IgG avidity has been developed and applied for determination of the avidity of anti-CMV and anti-T. gondii IgG.
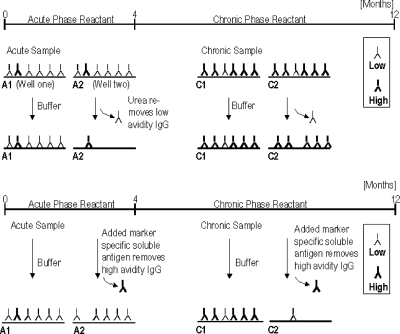
As shown in Fig. 1, low-avidity IgG, as an indicator for a recent infection, is detected indirectly by the conventional avidity method, since only high-avidity IgG remains bound to the solid phase and contributes to signal generation. In contrast, the newly developed AVIcomp (avidity competition) assay detects low-avidity IgG directly by blocking the specific high-avidity IgG of a specimen with soluble antigen and determining the concentration of the remaining marker-specific low-avidity IgG. The name AVIcomp reflects the competition of low- and high-avidity antibodies for binding sites on the soluble antigen. In both newly developed assays, the same antigen used in the pretreatment in soluble form is used as well for coating of the solid phase. The quality of the soluble antigen utilized is different in the two AVIcomp assays. In the CMV assay, the soluble antigen is a CMV lysate containing the CMV proteome, whereas a single recombinant T. gondii antigen is utilized in the Toxo AVIcomp assay. As a consequence, the CMV assay determinates the mean avidity against all CMV proteins, whereas the T. gondii assay determines only the avidity of the fraction of IgG directed against the antigen. In contrast to the Friguet method, the avidity of a polyclonal antibody response is determined using an AVIcomp assay, and the results obtained serve for the staging of an infection with a pathogen.
FIG. 1.
Comparison of conventional (chaotropic) and AVIcomp avidity assays. (Top) Principle of conventional (denaturing) avidity assays; (bottom) principle of the AVIcomp avidity assays.
For some avidity assays, it has been shown that the dilution of the specimen to a defined concentration of specific IgG prior to addition to the solid phase significantly improves the assay performance (3, 15). Accordingly, some assays utilize specific IgG concentration-dependent manual endpoint titration, while others only employ fixed dilution factors independent of the marker-specific IgG level (1, 9, 21). The AVIcomp assays rely on a number of dilution protocols that are automatically selected based on the concentration of marker-specific IgG.
In this report we describe the development and selected performance characteristics of the new Architect CMV and Toxo IgG avidity assays in comparison to commercially available avidity assays utilizing the chaotropic method.
MATERIALS AND METHODS
Development of the AVIcomp assay format.
In a first step, samples are diluted into suitable ranges (see the supplemental material) for the respective assays. Soluble antigen (for CMV, a CMV lysate manufactured at Abbott Park, IL, using CMV strain AD169 grown in cultivated human fibroblasts; for T. gondii, affinity-purified recombinant p30 antigen) is added to a first diluted aliquot of a sample, while a second aliquot is not pretreated with soluble antigen. After the concentrations of the CMV- or T. gondii-specific IgG of both differently pretreated samples are determined, the ratio of the two results allows one to differentiate between low- and high-avidity IgG in samples.
The avidity of the sample, expressed as a percentage, can then be calculated as 100 × [1 − (assay result obtained with soluble antigen/reference result obtained without soluble antigen)]. In this formula, the reduction in the signal due to the neutralization of high-avidity antibodies following the pretreatment with soluble antigen is expressed by the “assay result obtained with soluble antigen,” which is normalized by division by the “reference result obtained without soluble antigen.” The “1 − ratio” term reflects the fact that the AVIcomp format detects low-avidity IgG, in contrast to the chaotropic assay format, where high-avidity IgG is detected. This conversion makes the result directly comparable to the output of avidity assays using the chaotropic format.
For details of reagent composition, see the supplemental material for materials and methods.
Assay description.
The Architect IgG Avidity assays calculate avidity based on two replicates per sample, both of which are separately tested in two-step immunoassays with fully automated sample predilution and pretreatment steps (20).
For all samples that are reactive by the Architect CMV IgG or Toxo IgG assay, the instrument automatically selects one of five different dilution protocols for the avidity assay on the basis of the marker-specific IgG concentration. These protocols dilute the specific IgG concentration determined by the Architect CMV or Toxo IgG assay in every sample to the same final concentration range and are defined similarly for CMV and T. gondii (see the supplemental material). One aliquot of the prediluted sample is pretreated with soluble antigen in buffer as a blocking agent (for CMV, a CMV strain AD169-infected cell lysate; for T. gondii, purified recombinant p30 antigen). A second aliquot of the sample is pretreated with buffer without soluble antigen and serves as reference for the complete amount of marker-specific IgG in the sample. Both aliquots of the pretreated sample are incubated and then combined individually with the solid phase, consisting of antigen-coated paramagnetic microparticles (for CMV, a CMV strain AD169-infected cell lysate; for T. gondii, purified recombinant antigens). After additional incubation and washing, a murine acridinium-labeled anti-human IgG conjugate is added. Following another incubation and wash cycle, pretrigger and trigger solutions are added to the reaction mixture. The resulting chemiluminescent reaction is measured in relative light units. The avidity of anti-CMV (or anti-T. gondii) IgG in the sample is calculated using the relative light units of both tests according to the formula already mentioned: percent avidity = 100 × [1 − (result with soluble antigen/result without soluble antigen)]. As exemplified for the Architect CMV IgG Avidity assay, this formula can also be expressed utilizing the respective assay files employed: percent avidity = 100 × [1 − (CMVAvi1/CMVAvi2)]. Samples with an avidity result of <50% contain predominantly low-avidity IgG directed against the pathogen; samples above 60% avidity contain mainly high-avidity anti-CMV IgG; and samples between 50% and 60% avidity have specific IgG with a gray-zone avidity.
All the assays mentioned here are described in detail in the respective package inserts. Detailed information for the AVIcomp assays can be found in the supplemental material.
Architect instrument.
The Architect instrument is a random-access chemiluminescent microparticle immunoanalyzer with flexible pipetting protocols. A prerequisite for testing the AVIcomp assay format is that it supports automated sample dilution from multiple dilution protocols, sample pretreatment followed by a two-step immunoassay, and calculation of a result from two individual assays.
Specimens used for the development of the AVIcomp assay format. (i) Architect CMV IgG Avidity assay.
To demonstrate the effect of variation of CMV lysate concentrations in the pretreatment solution, three specimens were precharacterized utilizing the Radim CMV IgG Avidity assay (Radim SpA, Pometia, Italy) and were then tested with the Architect CMV IgG avidity assay in five (low- and gray-zone avidity samples) or three (high-avidity sample) replicates. The concentrations of CMV-specific IgG in the specimens, obtained by the Architect assay, and the avidities of the IgG, obtained by the Radim assay, are presented in Table 1.
