Abstract
We evaluated the Prodesse ProFlu-1 real-time reverse transcription-PCR multiplex assay with the SmartCycler instrument for the detection of human respiratory syncytial virus (RSV) and influenza A and B viruses in comparison to conventional cell culture and antigen immunoassays with the BD Directigen A+B and Binax NOW RSV assays over two successive respiratory virus seasons. Ninety-two percent of the 361 specimens tested were nasopharyngeal aspirates obtained from individual patients, of which 119 were positive for RSV and 59 were positive for influenza virus. The median age of the patients whose specimens were positive for RSV and influenza virus were 6.3 months and 42.4 years, respectively. The specificity of all of the methods tested was ≥99%, and the individual sensitivities of NOW RSV, RSV culture, Directigen A+B, influenza virus culture, and the Proflu-1 PCR for influenza/RSV were 82% (95% confidence interval [CI], 73 to 88), 57% (95% CI, 44 to 69), 59% (95% CI, 44 to 72), 54% (95% CI, 38 to 69), and 98% (95% CI, 93 to 100)/95% (95% CI, 85 to 99), respectively. In a clinical setting where viral isolation is performed to confirm rapid antigen immunoassay results for these common respiratory viruses, one-step real-time reverse transcriptase PCR testing can be a more sensitive and timely confirmatory method.
Human respiratory syncytial virus (RSV) and influenza A and B viruses are respiratory pathogens associated with substantial morbidity and mortality annually (43). Virtually all children become infected with RSV within 2 years after birth, and 1% require hospitalization (15). Although the importance of RSV as a cause of pneumonia and brochiolitis in young children is well recognized (21), the most serious morbidity and highest mortality associated with both RSV and influenza virus circulation occurs disproportionately among elderly persons (43). The first-line tests used to detect these virus infections in many hospitals are antigen-based immunoassays. It has been demonstrated that antigen immunoassays have exceedingly poor sensitivity in detecting RSV and influenza virus infections in the elderly, seriously limiting their utility for detecting and confirming institutional or community outbreaks (7, 13, 38). This study was intended to evaluate the performance of viral isolation in cell culture, one-step real-time multiplex reverse transcription-PCR (RT-PCR), and antigen immunoassays for the detection of influenza virus and RSV in respiratory specimens from adults and children during two respiratory virus seasons.
MATERIALS AND METHODS
Specimens.
Upper respiratory tract specimens were collected from 353 individual symptomatic patients during two successive winter respiratory virus seasons encompassing October 2006 to March 2007 and December 2007 to May 2008, when respiratory infection was highly prevalent in our community in southeastern Ontario. The antigen characterization and predominance of the influenza virus strains that were circulating in Canada over these respiratory seasons were 29% A(H1N1), 65% A(H3N2), and 6% B viruses for 2006 to 2007 and 36% A(N1N1), 17% A(H3N2), and 47% B viruses for 2007 to 2008. Patients were tested if they presented with acute respiratory symptoms and were under consideration for admission to the Kingston General Hospital, a 454-bed tertiary-care hospital (410 adult and 44 pediatric beds). Of the 361 specimens tested, 38 were collected by nasopharyngeal swabs, of which 15 (39%) tested positive for RSV or influenza virus. An additional 332 specimens were collected by nasopharyngeal aspiration, of which 178 (54%) tested positive for RSV or influenza virus. Specimens were tested directly within 0.5 h by antigen immunoassay for influenza virus and RSV with the Directigen A+B (BD) and NOW RSV (Binax) assays according to the respective manufacturers' instructions upon receipt at the microbiology laboratory. Specimen aliquots were also frozen at −80°C for subsequent nucleic acid purification and also forwarded at 4°C by courier twice a day at 1200 and 1400 h to a local reference laboratory for virus isolation.
RNA extraction.
Eighty-seven percent of the nucleic acid extractions from frozen specimens were performed within 1 week of collection. Specimens with inhibition as determined by failed amplification of the internal control in the PCR assay were reextracted. DNA extraction was performed with the MagNA Pure Compact instrument (Roche Applied Science, Indianapolis, IN) with Nucleic Acid Isolation Kit I. Briefly, a 400-μl respiratory specimen volume was used for extraction without a prior centrifugation step, and an elution volume of 100 μl was selected. The internal control provided with the ProFlu-1 real-time assay kit (Prodesse, Waukesha, WI) was initially diluted 1:10 according to the manufacturer's instructions and diluted a second time 1:2 with water, and 20 μl of it was included and automatically incorporated into the MagNA Pure Compact isolation process.
Real-time RT-PCR.