TABLE 1.
Precharacterization and testing of three specimens by the Radim CMV IgG Avidity assay and Architect CMV IgG assay
| Specimen | % Avidity of IgG by the Radim CMV IgG Avidity assay | IgG concn (AU/ml) by the Architect assay |
|---|---|---|
| High avidity | 77.7 | 185.4 |
| Gray-zone avidity | 39.6 | 201.6 |
| Low avidity | 34.2 | 174.3 |
To establish the optimal CMV-specific IgG concentration range to which all specimens should be automatically diluted by the Architect instrument, a number of manually diluted specimens were tested in triplicate and are represented by two high-avidity and two low-avidity specimens (according to precharacterization by the Radim CMV IgG Avidity assay). The concentrations and avidities of CMV-specific IgG in these specimens are presented in Table 2.
TABLE 2.
Characterization and testing of specimens to establish the optimal CMV-specific IgG concentration range for the Architect CMV IgG Avidity assay
| Specimen | % Avidity of IgG by the Radim CMV IgG Avidity assay | IgG concn (AU/ml) by the Architect CMV IgG assay |
|---|---|---|
| High-avidity specimen 1 | 89.4 | 248.3 |
| High-avidity specimen 2 | 71.7 | 229.7 |
| Low-avidity specimen 1 | 29.2 | 56.8 |
| Low-avidity specimen 2 | 21.7 | 40.8 |
(ii) Architect Toxo IgG Avidity assay.
To establish the optimal T. gondii-specific IgG concentration range to which all specimens should be diluted automatically by the Architect instrument, a single replicate (each) of two high-avidity specimens and one low-avidity specimen (as precharacterized by the Vidas Toxo IgG Avidity assay [bioMérieux, Marcy l'Etoile, France]) was tested. The avidity indices and concentrations of T. gondii-specific IgG in the three specimens used for assay development are presented in Table 3.
TABLE 3.
Characterization and testing of specimens to establish the optimal T. gondii-specific IgG concentration range for the Architect Toxo IgG assay
| Specimen | Avidity index by the Vidas Toxo IgG Avidity assay | IgG concn (IU/ml) by the Architect Toxo IgG assay |
|---|---|---|
| High-avidity specimen 1 | 0.575 | 260.3 |
| High-avidity specimen 2 | 0.353 | 160.7 |
| Low-avidity specimen | 0.045 | 40.5 |
Specimens for IgG avidity determination by the Architect CMV IgG Avidity assay. (i) Specificity testing.
Specimens from pregnant women (n = 312) were obtained from German hospitals and were tested. All specimens in this category were reactive with the Architect CMV IgG assay and nonreactive with the Architect CMV IgM assay. Clinical specificity, expressed as a percentage, was calculated for each assay as 100 × (number of specimens with a high-avidity result/number of all CMV IgG-reactive, non-CMV IgM-reactive specimens tested). For the Radim CMV IgG Avidity assay, the validity criterion for CMV IgG-reactive specimens was an optical density (OD) of >0.300, according to the package insert.
(ii) Sensitivity testing.
The sensitivity of the Architect CMV IgG Avidity assay was assessed by testing three commercially available CMV seroconversion panels (RP-003 and RP-019 [Profile Diagnostics] and PTC-901 [BBI]), with a total of 49 specimens. Clinical sensitivity, expressed as a percentage, was defined as 100 × (number of specimens drawn within 4 months after seroconversion in which CMV-specific IgG was detectable with low-avidity results/number of all specimens drawn within 4 months after seroconversion in which CMV-specific IgG was detectable).
The calculation was performed in two different ways. In calculation method 1, the number of all specimens drawn within 4 months after seroconversion in which CMV-specific IgG was detectable was restricted to those specimens that fulfilled the validity criterion for the respective assay. Per the respective package inserts, the validity criteria are as follows: for the Architect CMV IgG Avidity assay, a reactive assay result; for the Radim CMV IgG Avidity assay, an OD of >0.300; for the Vidas CMV IgG Avidity assay (bioMérieux, Marcy l'Etoile, France), a CMV-specific IgG concentration in the reference aliquot of ≥6 arbitrary units (AU)/ml. In calculation method 2, the number of all specimens drawn within 4 months after seroconversion in which CMV-specific IgG was detectable included all samples that fulfilled the validity criterion of any of the three avidity assays.
Specimens for IgG avidity determination by the Architect Toxo IgG Avidity assay.
The level of agreement between the Architect Toxo IgG Avidity assay and the Vidas Toxo IgG Avidity assay was determined by testing a total of 158 specimens. Fifty-one specimens were from cases of serologically confirmed seroconversion (all reactive with T. gondii-specific IgG and IgM) and were obtained from the Institut de Puériculture et de Périnatalogie, Paris, France. One-hundred seven specimens from pregnant women (T. gondii IgG reactive and T. gondii IgM nonreactive) were obtained from Horst-Schmitt Kliniken (Wiesbaden, Germany).
Additionally, seroconversion panel BW (Antibody Systems, Inc.) was tested on the Architect Toxo IgG, -IgM assay (in development) and the Architect Toxo IgG Avidity assay versus the Vidas Toxo IgG Avidity assay.
Competitor assays for the Architect CMV IgG and Toxo IgG Avidity assays.
The Architect CMV IgG Avidity assay was compared primarily to the Radim CMV IgG Avidity assay, a microtiter plate-based assay that employs the chaotropic assay format. The populations for specificity and sensitivity testing were tested completely by the Radim CMV IgG Avidity assay using the automated DSX microtiter plate system (Dynex Technologies, Inc., Chantilly, VA). Selected specimens were tested by the Vidas CMV IgG Avidity assay, a partially automated chaotropic CMV IgG Avidity assay on the bioMérieux mini-Vidas platform (bioMérieux, Marcy l'Etoile, France). All on-market assays were tested according to the respective package inserts.
For evaluation of the Architect Toxo IgG Avidity assay, the Vidas Toxo IgG Avidity assay was used.
RESULTS
Development of the AVIcomp assay format.
The decision to develop an AVIcomp avidity assay was made based on the initial observation that the addition of a soluble CMV lysate to a specimen before testing of its CMV-specific IgG concentration allows one to differentiate between low- and high-avidity specimens. In CMV-specific IgG-positive specimens that contained mainly low-avidity CMV-specific IgG, the CMV-specific IgG concentration remained nearly the same with and without the soluble antigen, while it was strongly reduced in specimens that contained primarily high-avidity antibodies.
The impact of two major factors was investigated to establish the assay principle: different concentrations of the soluble agent in the pretreatment solution and different concentrations of marker-specific IgG using selected high- and low-avidity specimens.