Three real-time reverse transcriptase PCR assays were used. The Prodesse Proflu-1 assay detects highly conserved regions of the RSV polymerase gene, influenza B virus nonstructural genes NS1 and NS2, and the influenza A virus matrix gene. The Cepheid RSV ASR (Cepheid, Sunnyvale, CA) assay detects nucleocapsid protein, and the gene targets for the Cepheid influenza virus (Flu A/B) assay are proprietary. The specificities of individual Cepheid RSV and influenza virus assays were evaluated, and both had 100% agreement with 30 specimens negative by viral culture, antigen testing, and Proflu-1 PCR testing. Similarly, these two assays each had 100% agreement with 30 specimens determined to be positive for their respective virus targets after testing by viral culture or antigen testing and Proflu-1 PCR. Specimens that produced discrepant results after initial testing with the one-step multiplex Prodesse ProFlu-1 real-time assay kit were tested further with uniplex real-time PCR assays for either influenza virus or RSV with the influenza virus (Flu A/B) (Cepheid) or RSV (Cepheid) primer and probe sets, respectively. The individual uniplex Cepheid ASR PCR assays for RSV and influenza virus that were performed with Proflu-1 PCR-positive specimens that were also negative by viral culture and antigen testing used the same extracted RNA. All three real-time reverse transcriptase PCR assays were performed with SmartCycler II instruments (Cepheid) and the respective reagent manufacturer's recommended cycling parameters. Each reaction mixture of the ProFlu-1 real-time reverse transcriptase PCR was prepared according to the manufacturer's instructions, which included 5 μl of extracted nucleic acid and 20 μl of a mixture containing Platinum Taq DNA polymerase (5 U/μl; Invitrogen, Carlsbad, CA), murine leukemia virus reverse transcriptase (50 U/μl; Applied Biosystems, Foster City, CA) diluted 1:10 with RT Enzyme Dilution Buffer (Prodesse), and IA/IB/RSV Mix (Prodesse). Each reaction mixture of the assays developed with the influenza virus (Flu A/B) and RSV assay primer and probe sets was prepared according to the manufacturer's instructions and included 5 μl of extracted nucleic acid and 20 μl of a mixture containing one Flu A/B or RSV ASR lyophilized bead, 50 mM MgCl2 (Invitrogen) for Flu A/B only, RNase inhibitor (20 U/μl; Applied Biosystems), RNase-free water (Qiagen), and OneStep RT-PCR reagents consisting of deoxynucleoside triphosphate mix, enzyme mix, and buffer (5×) (Qiagen, Valencia, CA).
Viral isolation.
WI38 (human lung fibroblast) and rhesus monkey kidney cell monolayers in culture tubes were inoculated with 4 drops of antibiotic-treated specimen and incubated at 37°C for 1 h. The cells were then fed with 1.5 ml of cell culture maintenance medium consisting of Eagle's minimum essential medium (Lonaz, Walkersville, MD). The cultures were examined daily for a cytopathic effect. Additionally, cell cultures that were negative for a cytopathic effect at days 5 and 10 had cells scraped off and tested with the D3 Ultra DFA Respiratory Virus Screening and ID kit (Diagnostic HYBRIDS, Athens, OH) as described by the manufacturer. This culture confirmation immunostaining detected viral antigens for influenza A and B viruses, RSV, parainfluenza virus types 1 to 3, and adenovirus.
Determination of test accuracy.
In addition to viral culture, specimens were also defined as true positive for influenza virus or RSV if the Proflu-1 multiplex assay was positive in combination with either a positive antigen immunoassay or a second real-time RT-PCR positive result obtained with a virus-specific real-time RT-PCR assay developed with Cepheid analyte-specific reagents. Data were obtained by testing single specimens from individual patients without repeats.
Statistical analysis.
Statistical analysis was performed on all quantitative data which were considered parametric. The results of different comparisons were analyzed by performing the Student t test on paired data. All P values are two tailed. Calculations were performed with InStat3 (GraphPad Software, La Jolla, CA). Sensitivity, specificity, and positive and negative predictive values were calculated from two-by-two contingency tables for each test. Statistical comparisons were performed on the mean cycle threshold (CT) values of specimens that were positive and negative by viral culture and antigen testing, respectively. Coefficients of determination (r2 values) were determined from the linear correlation of CT values obtained by comparing sequential Proflu-1 and Cepheid singleplex PCR assays by using Microsoft Excel (Microsoft Office 2003; Microsoft Corp., Redmond, WA).
RESULTS AND DISCUSSION
Ninety-two percent of the 361 specimens tested were nasopharyngeal aspirates, and 8% were nasopharyngeal swabs. Of the 38 nasopharyngeal swabs, 5 were positive for RSV and another 10 were positive for influenza virus. The Proflu-1 real-time RT-PCR assay had a specificity of 100% and sensitivities of 94.7% (95% confidence interval [CI], 84.5 to 98.6) and 98.2% (95% CI, 93.0 to 99.7) for the detection of influenza virus and RSV, respectively (Table 1). The accuracy of these results agrees with a previous study of the Proflu-1 assay by LeGoff et al. of a severely diseased pediatric population (28). For our 54 RSV-positive specimens tested by all three methods (119 overall), the RSV positivity rates were 94.7% for PCR, 81.7% for antigen immunoassay, and 56.9% for viral isolation. Similarly, for the 32 influenza virus-positive specimens tested by all three methods (59 overall), the detection rates were 94.7% for PCR, 58.8% for antigen immunoassay, and 53.5% for viral isolation. Other viruses that were isolated in cell culture from individual specimens included 3 isolates of adenovirus, 19 isolates of rhinovirus-like virus, 17 isolates of parainfluenza virus type 3, and 1 isolate each of parainfluenza virus types 1 and 2. One specimen that was positive for RSV by antigen immunoassay and PCR testing was considered a false negative by viral isolation, but a rhinovirus-like virus also grew in cell culture. A second specimen was identified by cell culture as a dual infection with RSV and rhinovirus-like virus. A dual infection with RSV and influenza A virus was detected in only one specimen.
TABLE 1.