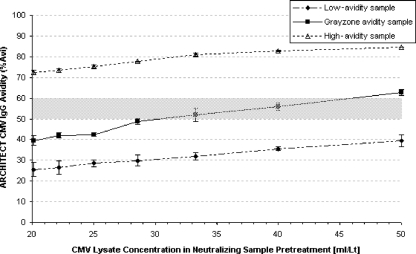
The results of the variation of the soluble CMV antigen concentration with two low-, one gray-zone, and three high-avidity specimens are shown in Fig. 2. The avidity result obtained for a specimen strongly depends on the amount of soluble antigen in the pretreatment blocking solution. Generally, a larger amount of antigen in the blocking reagent leads to more-effective blocking of specific antibodies and therefore to a lower signal in the blocked part of the assay. This causes an increase in the avidity result. For concentrations of the CMV lysate used as the soluble antigen below the range from 30 to 100 ml/liter, the separation of low- and high-avidity specimens decreases markedly. However, within this range, low- and high-avidity specimens can be sufficiently discriminated. Thus, titration of the soluble-antigen concentration within this range is performed to adjust the cutoff of newly manufactured reagent lots. To standardize the adjustment, a specimen with gray-zone avidity was selected, which aids in anchoring the performance of every new reagent lot to those reagents that served to determine the optimal position of the cutoff according to a ROC (receiver operator characteristic) analysis performed early in the development process. The results of this ROC analysis have been confirmed by the data presented in this study (see below).
FIG. 2.
CMV IgG avidity as a function of CMV antigen concentration. Three samples precharacterized as low, gray zone, or high avidity by the Radim CMV IgG Avidity assay were tested using seven different antigen concentrations in the neutralizing pretreatment solution. The error bars indicate 1 standard deviation. The gray field between 50 and 60% avidity represents the gray zone of the Architect CMV IgG Avidity assay.
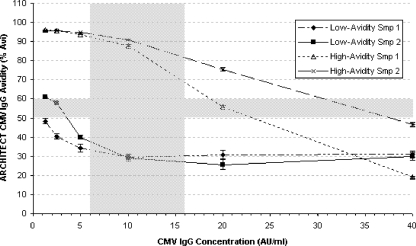
The effect of alteration of the concentration of the marker-specific IgG in the specimen is demonstrated for the Architect CMV IgG Avidity assay by the testing of two specimens, each precharacterized as low or high avidity, respectively. Before testing, these specimens were diluted in Architect wash buffer to a known specific IgG concentration. Figure 3 shows that the avidity results obtained for high- and low-avidity specimens depend on the concentration of CMV-specific IgG in the specimen at a fixed concentration of soluble antigen. Specimens with low marker-specific IgG concentrations are more efficiently blocked than specimens with high marker-specific IgG concentrations. Specimens precharacterized as having high avidity show a tendency to give false low-avidity results when the specific-IgG concentration rises. Specimens precharacterized as having low avidity tend to give false high-avidity results when the specific-IgG concentration decreases. These results revealed a range of specific-IgG concentrations within which all specimens correlated well with the precharacterization results. Based on similar results for a number of specimens tested in this way (data not shown), an optimal concentration range between 0.6 and 1.9 AU/ml was established for specimens with a CMV-specific IgG concentration from 6 to 500 AU/ml, corresponding to dilution protocols 1 to 4. Specimens with a CMV-specific IgG concentration from 500 to 2,500 AU/ml are diluted into the range from 0.6 to 3.0 AU/ml (dilution protocol 5). See the supplemental materials for details.
FIG. 3.
Impact of the CMV IgG concentration on the CMV IgG Avidity assay result. Four samples (Smp) precharacterized by the Radim CMV IgG Avidity assay as low or high avidity (two samples at each level) were diluted to six concentrations of specific IgG before testing. % Avi, percent avidity. The error bars represent 1 standard deviation; the horizontal gray field represents the gray zone of the assay; and the vertical gray field represents the target dilution range, into which the final assay dilutes every sample automatically before the avidity of the specific IgG is determined.
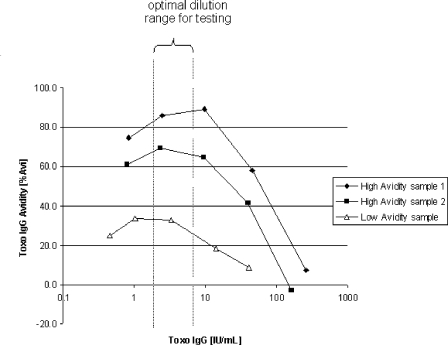
Similar experiments were performed for T. gondii-specific IgG avidity using the Architect Toxo IgG avidity assay as a measuring system. Figure 4 shows the effect of variation of the T. gondii-specific IgG concentration on the avidity results obtained for high- and low-avidity specimens. The three specimens also show marker-specific IgG concentration-dependent avidity results, as has been found for CMV; however, the corresponding curves for high- and low-avidity specimens all reflect a plateau in the optimal dilution range, and below or above this concentration range, avidity values tend to become lower. The concentrations of anti-T. gondii IgG in the samples in this experiment are remarkably lower than the concentrations obtained by automated dilution performed by the Architect instrument. With decreasing concentrations of IgG, the proportion of the instrument background signal rises and leads to low-avidity results. This effect does not occur for CMV, where, at the highest dilutions tested, the specific signal is still remarkably higher than the background.
FIG. 4.
Impact of the T. gondii IgG concentration on the Toxo IgG Avidity assay result. Three samples precharacterized by the Biomerieux Vidas Toxo IgG Avidity assay as low (one sample) or high (two samples) avidity were diluted to five concentrations of specific IgG before testing using the Architect Toxo IgG Avidity assay. % Avi, percent avidity.
Dilution protocols for the Architect Toxo IgG Avidity assay for T. gondii-specific IgG concentrations between 1.6 and 2,000.0 IU/ml were determined analogously to those for the Architect CMV IgG avidity assay. See the supplemental materials for details.
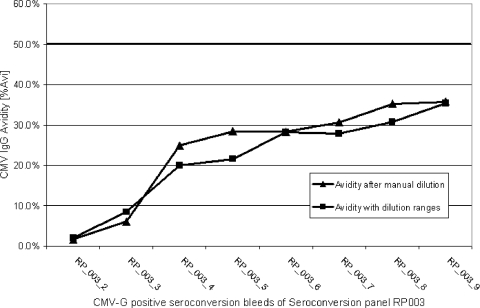
The Architect instrument allows one to perform specimen-specific dilution protocols that are selected automatically based on the IgG result obtained previously (e.g., CMV-specific IgG) for the specimen as described above. Figure 5 demonstrates that there are no qualitative differences, and only minor quantitative differences, between specimens diluted manually to an endpoint (1.2 AU/ml) or diluted within specific ranges using the automated dilution protocols on the instrument, as shown for selected early-seroconversion specimens with quickly rising marker-specific IgG titers (see also Table 5).