Accuracy of the Prodesse Proflu-1, Binax NOW RSV, and BD Directigen Flu A+B assays and conventional virus culturea
| Test method | No. of samples | % Sensitivity | % Specificity | PPV | NPV | TP | FP | TN | FN | Age TP |
|---|---|---|---|---|---|---|---|---|---|---|
| RSV RT-PCR (Proflu-1) | 318 | 98.2 (93.0-99.7) | 100.0 (97.7-100) | 100.0 (95.8-100) | 99.0 (96.2-99.8) | 109 | 0 | 207 | 2 | 3.6 |
| RSV RT-PCR (Proflu-1) without 2nd PCR | 318 | 98.0 (92.4-99.7) | 95.8 (92.0-98.0) | 91.7 (84.5-96.0) | 99.0 (96.2-99.8) | 100 | 9 | 207 | 2 | 3.8 |
| RSV antigen EIAb (NOW RSV) | 270 | 81.7 (73.2-88.1) | 98.7 (94.9-99.8) | 97.9 (92.0-99.6) | 87.9 (81.9-92.2) | 94 | 2 | 153 | 21 | 1.5 |
| RSV culture | 332 | 56.9 (44.1-68.9) | 100.0 (98.2-100) | 100.0 (88.3-100) | 90.5 (86.4-93.5) | 37 | 0 | 267 | 28 | 6.4 |
| Influenza virus RT-PCR (Proflu-1) | 286 | 94.7 (84.5-98.6) | 100.0 (97.9-100) | 100.0 (91.7-100) | 98.7 (96.0-99.7) | 54 | 0 | 229 | 3 | 45.5 |
| Influenza virus RT-PCR (Proflu-1) without 2nd PCR | 286 | 93.3 (80.7-98.3) | 95.0 (91.3-97.3) | 77.8 (64.1-87.5) | 98.7 (96.0-99.7) | 42 | 12 | 229 | 3 | 45.5 |
| Influenza virus antigen EIA (Directigen Flu A+B) | 180 | 58.8 (44.2-72.1) | 99.2 (95.1-100) | 96.8 (81.5-99.8) | 85.9 (79.0-90.9) | 30 | 1 | 128 | 21 | 44.0 |
| Influenza virus culture | 329 | 53.5 (37.8-68.5) | 100.0 (98.3-100) | 100.0 (82.2-100) | 93.5 (89.9-95.9) | 23 | 0 | 286 | 20 | 33.6 |
PPV and NPV are positive and negative predictive values, TP and FP are true and false positives, and TN and FN are true and false negatives, respectively. The age in years for the true positive results of each testing method is given as a mean. Values in parentheses are 95% CIs. The accuracy of each test method was determined without repeated testing of the same specimen or patient.
EIA, enzyme immunoassay.
Seventy-one percent of patients who were tested for RSV and 65.6% of those tested for influenza virus were less than 18 years old. Overall, the highest incidence of RSV positivity was found in pediatric patients, of whom 98 (82.4%) were between 1 month and 2 years old and of whom 10 (8.4%) were <1 month old. The mean/median patient ages associated with all of the specimens tested for RSV and the ages of the patients who tested positive for RSV were 19.7 years/1.5 years and 38.1 months/6.3 months, respectively. In contrast, 38 (64.4%) of the influenza virus-positive samples were from individuals >18 years old, with 20 of those being >65 years old. Overall, with the Directigen A+B assay, the mean/median patient ages associated with the specimens tested and the ages of those patients who tested positive for influenza virus were 22.9 years/1.8 years and 42.0 years/40.5 years, respectively. As a consequence, since viral shedding is generally higher in children, the accuracy of RSV testing in this study should not be generalized to adults and, conversely, the results of influenza virus testing should be generalized to a pediatric population with caution.
An additional 12 influenza virus (22.2% of the total PCR positives)- and 8 RSV (7.3% of total PCR positives)-positive results with the Proflu-1 assay were reclassified from potential false positive to true positive after agreement with either the Cepheid influenza virus A/B or the Cepheid RSV PCR assay. There was highly significant agreement for the correlation of PCR CT measurements between sequential PCR tests for influenza virus (r2 = 0.80, P < 0.0001) and RSV (r2 = 0.84, P < 0.0001) with the Proflu-1 and Cepheid analyte-specific reagent-derived assays. These CT values ranged from 26.47 to 35.98 for RSV and from 20.38 to 35.95 for influenza virus. This correlation of sequential PCR CT values supports the robustness of the determined PCR specificity since CT values are inversely proportional to the amount of target nucleic acid detected. This type of supporting evidence for the use of PCR for influenza virus and RSV testing has not been previously demonstrated. For these positive results obtained only by sequential PCR testing, the CT levels were indicative of an abundant nucleic acid target, defined here as a CT of <29, in 58% and 38% of the nucleic acid extractions for influenza virus and RSV, respectively.
The sensitivity of the Proflu-1 RT-PCR was substantially higher in comparison to viral culture or the Binax NOW RSV and BD Directigen A+B antigen immunoassays (Table 1). The improved sensitivity of PCR over antigen testing and virus isolation for RSV (14, 16, 44, 45) and influenza virus (17, 20, 40, 41, 48) has been previously demonstrated by uniplex, as well as multiplex, testing (4, 23, 28, 32). Studies have also demonstrated improved sensitivity of PCR in combination with culture as a composite “gold standard” or as the alternate reference test for RSV or influenza virus (1, 19, 33, 36, 38). A very small minority of previous studies evaluating PCR detection of influenza virus and RSV have included controls to monitor inhibition due to inadequate extraction and purification of nucleic acids (16, 23, 28, 36). Estimates of sensitivity are incomplete or compromised without the inclusion of an internal control to monitor these potential false-negative results. The extraction of nucleic acids did not include an initial centrifugation of the respiratory specimens. Less inhibition was seen when respiratory specimens were first centrifuged to remove the inhibitors in cellular debris. However, this cellular material contains respiratory virus and the centrifugation step was found to decrease detection sensitivity by removing it (data not shown). In this study, 13% of the specimens tested by PCR had a failed internal control upon initial testing, which was reduced to 9% on repeat testing of the extracts after a freeze-thaw cycle and reduced further to 4% after repetition of the nucleic acid extraction and purification steps. In this study, 25% of the specimens that were initially negative for RSV or influenza virus by PCR in combination with a failed internal control were subsequently determined to be positive on repeat PCR testing with or without repeated extraction.