FIG. 5.
Manual versus automated sample dilution in Architect wash buffer exemplified for the Architect CMV IgG Avidity assay. Shown is a comparison of automatically performed dilution of samples to the optimal testing range versus manual endpoint dilution to a fixed concentration of CMV-specific IgG (10 AU/ml). % Avi, percent avidity.
TABLE 5.
Performances of different CMV assays on the commercially available seroconversion panel RP-003 (Profile Diagnostics)
| Day of sampling | Resulta by the following assay:
|
||||
|---|---|---|---|---|---|
| Architect CMV IgG (IgG concn [AU/ml]) | Architect CMV IgM (IgM index) | Architect CMV IgG Avidity (% avidity) | Radim CMV IgG Avidity (% avidity) | Vidas CMV IgG Avidity (% avidity) | |
| 1 | 8.1 | 1.65 | 1.0 | Invalid | ND |
| 4 | 21.8 | 4.29 | 2.4 | Invalid | 3 |
| 8 | 51.1 | 4.42 | 10.5 | Invalid | 5 |
| 51 | 86.2 | 1.40 | 25.4 | 24.1 | 30 |
| 55 | 94.1 | 1.32 | 24.7 | 31.2 | 31 |
| 59 | 91.2 | 1.42 | 30.4 | 27.9 | 31 |
| 65 | 84.0 | 1.20 | 31.8 | 29.6 | 36 |
| 67 | 85.1 | 1.19 | 32.7 | 30.3 | 36 |
| 72 | 80.8 | 1.12 | 37.9 | 50.2 | 34 |
| 74 | 78.4 | 1.10 | 39.9 | 28.4 | 33 |
| 79 | 80.3 | 1.11 | 40.5 | 31.0 | 38 |
| 84 | 85.1 | 1.11 | 39.4 | 43.3 | 39 |
| 88 | 94.7 | 1.13 | 40.3 | 26.7 | 43 |
| 95 | 87.1 | 1.09 | 45.1 | 45.7 | 39 |
| 99 | 89.4 | 1.03 | 44.9 | 44.1 | 43 |
Boldface data represent correct interpretations (with respect to the sensitivity definition for the Architect CMV IgG avidity assay). The cutoff for the Architect CMV IgG assay was a CMV-specific IgG concentration of 6 AU/ml. For the Architect CMV IgM assay, the cutoff was an IgM index of 1.00, and the upper limit of the gray zone was an index of 0.85. For the Architect, Radim, and Vidas CMV IgG Avidity assays, the cutoff and upper limit of the gray zone for specific IgG avidity were 50 and 60%, 35 and 45%, and 20 and 80%, respectively. ND, not determined.
Performance results for the Architect CMV IgG Avidity assay. (i) Specificity.
The specificity of the Architect CMV IgG Avidity assay was evaluated by testing specimens from pregnant women that were reactive with the Architect CMV IgG assay and nonreactive with the Architect CMV IgM assay. This precharacterization reflects the assumption that the serological state of being IgG reactive and non-IgM reactive represents specimens with past infection that should have marker-specific IgG of high avidity. A total of 312 specimens from pregnant women with this serological precharacterization were tested for CMV. The specificity of the Architect CMV IgG Avidity assay was primarily determined versus the result that should be expected based on the serological precharacterization as “clinical specificity.” The same strategy was applied using the data of the Radim CMV IgG Avidity assay. Specimens with gray-zone avidity results were removed from the observed-specificity calculations of both assays.
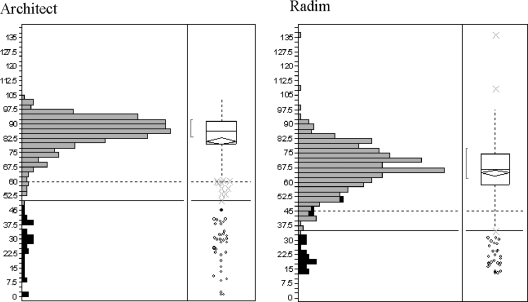
The distribution of results and the specificity for the tested population are shown in Table 4 and Fig. 6. The clinical specificity of the Architect CMV IgG Avidity assay on 312 specimens from CMV-immune donors versus serological precharacterization was 100% (309/309 specimens, with 3 gray-zone specimens excluded). The Radim assay showed a slightly lower clinical specificity of 99.7% (299/300 specimens, with 11 gray-zone specimens and 1 specimen in which specific IgG was not detectable excluded), with more gray-zone results. Different specimens were identified as having gray-zone avidity in the two avidity assays. The histograms show a tighter distribution of results by the Architect CMV IgG Avidity assay than by the Radim assay (Fig. 6).
TABLE 4.
Specificity results for the Architect versus the Radim CMV IgG Avidity assay
| Avidity by the Architect assay | No. of specimensa with the following level of avidity by the Radim assay:
|
||||
|---|---|---|---|---|---|
| Low | Gray zone | High | Not detectable | Total | |
| Low | 0 | 0 | 0 | 0 | 0 |
| Gray zone | 0 | 0 | 3 | 0 | 3 |
| High | 1 | 11 | 296 | 1 | 309 |
| Not detectable | 0 | 0 | 0 | 0 | 0 |
| Total | 1 | 11 | 299 | 1 | 312 |
A total of 312 specimens from pregnant women, all positive for CMV-specific IgG and negative for CMV-specific IgM, were tested.
FIG. 6.
IgG avidity by the Architect and Radim CMV IgG Avidity assays. Histograms show data from ROC analysis. Light shaded bars, specimens tested for specificity; dark shaded bars, specimens tested for seroconversion sensitivity. % Avi, percent avidity. Numbers of specimens, given below the histograms, include all specimens from specificity and sensitivity testing with a valid avidity result (IgG reactivity for the Architect CMV IgG Avidity assay; an OD of >0.300 for the Radim CMV IgG Avidity assay).
(ii) Seroconversion sensitivity.
Specimens drawn within 4 months after seroconversion should be of low avidity to allow a retrospective diagnosis at the end of the first trimester of pregnancy, including the time shortly before conception. Therefore, sensitivity has been defined as the ratio of the number of early-seroconversion specimens identified as having low-avidity marker-specific IgG to the number of all early-seroconversion specimens in which marker-specific IgG was detected. See Materials and Methods for the explanation of the two alternative calculation approaches.
The ability to detect low-avidity marker-specific IgG in early-phase seroconversion blood samples was evaluated by testing three commercially available seroconversion panels (RP-003, RP-019, and PTC901). The results for RP-003 are shown in Table 5; a summary of the results of all seroconversion panel tests is given in Table 6.
TABLE 6.
Summary of the seroconversion sensitivities of the Architect, Radim, and Vidas CMV IgG Avidity assays
| Assay and time of blood draw after seroconversiona | No. of blood specimens:
|
Sensitivity (%) by:
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | With detectable CMV-specific IgG
|
Calculation method 1b | Calculation method 2b | |||||||||||
| Total | Low avidity | Gray-zone avidity | High avidity | |||||||||||
| Architect CMV IgG Avidity assay | ||||||||||||||
| 0-3 mo | 35 | 34 | 34 | 0 | 0 | 100.0 | 97.1 | |||||||
| 0-4 mo | 37 | 36 | 36 | 0 | 0 | 100.0 | 97.3 | |||||||
| Radim CMV IgG Avidity assay | ||||||||||||||
| 0-3 mo | 35 | 30 | 28 | 1 | 1 | 93.3 | 80.0 | |||||||
| 0-4 mo | 37 | 32 | 28 | 2 | 2 | 87.5 | 75.7 | |||||||
| Vidas CMV IgG Avidity assay | ||||||||||||||
| 0-3 mo | 35 | 31 | 5 | 26 | 0 | 16.1 | 14.3 | |||||||
| 0-4 mo | 37 | 33 | 5 | 28 | 0 | 15.2 | 13.5 | |||||||
All the assays tested were able to detect the early stage of seroconversion, since the validity criteria of the Architect (CMV-specific IgG concentration, ≥6.0 AU/ml), Radim (OD, >0.300), and Vidas (CMV-specific IgG concentration in the reference aliquot, ≥6 AU/ml) CMV IgG avidity assays are fulfilled.
For an explanation of the different calculation methods, see Materials and Methods.
The Architect CMV IgG Avidity assay yielded low-avidity results for 36 of 36 seroconversion specimens that fulfilled the validity criteria of this assay (100%). The Radim CMV IgG Avidity assay yielded low-avidity results for 28 of 32 specimens in which CMV-specific IgG was detectable (87.5%); 2 specimens were in the gray zone (6.3%), and 2 had high avidity (6.3%). Of the total number of 37 specimens in which CMV-specific IgG was detectable by any of the assays evaluated following seroconversion, 1 specimen could not be measured by the Architect CMV IgG Avidity assay, because it was nonreactive in the Architect CMV IgG assay. The Radim assay failed to detect CMV-specific IgG in five specimens in which it was detectable by the Architect assay based on the validity criteria of the respective assays.
The Vidas CMV IgG Avidity assay was tested on the three commercially available seroconversion panels, too. For 5 of 33 specimens in which CMV-specific IgG was detectable (15.2%), a low-avidity result was obtained, allowing the identification of a primary infection. The Vidas assay was not able to detect 4 of the 37 specimens in which CMV-specific IgG was detectable following seroconversion by any of the assays evaluated.
The alternative calculation method for sensitivity, method 2, yielded a sensitivity of 97.3% (36/37) for the Architect, 75.7% (28/37) for the Radim, and 13.5% (5/37) for the Vidas CMV IgG Avidity assay (Table 6).
(iii) ROC analysis.
A ROC curve is a plot of the true-positive rate against the false-positive rate for the different possible cut points of a diagnostic test. Specificity and sensitivity populations were utilized to perform a ROC analysis to confirm the correct position of the cutoff for the Architect CMV IgG Avidity assay. On the population tested, the sensitivity (determined according to calculation method 1) and specificity were 100% for a cutoff position between 45.1 and 53.2% avidity, with an area under the ROC curve of 1.0, which confirms the cutoff at 50% avidity. The corresponding result for the Radim assay was an area under the curve of 0.995, reflecting the fact that there is no cutoff that differentiates completely between low- and high-avidity samples. Figure 6 shows the corresponding histograms of the distributions of all specificity and sensitivity data for both assays in relation to the cutoff values at 50% and 35% avidity, respectively.
Performance results for the Architect Toxo IgG avidity assay.
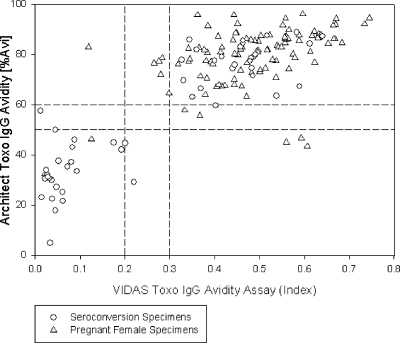
The level of agreement between the Architect Toxo IgG avidity assay and the Vidas Toxo IgG Avidity assay was 97.2% (140/144 specimens) when gray-zone results in both assays were excluded and 90.3% when gray-zone results were included (140/155 specimens). The results are shown in Fig. 7. Four specimens from pregnant women showed discordant results between the two assays. For one specimen, the Architect Toxo IgG Avidity assay gave a high-avidity result (82.9% avidity), whereas the Vidas Toxo IgG Avidity assay gave a low-avidity result (avidity index, 0.119). In three other cases, the Vidas Toxo IgG Avidity assay yielded high-avidity results whereas the Architect Toxo IgG Avidity assay showed low-avidity results.
FIG. 7.
Agreement between the Architect and Vidas Toxo IgG Avidity assays on specimens from pregnant females (n = 104) and on seroconversion specimens (n = 51). The gray zones of both assays are indicated by dashed lines (avidity index, 0.2 to 0.3; percent avidity, 50 to 60%). The relative agreement between the Architect and Vidas Toxo IgG Avidity assays was 97.2% (140/144 specimens). % Avi, percent avidity.
The results obtained for seroconversion panel BW are shown in Table 7. Both avidity assays yielded the same interpretation for every specimen, with one exception: The first Architect Toxo IgG assay-positive specimen with a low-avidity interpretation tested negative for T. gondii-specific IgG by the Vidas Toxo IgG assay and therefore could not be tested for avidity using the Vidas Toxo IgG Avidity assay.
TABLE 7.