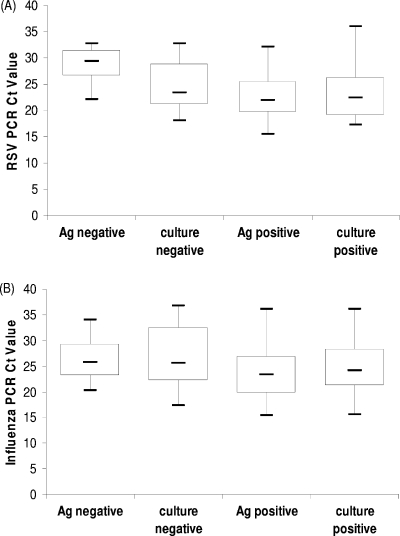
As might be expected for a test with higher sensitivity, the mean PCR CT values were lower for the specimens that also tested positive for RSV or influenza virus by antigen testing or cell culture than for those specimens that tested negative (Fig. 1A and B). Significant differences were observed between the mean PCR CT values of specimens antigen positive and negative by the NOW RSV (P < 0.0001) and Directigen A+B (P = 0.0235) assays. The simplest explanation for these results is found in the larger number of pediatric patients who tested positive for RSV than for influenza virus and the well-described association of high viral shedding in pediatric patients with RSV infections (13).
FIG. 1.
Comparison of PCR CT values obtained with the Proflu-1 multiplex assay for specimens that tested positive or negative for RSV (A) or influenza virus (B) by virus isolation or antigen immunoassay. The differences between the mean PCR CT values of antigen (Ag)-positive and -negative specimens were significant (NOW RSV, P < 0.0001; Directigen A+B, P = 0.0235).
The performance of both the NOW RSV and Directigen A+B assays is at least equal to that of other commercially available antigen immunoassays (2, 6, 7, 25, 38, 47). In this study, the NOW RSV assay had a sensitivity of 81.7% (95% CI, 73.2 to 88.1), which agrees well with the results obtained previously by other laboratories when testing nasopharyngeal aspirates from pediatric patients (Table 2). The Directigen A+B assay had an overall sensitivity of 58.8% (95% CI, 44.2 to 72.1) for the detection of influenza virus. Separate sensitivities of 61.4% (95% CI, 45.5 to 75.3) for the detection of influenza A virus and 42.9% (95% CI, 11.8 to 79.8) for the detection of influenza B virus were obtained, although the number of influenza B virus-positive specimens tested was very small (Table 3). While these results compare well with recent studies (7, 25, 35, 39), there nevertheless exists substantial variability in the reported sensitivity of the Directigen A+B assay for the detection of influenza virus (Table 3). Differences due to both the specimen type tested (24) and patient age (8, 26, 38, 41) have been postulated to affect the sensitivity reported for influenza virus antigen immunoassays with the Directigen A+B and other, similar, commercial products. Neither the association nor a definitive explanation for the observed decrease in antigen immunoassay sensitivity for influenza virus detection in adult versus pediatric patients has been conclusively demonstrated to date. Young children have been reported to have higher attack rates and more prolonged viral shedding (19). Similarly, it has been empirically shown that the sensitivity of influenza virus antigen immunoassays is highest in patients ≤5 years old (38, 41). Patterns of virus shedding and differences in the quality of specimens between age groups are plausible explanations (41). This would help explain the low Directigen A+B assay sensitivity reported here, since despite the testing of samples from patients with a wide age range, the mean age of the influenza virus-positive patients was 42 years. Nevertheless, it is important to recognize that greater than 90% of the deaths due to influenza and its complications occur among elderly persons and nursing home residents are at higher risk of serious influenza-related complications than are elderly persons living in the community (18, 46). Oseltamivir postexposure prophylaxis during nursing home outbreaks is used to reduce serious complications and death but must commence within 48 h of the onset of symptoms (18, 46). In this study, PCR was the only method which provided results both rapid and accurate enough for the effective initiation of antiviral therapy.
TABLE 2.
Comparison of reports of the accuracy of the Binax NOW RSV antigen immunoassay
| Reference | Yr | % Sensitivity | % Specificity | Age when tested | Total no. of tests | No. antigen positive | Specimen(s)a | Reference test(s) |
|---|---|---|---|---|---|---|---|---|
| 31 | 2004 | 87.0 | 94.0 | 6.9 mo | 306 | Not available | NPA | DFA |
| 34 | 2004 | 89.0 | 100.0 | 6 days to adultb | 118 | 31 | NPA, NPW, N | Culture |
| 47 | 2004 | 94.6 | 88.5 | <17.0 yrc | 84 | 35 | NPW | Shell vial |
| 2 | 2004 | 89.2 | 100.0 | <18.0 yrd | 310e | 102 | NPWf | DFA, PCR, cultureg |
| 22 | 2006 | 87.5 | 100.0 | <5.0 yr | 91 | 14 | NPA | DFA, culture |
| 6 | 2006 | 73.0 | 100.0 | <18.0 yr | 130 | 33 | NPAf | DFA, shell vial |
| 10 | 2007 | 81.0 | 93.0 | Not availableh | 14,756 | 794 | NWi | Culture |
| This study | 2008 | 84.0 | 99.0 | Median, 1.5 yrj | 256 | 92 | NPA, NPSj | Culture, PCR |
NPA is nasopharyngeal aspirate, NW is nasal wash, NPS is nasopharyngeal swab, N is nasal, and NPW is nasopharyngeal wash.