Performances of different T. gondii assaysa on the commercially available seroconversion panel BW
| Specimen | Time since last negative bleed
|
Result by the following assayb:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Architect Toxo IgG (IgG concn [IU/ml]) | Vidas Toxo IgG (IgG concn [IU/ml]) | Architect Toxo IgM (IgM index) | Architect Toxo IgG Avidity
|
Vidas Toxo IgG Avidity
|
|||||
| Days | Mo | % Avidity | Avidity interpretation | Avidity index | Avidity interpretation | ||||
| BW01 | N/A | N/A | 0.0 | Neg | 0.59 | NT | N/A | NT | N/A |
| BW02 | N/A | N/A | 1.1 | Neg | 7.18 | NT | N/A | NT | N/A |
| BW03 | 0 | 0 | 3.7 | Neg | 7.79 | 2 | Low | NT | N/A |
| BW04 | 44 | 1.4 | 119.9 | 200 | 5.22 | 32 | Low | 0.070 | Low |
| BW05 | 46 | 1.5 | 120.9 | 210 | 5.00 | 32 | Low | 0.067 | Low |
| BW06 | 55 | 1.8 | 144.2 | 300 | 4.63 | 33 | Low | 0.073 | Low |
| BW07 | 57 | 1.9 | 143.5 | 275 | 4.66 | 36 | Low | 0.077 | Low |
| BW08 | 65 | 2.1 | 127.5 | 271 | 3.97 | 39 | Low | 0.095 | Low |
| BW09 | 87 | 2.9 | 104.6 | 278 | 3.67 | 40 | Low | 0.120 | Low |
| BW10 | 132 | 4.3 | 74.9 | 256 | 2.92 | 50 | Gray zone | 0.208 | Intermediate |
| BW11 | 200 | 6.6 | 47.1 | 185 | 2.09 | 65 | High | 0.378 | High |
| BW12 | 211 | 6.9 | 36.7 | 154 | 2.02 | 69 | High | 0.365 | High |
| BW13 | 226 | 7.4 | 31.0 | 130 | 1.93 | 65 | High | 0.370 | High |
Including the newly developed Architect Toxo panel consisting of IgG and IgM (in development). N/A, not applicable; Neg, negative result; NT, not tested.
For the Architect and Vidas Toxo IgG assays, the cutoffs were 3 and 8 IU of specific IgG/ml, respectively, and the upper limits of the gray zones were 1.6 and 4 IU/ml, respectively. For the Architect Toxo IgM assay, the cutoff was an IgM index of 0.35 and the upper limit of the gray zone was an IgM index of 0.25. For the Architect Toxo IgG Avidity assay, an avidity of <50% was interpreted as low, and the gray zone extended to 60% avidity (i.e., an avidity of >60% was considered high). For the Vidas Toxo IgG Avidity assay, an avidity index of <0.20 was considered low, and the gray zone extended to an index of 0.30 (i.e., an avidity index of >0.30 was considered high).
DISCUSSION
Development of the new AVIcomp assay format.
The newly developed method to determine the mean avidity of polyclonal antibodies in a patient specimen is based on the removal of the high-avidity fraction of marker-specific IgG by pretreatment of the specimen with soluble antigen prior to its addition to the solid phase. A surplus of soluble antigen needs to be added to every specimen in order to allow for sufficient blocking of high-avidity antibodies. On the other hand, an excessive surplus of soluble antigen will lead to the blocking of low-avidity antibodies as well.
Naturally occurring concentrations of anti-CMV IgG and anti-T. gondii IgG in human blood span more than 3 orders of magnitude, posing a general challenge for all avidity assays. Therefore, a dilution of the specimens based on the marker-specific IgG concentration is required in order to produce constant reaction conditions. As observed for both AVIcomp IgG avidity assays, a high-avidity specimen with too much marker-specific IgG will give a false low-avidity reading, because the blocking capacity of the soluble antigen is limiting. On the other hand, a low-avidity specimen tends to be read as high avidity in the CMV assay if the specimen is overdiluted and the level of low-avidity antibodies is underestimated on the solid phase. This observation does not apply for the determination of the avidity of IgG antibodies to T. gondii, where no false high-avidity results can be obtained by excessive dilution of samples with a low-avidity precharacterization. A potential reason for the greater robustness of the T. gondii AVIcomp assay is the utilization of only one selected recombinant antigen. This antigen can be added at a high concentration to the pretreatment solution, whereas the CMV assay works with a relatively low concentration of a multiplicity of antigens with a limited blocking capacity.
During the assay automation process, five automated dilution protocols were implemented that use the specific-IgG result for the specimen to order the suitable dilution protocol for the avidity assay. The dilution protocols were optimized by running precharacterized specimens using manual and automated dilution in parallel and minimizing the difference between the results.
Testing of a specimen that runs at the border of a dilution range in both adjacent dilution protocols potentially causes shifts, which may in rare cases remarkably impact the quantitative evaluation of the specimen. This problem can be eliminated by manual dilution in Architect wash buffer targeting the center of dilution range 1 (10 AU/ml), based on the Architect CMV-specific IgG result, and testing of the specimen in dilution range 1.
In contrast to dilution protocols 1 to 4, which cover the range from 6 to 500 AU/ml, for which the ratio of the higher to the lower range limit is about 3, the fifth dilution range is larger, with a ratio of 5. This reflects the experience that the robustness of the Architect CMV IgG Avidity assay is greater for specimens with CMV-specific IgG concentrations of >500 AU/ml, which may be due to the fact that these specimens contain predominantly marker-specific IgG of very high avidity. For the Architect Toxo IgG Avidity assay, analogous observations were made and assay-specific ranges were established.
IgG titer-dependent specimen dilution has been proposed in recent publications for the chaotropic avidity format as well (3, 15), in order to improve performance.
The automated dilution protocol ordering based on the respective IgG test result, which is embedded in a general reflex testing algorithm for the entire CMV or Toxo IgG, IgM and IgG Avidity assay set, simplifies the overall handling requirements. Reflex testing allows one to proceed from obtaining the initial marker-specific IgG test result to the final avidity determination without the need for any additional manual handling step.
Mode of operation of the AVIcomp format.
Upon the addition of a corresponding soluble antigen, marker-specific IgG with high avidity will form immune complexes and show a slight tendency to dissociate from the soluble antigen based on the low KD (equilibrium dissociation constant). In contrast, marker-specific IgG of low avidity tends to bind less stably to the soluble antigen, leading to a higher concentration of unbound low-avidity IgG than of unbound high-avidity IgG in the solution under equilibrium conditions.
The coating of the solid phase consists of antigen that is adsorbed onto the surfaces of microparticles with high density, allowing specific antibodies to bind with both binding sites. This is in contrast to the relatively low concentration of soluble antigen in solution, where only antibodies with high avidity will be able to saturate both valencies. Accordingly, the solid phase has been described as a trap for antibodies (5), because the local antigen concentration is supposed to be high enough to efficiently prevent the dissociation of antibodies. Upon the addition of a sample containing specific antibodies, a boundary layer forms on the microparticle surface. Antibodies from the solution are rapidly depleted by the high local concentration of surface-bound antigen, and replenishment is limited by diffusion. However, the homogeneous distribution of antigen-coated microparticles during the incubation phase and mixing steps reduces the influence of diffusion in the Architect assays, thus aiding in the rapid achievement of equilibrium conditions.
Performance of the Architect IgG Avidity assays.
The specificity data obtained for specimens from pregnant women in the CMV AVIcomp format and by the Radim CMV IgG Avidity assay differ slightly. Based on the high-avidity result obtained by the Architect CMV IgG Avidity assay, a recently acquired primary CMV infection (target, 4 months) can be excluded for 309 of 312 specimens (99.0%), whereas the Radim assay allows this conclusion only for 299 of 312 specimens (95.8%), a difference caused predominantly by the higher number of specimens with gray-zone results, which require further testing to clarify the stage of the CMV infection.
Testing of specimens from the first 4 months after seroconversion revealed a higher seroconversion sensitivity for the AVIcomp format. According to the package insert for the Radim assay, it has been optimized for the indication of a (primary) infection within the past 3 months, whereas blood specimens collected up to 4 months after seroconversion were tested in this study.
Generally, there were two reasons for the performance differences observed during sensitivity testing. For very early specimens, the sensitivity of both competitor CMV IgG avidity assays in the early stage of seroconversion (analytical sensitivity for CMV IgG) was too low to report a valid avidity result (Tables 5 and 6). Apart from one exceptional specimen, the Architect CMV IgG Avidity assay is able to detect low-avidity IgG earlier than the competitors. In addition, the Radim assay revealed a faster increase in IgG avidity on one of the seroconversion panels tested, leading to gray-zone and high-avidity results 84 days after seroconversion, whereas the Architect CMV IgG Avidity assay reported low avidity until the last specimen of the seroconversion panels tested, 99 days after seroconversion. Comparison with the partially automated Vidas CMV IgG Avidity assay is difficult, because for specimens with a result within the range of 20 to 80% avidity, no conclusion regarding the presence or absence of a recent CMV infection can be drawn, according to the package insert. On the seroconversion panels tested, only 5 of 33 specimens in which specific IgG was detectable (15.2%) gave low-avidity results, while 28 of these specimens (84.8%) gave gray-zone results. This assay has possibly been developed to exclude primary infections by a high-avidity result, whereas the Radim and Architect CMV IgG Avidity assays are designed to be utilized for the identification of primary infections by a low-avidity result and for the exclusion of a recently acquired primary infection by a high-avidity result.
The Architect CMV IgG Avidity assay reports low avidity for a longer period of time, and fewer samples from the specificity population give gray-zone or low-avidity results, than in the chaotropic competitor assays. This indicates that the Architect CMV IgG Avidity assay identifies or excludes primary CMV infections in pregnant women more reliably than the chaotropic assay format.
Comparison of the results of the T. gondii AVIcomp assay with those of the competitor assay (the Vidas Toxo IgG assay) demonstrates excellent agreement between the assays. This surprisingly high agreement for two completely different methods allows the conclusion that the two assay formats determine the same property of antibodies by utilizing completely different methods. No further interpretation for the four discordant specimens obtained from pregnant women is available, since precharacterization of the stage of infection is missing. The interpretation of the results on seroconversion panel BW is exactly the same for both assays, except for the first T. gondii-specific IgG-positive specimen, which can be tested only on the Architect instrument due to a higher seroconversion sensitivity of the Architect Toxo IgG assay. The fact that the Architect and other T. gondii-specific IgG assays have higher seroconversion sensitivities than the Vidas Toxo IgG assay has already been observed and described in other studies (24).
Taken together, the competitive antigen format of the Architect IgG avidity assays has several advantages over the chaotropic format of other IgG avidity assays.
No chaotropic reagents need be handled in the AVIcomp assays. These reagents are hazardous or corrosive to automated immunoassay instruments and may also easily cross-contaminate other assays, potentially causing aberrant results.
The AVIcomp format enables one to place the cutoff in the middle of the measuring range (50% avidity) by varying the concentration of soluble antigen in the specimen pretreatment, thus resulting in high- and low-avidity ranges of equal sizes.
The analytical sensitivity of the AVIcomp assays is superior to those of the competitor assays tested, allowing an earlier avidity determination with the new method. Since the AVIcomp format directly detects specific low-avidity IgG without the detour via the determination of high-avidity IgG, higher reliability for the identification of primary infections is to be expected.
In addition, only pathogen-specific high-avidity antibodies are removed by soluble antigen in the AVIcomp format. This is potentially a more specific process than the elution of all specific low-avidity and nonspecific weakly binding antibodies from the solid phase using a chaotropic wash.
In conclusion, the excellent agreement between the chaotropic method and the AVIcomp method suggests additional applications for the new method. The data presented show that the AVIcomp method works with crude lysates from CMV-infected cells as well as with a purified recombinant antigen. We hypothesize that it may be generally possible to use the AVIcomp format for determination of the avidity of IgG directed against infectious agents and to draw a conclusion about the time that has elapsed since the infection was acquired.
Supplementary Material
Acknowledgments
We thank Andrea Karn, Peter Keller, Ingo Stenzel, Christian Awenius, Cordelia Gundlach, Anja Kaufmann, Miriam Gerlach, Claudia Birkenbach, Karina Cordier, and Mirjana Jelovac for excellent technical support during assay development and performance evaluation.
Footnotes
Published ahead of print on 7 January 2009.
Supplemental material for this article may be found at http://jcm.asm.org/.