71% of the specimens were from persons <32 months old.
81% of the specimens were from persons <3 years old.
80% of the specimens were from persons <12 months old.
Specimens for antigen immunoassays were initially frozen.
Specimens were in 3 ml of viral transport medium.
Viral culture was performed after shipment to referral laboratory with average 36-h delay before setup.
6.5% of the specimens were from persons <1 month old.
96.2% of the specimens were nasal washes.
Data from this study (the median age of RSV-positive patients was 6.2 months) include the testing of 38 nasopharyngeal swab samples, of which 5 were RSV positive.
TABLE 3.
Comparison of reports of the accuracy the BD Directigen A+B antigen immunoassay
| Reference | Yr | Influenza A virus
|
Influenza B virus
|
Age when tested | No. of samples | No. influenza A virus antigen positive | No. influenza B virus antigen positive | Specimena | Reference test(s) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| % Sensitivity | % Specificity | % Sensitivity | % Specificity | ||||||||
| 8 | 2002 | 96.0 | 99.6 | 87.5 | 96.8 | <2 yr to adultb | 250 | 24 | 28 | NPA | Culture, PCRc |
| 37 | 2002 | 82.9 | 100.0 | 51.5 | 100.0 | Not availabled | 160 | 34 | 17 | NPA, TSd | Shell vial |
| 11 | 2003 | 82.4 | 100.0 | 70.0 | 99.6 | Not available | 155 | 14 | 7 | Not available | Shell vial, DFA |
| 38 | 2003 | 86.7 | 97.7 | 86.3 | 97.8 | 1 day to 31 yre | 200 | 13 | 44 | NPA | Culture |
| 25 | 2004 | 61.3 | 100.0 | 50.0 | 100.0 | Not availablef | 77 | 19 | 14 | NPA, NPSg | Culture, DFAg |
| 7 | 2004 | 43.0 | 99.8 | 44.8 | 99.9 | Mean, 3.2 yrh | 4,092 | 49 | 47 | NWi | Culture |
| 35 | 2007 | 41.0 | 98.0 | 50.0 | 99.0 | Median, 44 yrj | 118 | 15 | 3 | NPS | Culture |
| 39 | 2007 | 53.0 | 99.7 | 33.0 | 100.0 | Median, 34 yr | 354 | 38 | 2 | NPW, TS, NW | Culture |
| This study | 2008 | 61.4 | 99.2 | 42.9 | 100.0 | Median, 1.8 yrk | 180 | 27 | 3 | NPA, NPS | Culture, PCR |
NPA is nasopharyngeal aspirate, TS is throat swab, NPS is nasopharyngeal swab, and NW is nasal wash.
80% of specimens from persons <6 years old.
PCR used for specimens that were culture negative but positive by antigen immunoassay or direct immunofluorescence antigen microscopy.
Nasopharyngeal aspirates from pediatric patients (62.2% of positives) and throat swabs from adults (37.8% of positives).
36.5% of the specimens were from pediatric patients.
56.2% of the specimens were from pediatric patients.
Specimens for antigen testing and culture were initially frozen.
2% of the patients were >18 years old.
98% nasal wash, 0.22% nasopharyngeal swab, 1.7% tracheal aspirate, 0.17% bronchoalveolar lavage, 0.1% sinus wash, and 0.06% sputum samples.
Overall estimate with combined specimens for antigen testing and DFA.
Data are from this study (the mean and median ages of influenza virus-positive patients were 42.7 and 42.4 years, respectively), where 33 of 50 samples were influenza A virus positive, 3 of 6 samples were influenza B virus positive, and 10 of 38 nasopharyngeal swab samples were influenza virus positive.
The Binax NOW RSV and Directigen Flu A+B antigen immunoassays had sensitivities that were 25% and 5% higher than that of culture, respectively (Table 1). The thermolability of RSV is well described, and samples must be kept cold during transport without freeze-thawing and be inoculated onto a cell culture as quickly as possible (2, 9). This fact may account for the lower sensitivity of RSV isolation in this study since the inoculation of our specimens onto a cell culture was delayed. The difference between the mean PCR CT values of culture-positive and -negative specimens was not statistically significant for RSV or influenza virus. When this observation is considered together with the similar wide distribution of the interquartile range of PCR CT values (Fig. 1), it suggests that viral nucleic acid was present in a range of quantities in both culture-positive and culture-negative specimens. The simplest explanation for this observation is that the loss of virus culturability occurred with minimal RNA and antigen degradation and that this was an important contributing cause of the lower sensitivities observed with culture. A range of different sensitivities for virus isolation have been reported in other studies. Importantly, a small number of studies have described the immediate culture of pediatric patient specimens as having a very high sensitivity for RSV and influenza virus detection when cell culture was performed on site (20, 37). In addition to the importance of immediate virus isolation for accurate cell culture results, the methodology is also heterogeneous and the sensitivity for the detection of these viruses differs when different cell culture methods are used (12, 27, 29, 42).