REFERENCES
- 1.Blackburn, N. K., T. G. Besselaar, B. D. Schoub, and K. F. O'Connell. 1991. Differentiation of primary cytomegalovirus infection from reactivation using the urea denaturation test for measuring antibody avidity. J. Med. Virol. 336-9. [DOI] [PubMed] [Google Scholar]
- 2.Candolfi, E., R. Pastor, R. Huber, D. Filisetti, and O. Villard. 2007. IgG avidity assay firms up the diagnosis of acute toxoplasmosis on the first serum sample in immunocompetent pregnant women. Diagn. Microbiol. Infect. Dis. 5883-88. [DOI] [PubMed] [Google Scholar]
- 3.Dangel, V., U. Bader, and G. Enders. 2006. Improvement of cytomegalovirus avidity testing by adjusting the concentration of CMV-specific IgG in test samples. J. Clin. Virol. 35303-309. [DOI] [PubMed] [Google Scholar]
- 4.Friguet, B., A. F. Chaffotte, L. Djavadi-Ohaniance, and M. E. Goldberg. 1985. Measurements of the true affinity constant in solution by antigen-antibody complexes by enzyme-linked immunosorbent assay. J. Immunol. Methods 77305-319. [DOI] [PubMed] [Google Scholar]
- 5.Harlow, E., and D. Lane. 1988. Antibodies: a laboratory manual, p. 23-52. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 6.Hedman, K., M. Lappalainen, I. Seppälä, and O. Mäkelä. 1989. Recent primary toxoplasma infection indicated by a low avidity of specific IgG. J. Infect. Dis. 159736-740. [DOI] [PubMed] [Google Scholar]
- 7.Hedman, K., M. Lappalainen, M. Söderlund, and L. Hedman. 1993. Avidity of IgG in serodiagnosis of infectious diseases. Rev. Med. Microbiol. 4123-129. [Google Scholar]
- 8.Horváth, K. N., Z. Szénási, J. Danka, and I. Kucsera. 2005. Value of the IgG avidity in the diagnosis of recent toxoplasmosis: a comparative study of four commercially available anti-Toxoplasma gondii IgG avidity assays. Acta Parasitol. 50255-260. [Google Scholar]
- 9.Inouye, S., A. Hasegawa, S. Matsuno, and S. Katow. 1984. Changes in antibody avidity after virus infections: detection by an immunosorbent assay in which a mild protein-denaturing agent is employed. J. Clin. Microbiol. 20525-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamoun, P. P. 1988. Denaturation of globular proteins by urea: breakdown of hydrogen or hydrophobic bonds? Trends Biochem. Sci. 13424-425. [DOI] [PubMed] [Google Scholar]
- 11.Kangro, H. O., S. Manzoor, and D. R. Harper. 1991. Antibody avidity following varicella-zoster virus infections. J. Med. Virol. 33100-105. [DOI] [PubMed] [Google Scholar]
- 12.Kneitz, R. H., J. Schubert, F. Tollmann, W. Zens, K. Hedman, and B. Weissbrich. 2004. A new method for determination of varicella-zoster virus immunoglobulin G avidity in serum and cerebrospinal fluid. BMC Infect. Dis. 433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lazzarotto, T., B. Guerra, M. Lanari, L. Gabrielli, and M. P. Landini. 2008. New advances in the diagnosis of congenital cytomegalovirus infection. J. Clin. Virol. 41192-197. [DOI] [PubMed] [Google Scholar]
- 14.Lazzarotto, T., C. Galli, R. Pulvirenti, R. Rescaldani, R. Vezzo, A. La Gioia, C. Martinelli, S. La Rocca, G. Agresti, L. Grillner, M. Nordin, M. van Ranst, B. Combs, G. T. Maine, and M. P. Landini. 2001. Evaluation of the Abbott AxSYM cytomegalovirus (CMV) immunoglobulin M (IgM) assay in conjunction with other CMV IgM tests and a CMV IgG avidity assay. Clin. Diagn. Lab. Immunol. 8196-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montoya, J. G., O. Liesenfeld, S. Kinney, C. Press, and J. S. Remington. 2002. VIDAS test for avidity of Toxoplasma-specific immunoglobulin G for confirmatory testing of pregnant women. J. Clin. Microbiol. 402504-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petersen, E., M. V. Borobio, E. Guy, O. Liesenfeld, V. Meroni, A. Naessens, E. Spranzi, and P. Thulliez. 2005. European multicenter study of the LIAISON automated diagnostic system for determination of Toxoplasma gondii-specific immunoglobulin G (IgG) and IgM and the IgG avidity index. J. Clin. Microbiol. 431570-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polanec, J., I. Seppala, S. Rousseau, and K. Hedman. 1994. Evaluation of protein-denaturing immunoassays for avidity of immunoglobulin G to rubella virus. J. Clin. Lab. Anal. 816-21. [DOI] [PubMed] [Google Scholar]
- 18.Press, C., J. G. Montoya, and J. S. Remington. 2005. Use of a single serum sample for diagnosis of acute toxoplasmosis in pregnant women and other adults. J. Clin. Microbiol. 433481-3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pullen, G. R., M. G. Fitzgerald, and C. S. Hosking. 1986. Antibody avidity determination by ELISA using thiocyanate elution. J. Immunol. Methods 8683-87. [DOI] [PubMed] [Google Scholar]
- 20.Quinn, F. A. 2001. Bulk reagent random-access analyzer: ARCHITECT I 2000, p. 363-367. In D. Wild (ed.), The immunoassay handbook, 2nd ed. Nature Publishing Group, London, England.
- 21.Revello, M. G., G. Gorini, and G. Gerna. 2004. Clinical evaluation of a chemiluminescence immunoassay for determination of immunoglobulin G avidity to human cytomegalovirus. Clin. Diagn. Lab. Immunol. 11801-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roque-Afonso, A. M., L. Grangeot-Keros, B. Roquebert, D. Desbois, J. D. Poveda, V. Mackiewicz, and E. Dussaix. 2004. Diagnostic relevance of immunoglobulin G avidity for hepatitis A virus. J. Clin. Microbiol. 425121-5124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scatchard, G. 1949. The attractions of proteins for small molecules and ions. Ann. N. Y. Acad. Sci. 51660-672. [Google Scholar]
- 24.Sickinger, E., F. Gay-Andrieu, G. Jonas, J. Schultess, M. Stieler, D. Smith, M. Hausmann, R. Stricker, R. Stricker, J. Dhein, and H.-B. Braun. 2008. Performance characteristics of the new ARCHITECT Toxo IgG and Toxo IgG avidity assays. Diagn. Microbiol. Infect. Dis. 62235-244. [DOI] [PubMed] [Google Scholar]
- 25.Suligoi, B., C. Galli, M. Massi, F. Di Sora, M. Sciandra, P. Pezzotti, O. Recchia, F. Montella, A. Sinicco, and G. Rezza. 2002. Precision and accuracy of a procedure for detecting recent human immunodeficiency virus infections by calculating the antibody avidity index by an automated immunoassay-based method. J. Clin. Microbiol. 404015-4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas, H. I. 1997. Relative functional affinity of specific anti-core IgG in different categories of hepatitis B virus infection. J. Med. Virol. 51189-197. [DOI] [PubMed] [Google Scholar]
- 27.Ward, K. N., W. Dhaliwal, K. L. Ashworth, E. J. Clutterbuck, and C. G. Teo. 1994. Measurement of antibody avidity for hepatitis C virus distinguishes primary antibody responses from passively acquired antibody. J. Med. Virol. 43367-372. [DOI] [PubMed] [Google Scholar]
- 28.Weissbrich, B. 1998. The use of semi-automated EBV IgG avidity determination for the diagnosis of infectious mononucleosis. J. Med. Virol. 54145-153. [DOI] [PubMed] [Google Scholar]
- 29.Zhang, J. Z., S. W. Im, S. H. Lau, T. N. Chau, S. T. Lai, S. P. Ng, M. Peiris, C. Tse, T. K. Ng, and M. H. Ng. 2002. Occurrence of hepatitis E virus IgM, low avidity IgG serum antibodies, and viremia in sporadic cases of non-A, -B, and -C acute hepatitis. J. Med. Virol. 6640-48. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.