Isolation of RSV and influenza virus with R-mix cells can provide positive culture results in as little as 1 to 2 days, whereas conventional tube culture methods typically provide a 5- to 6-day turnaround time for positive specimens (12, 42). The average turnaround times in this study for specimens positive by tube cell culture for RSV and influenza virus were 10.6 and 8.8 days, respectively. This prolonged turnaround time for viral isolation reduced its usefulness in patient management. Moreover, in this study, the sensitivity of viral isolation performed off site was shown to be 53.5% (95% CI, 37.8 to 68.5) and 56.9% (95% CI,44.1 to 68.9) for influenza virus and RSV, respectively, greatly diminishing the use of viral culture as a confirmatory method. In contrast, with real-time RT-PCR, eight specimens required an average of 45 min for the extraction and purification of nucleic acids by a semiautomated method, which was followed by 45 min for the preparation of the Proflu-1 assay and 97 min of cycling run time (a total of 3 h).
The definitive diagnosis of RSV and influenza virus infections depends on the microbiology laboratory. A rapid diagnosis of infections with these viruses is required to implement effective infection control measures to limit nosocomial transmission but is also associated with a reduced length of hospitalization and other hospital-related costs (3, 5, 30). In the case of influenza virus testing in particular, the annual impact of this virus is expected to intensify since people aged ≥80 years are the fastest growing segment of the U.S. population (18). PCR testing represents an alternative to the unacceptably low sensitivity of rapid antigen immunoassays for influenza virus in this high-risk group of people. In clinical practice, antigen immunoassays and direct immunofluorescence antigen (DFA) testing with fluorescent antibody have often been relied upon to make a rapid diagnosis of influenza virus and RSV infections. DFA testing with fluorescent antibody remains an excellent screening test, although the technical time required to obtain results and the subjective nature of the results can be serious limitations. Rapid antigen testing by immunoassay methods remains a very attractive option for laboratories because they are convenient and rapid and possess a high positive predictive value. Although rapid results can be obtained in 30 min by this method, the sensitivity can be low and negative results require confirmation by more sensitive testing. Viral isolation by cell culture has traditionally been the method used for this purpose; however, these results can be delayed, thereby negating the potential impact of confirmatory testing on patient care. In a clinical setting where the sensitivity of viral isolation is less than optimal, real-time reverse transcriptase PCR testing is a more accurate and timely confirmatory test for influenza virus and RSV antigen testing.
Footnotes
Published ahead of print on 7 January 2009.
REFERENCES
- 1.Agoritsas, K., K. Mack, B. K. Bonsu, D. Goodman, D. Salamon, and M. J. Marcon. 2006. Evaluation of the Quidel QuickVue test for detection of influenza A and B viruses in the pediatric emergency medicine setting by use of three specimen collection methods. J. Clin. Microbiol. 442638-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aldous, W. K., K. Gerber, E. W. Taggart, J. Thomas, D. Tidwell, and J. A. Daly. 2004. A comparison of Binax NOW to viral culture and direct fluorescent assays for respiratory syncytial virus. Diagn. Microbiol. Infect. Dis. 49265-268. [DOI] [PubMed] [Google Scholar]
- 3.Barenfanger, J., C. Drake, N. Leon, T. Mueller, and T. Troutt. 2000. Clinical and financial benefits of rapid detection of respiratory viruses: an outcomes study. J. Clin. Microbiol. 382824-2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boivin, G., S. Cote, P. Dery, G. De Serres, and M. G. Bergeron. 2004. Multiplex real-time PCR assay for detection of influenza and human respiratory syncytial viruses. J. Clin. Microbiol. 4245-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonner, A. B., K. W. Monroe, L. I. Talley, A. E. Klasner, and D. W. Kimberlin. 2003. Impact of the rapid diagnosis of influenza on physician decision-making and patient management in the pediatric emergency department: results of a randomized, prospective, controlled trial. Pediatrics 112363-367. [DOI] [PubMed] [Google Scholar]
- 6.Borek, A. P., S. H. Clemens, V. K. Gaskins, D. Z. Aird, and A. Valsamakis. 2006. Respiratory syncytial virus detection by Remel Xpect, Binax NOW RSV, direct immunofluorescent staining, and tissue culture. J. Clin. Microbiol. 441105-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cazacu, A. C., S. E. Chung, J. Greer, and G. L. Demmler. 2004. Comparison of the Directigen Flu A+B membrane enzyme immunoassay with viral culture for rapid detection of influenza A and B viruses in respiratory specimens. J. Clin. Microbiol. 423707-3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan, K. H., N. Maldeis, W. Pope, A. Yup, A. Ozinskas, J. Gill, W. H. Seto, K. F. Shortridge, and J. S. M. Peiris. 2002. Evaluation of the Directigen Flu A+B test for rapid diagnosis of influenza virus type A and B infections. J. Clin. Microbiol. 401675-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins, P. L., and J. E. Crowe, Jr. 2007. Respiratory syncytial virus and metapneumovirus, p. 1601-1646. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 10.Cruz, A. T., A. C. Cazacu, J. M. Greer, and G. J. Demmler. 2007. Performance of a rapid assay (Binax NOW) for detection of respiratory syncytial virus at a children's hospital over a 3-year period. J. Clin. Microbiol. 451993-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunn, J. J., C. Gordon, C. Kelley, and K. C. Carroll. 2003. Comparison of the Denka-Seiken INFLU A·B-Quick and BD Directigen Flu A+B kits with direct fluorescent-antibody staining and shell vial culture methods for rapid detection of influenza viruses. J. Clin. Microbiol. 412180-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunn, J. J., R. D. Woolstenhulme, J. Langer, and K. C. Carroll. 2004. Sensitivity of respiratory virus culture when screening with R-mix fresh cells. J. Clin. Microbiol. 4279-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falsey, A. R., and E. E. Walsh. 2000. Respiratory syncytial virus infection in adults. Clin. Microbiol. Rev. 13371-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falsey, A. R., M. A. Formica, and E. E. Walsh. 2002. Diagnosis of respiratory syncytial virus infection: comparison of reverse transcription-PCR to viral culture and serology in adults with respiratory illness. J. Clin. Microbiol. 40817-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glezen, W. P. 1990. Morbidity associated with the major respiratory viruses. Pediatr. Ann. 19535-540. [DOI] [PubMed] [Google Scholar]
- 16.Goodrich, J. S., and M. B. Miller. 2007. Comparison of Cepheid's analyte-specific reagents with BD Directigen for detection of respiratory syncytial virus. J. Clin. Microbiol. 45604-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gooskens, J., C. M. Swaan, E. C. Claas, and A. C. Kroes. 2008. Rapid molecular detection of influenza outbreaks in nursing homes. J. Clin. Virol. 417-12. [DOI] [PubMed] [Google Scholar]
- 18.Gravenstein, S., and H. E. Davidson. 2002. Current strategies for management of influenza in the elderly population. Clin. Infect. Dis. 35729-737. [DOI] [PubMed] [Google Scholar]
- 19.Grijalva, C. G., K. A. Poehling, K. M. Edwards, G. A. Weinberg, M. A. Staat, M. K. Iwane, W. Schaffner, and M. R. Griffin. 2007. Accuracy and interpretation of rapid influenza tests in children. Pediatrics 119e6-e11. [DOI] [PubMed] [Google Scholar]
- 20.Habib-Bein, N. F., W. H. Beckwith III, D. Mayo, and M. L. Landry. 2003. Comparison of SmartCycler real-time reverse transcription-PCR assay in a public health laboratory with direct immunofluorescence and cell culture assays in a medical center for detection of influenza A virus. J. Clin. Microbiol. 413597-3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall, C. B. 2001. Respiratory syncytial virus and parainfluenza virus. N. Engl. J. Med. 3441917-1928. [DOI] [PubMed] [Google Scholar]
- 22.Jonathan, N. 2006. Diagnostic utility of BINAX NOW RSV—an evaluation of the diagnostic performance of BINAX NOW RSV in comparison with cell culture and direct immunofluorescence. Ann. Clin. Microbiol. Antimicrob. 513-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuypers, J., N. Wright, J. Ferrenberg, M. Huang, A. Cent, L. Corey, and R. Morrow. 2006. Comparison of real-time PCR assays with fluorescent-antibody assays for diagnosis of respiratory virus infections in children. J. Clin. Microbiol. 442382-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Landry, M. L., S. Cohen, and D. Ferguson. 2000. Impact of sample type on rapid detection of influenza virus A by cytospin-enhanced immunofluorescence and membrane enzyme-linked immunosorbent assay. J. Clin. Microbiol. 38429-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Landry, M. L., S. Cohen, and D. Ferguson. 2004. Comparison of Binax NOW and Directigen for rapid detection of influenza A and B. J. Clin. Virol. 31113-115. [DOI] [PubMed] [Google Scholar]
- 26.Landry, M. L., and D. Ferguson. 2003. Suboptimal detection of influenza virus in adults by the Directigen Flu A+B enzyme immunoassay and correlation of results with number of antigen-positive cells detected by cytospin immunofluorescence. J. Clin. Microbiol. 413407-3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LaSala, P. R., K. K. Bufton, N. Ismail, and M. B. Smith. 2007. Prospective comparison of R-mix shell vial system with direct antigen tests and conventional cell culture for respiratory virus detection. J. Clin. Virol. 38210-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LeGoff, J., R. Kara, F. Moulin, A. Si-Mohamed, A. Krivine, L. Bélec, and P. Lebon. 2008. Evaluation of the one-step multiplex real-time reverse transcription-PCR ProFlu-1 assay for detection of influenza A and influenza B viruses and respiratory syncytial viruses in children. J. Clin. Microbiol. 46789-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leland, D. S., and C. C. Ginocchio. 2007. Role of cell culture for virus detection in the age of technology. Clin. Microbiol. Rev. 2049-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macartney, K. K., M. H. Gorelick, M. L. Manning, R. L. Hodinka, and L. M. Bell. 2000. Nosocomial respiratory syncytial virus infections: the cost-effectiveness and cost-benefit of infection control. Pediatrics 106520-526. [DOI] [PubMed] [Google Scholar]
- 31.Mackie, P. L., E. M. McCormick, and C. Williams. 2004. Evaluation of Binax NOW RSV as an acute point-of-care screening test in a paediatric accident and emergency unit. Commun. Dis. Public Health 7328-330. [PubMed] [Google Scholar]
- 32.Marshall, D. J., E. Reisdorf, G. Harms, E. Beaty, M. J. Moser, W. Lee, J. E. Gern, F. Nolte, P. Shult, and J. R. Prudent. 2007. Evaluation of a multiplexed PCR assay for detection of respiratory viral pathogens in a public health laboratory setting. J. Clin. Microbiol. 453875-3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nilsson, A. C., B. Alemo, P. Björkman, L. Dillner, A. Melhus, B. Nilsson, and A. Widell. 2008. Around-the-clock, rapid diagnosis of influenza by means of membrane chromatography antigen testing confirmed by polymerase chain reaction. Infect. Control Hosp. Epidemiol. 29177-179. [DOI] [PubMed] [Google Scholar]
- 34.Ohm-Smith, M. J., P. S. Nassos, and B. L. Haller. 2004. Evaluation of the Binax NOW, BD Directigen, and BD Directigen EZ assays for detection of respiratory syncytial virus. J. Clin. Microbiol. 422996-2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rahman, M., B. A. Kieke, M. F. Vandermause, P. D. Mitchell, R. T. Greenlee, and E. A. Belongia. 2007. Performance of Directigen flu A+B enzyme immunoassay and direct fluorescent assay for detection of influenza infection during the 2004-2005 season. Diagn. Microbiol. Infect. Dis. 58413-418. [DOI] [PubMed] [Google Scholar]
- 36.Rahman, M., M. F. Vandermause, B. A. Kieke, and E. A. Belongia. 2008. Performance of Binax NOW Flu A and B and direct fluorescent assay in comparison with a composite of viral culture or reverse transcription polymerase chain reaction for detection of influenza infection during the 2006 to 2007 season. Diagn. Microbiol. Infect. Dis. 62162-166. [DOI] [PubMed] [Google Scholar]
- 37.Reina, J., E. Padilla, F. Alonso, E. Ruiz De Gopegui, M. Munar, and M. Mari. 2002. Evaluation of a new dot blot enzyme immunoassay (Directigen Flu A+B) for simultaneous and differential detection of influenza A and B virus antigens from respiratory samples. J. Clin. Microbiol. 403515-3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruest, A., S. Michaud, S. Deslandes, and E. H. Frost. 2003. Comparison of the Directigen flu A+B test, the QuickVue influenza test, and clinical case definition to viral culture and reverse transcription-PCR for rapid diagnosis of influenza virus infection. J. Clin. Microbiol. 413487-3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smit, M., K. A. Beynon, D. R. Murdoch, and J. C. Jennings. 2007. Comparison of the NOW Influenza A & B, NOW Flu A, NOW Flu B, and Directigen Flu A+B assays, and immunofluorescence with viral culture for the detection of influenza A and B viruses. Diagn. Microbiol. Infect. Dis. 5767-70. [DOI] [PubMed] [Google Scholar]
- 40.Smith, A. B., V. Mock, R. Melear, P. Colarusso, and D. E. Willis. 2003. Rapid detection of influenza A and B viruses in clinical specimens by Light Cycler real time RT-PCR. J. Clin. Virol. 2851-58. [DOI] [PubMed] [Google Scholar]
- 41.Steininger, C., M. Kundi, S. W. Aberle, J. H. Aberle, and T. Popow-Kraupp. 2002. Effectiveness of reverse transcription-PCR, virus isolation, and enzyme-linked immunosorbent assay for diagnosis of influenza A virus infection in different age groups. J. Clin. Microbiol. 402051-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.St. George, K., N. M. Patel, R. A. Hartwig, D. R. Scholl, J. A. Jollick, L. M. Kauffmann, M. R. Evans, and C. R. Rinaldo. 2002. Rapid and sensitive detection of respiratory virus infections for directed antiviral treatment using R-Mix cultures. J. Clin. Virol. 24107-115. [DOI] [PubMed] [Google Scholar]
- 43.Thompson, W. W., D. K. Shay, E. Weintraub, L. Brammer, C. B. Bridges, N. J. Cox, and K. Fukuda. 2004. Influenza-associated hospitalizations in the United States. JAMA 2921333-1340. [DOI] [PubMed] [Google Scholar]
- 44.van Elden, L. J., A. M. van Loon, A. van der Beek, K. A. Hendriksen, A. I. Hoepelman, M. G. van Kraaij, P. Schipper, and M. Nijhuis. 2003. Applicability of a real-time quantitative PCR assay for diagnosis of respiratory syncytial virus infection in immunocompromised adults. J. Clin. Microbiol. 414378-4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whiley, D. M., M. W. Syrmis, I. M. Mackay, and T. P. Sloots. 2002. Detection of human respiratory syncytial virus in respiratory samples by LightCycler reverse transcriptase PCR. J. Clin. Microbiol. 404418-4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whitley, R. J., and A. S. Monto. 2006. Prevention and treatment of influenza in high-risk groups: children, pregnant women, immunocompromised hosts, and nursing home residents. J. Infect. Dis. 194(Suppl. 2)S133-S138. [DOI] [PubMed] [Google Scholar]
- 47.Zheng, X., S. Quianzon, Y. Mu, and B. Z. Katz. 2004. Comparison of two new rapid antigen detection assays for respiratory syncytial virus with another assay and shell vial culture. J. Clin. Virol. 31130-133. [DOI] [PubMed] [Google Scholar]
- 48.Zitterkopf, N. L., S. Leekha, M. J. Espy, C. M. Wood, P. Sampathkumar, and T. F. Smith. 2006. Relevance of influenza A virus detection by PCR, shell vial assay, and tube cell culture to rapid reporting procedures. J. Clin. Microbiol. 443366-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]