Abstract
Cross-priming amplification (CPA) technology for tuberculosis diagnosis from sputum specimens was evaluated. The sensitivity of CPA from smear- and liquid culture-positive specimens was 96.9%, and that from smear-negative and liquid culture-positive specimens was 87.5%. The specificity of CPA in culture-negative specimens was 98.8%.
The diagnosis of tuberculosis (TB) is based on phenotypic and genotypic methods. Light microscopy for the detection of acid-fast bacilli (AFB) in sputum smears is a rapid and specific tool commonly used all over the world, but it only detects 30% to 40% of TB patients (10). While the sensitivity of Löwenstein-Jensen (L-J) solid or liquid culture methods is higher than that of AFB microscopy, culture-based methods require several weeks of incubation time. Several genotypic assays, mostly based on nucleic acid amplification tests, have been developed for rapid TB diagnosis, including the GenProbe amplified Mycobacterium tuberculosis direct test, Roche Amplicor MTB test, Cobas Amplicor test, Abbott LCx test, and the BD-ProbeTec (strand displacement amplification) test (1, 2, 5, 8, 12). However, the cost of equipment needed for these methods is a barrier to their widespread use, especially in developing countries. A sensitive, accurate, rapid, and affordable diagnostic tool that will work in resource-limited settings is urgently needed.
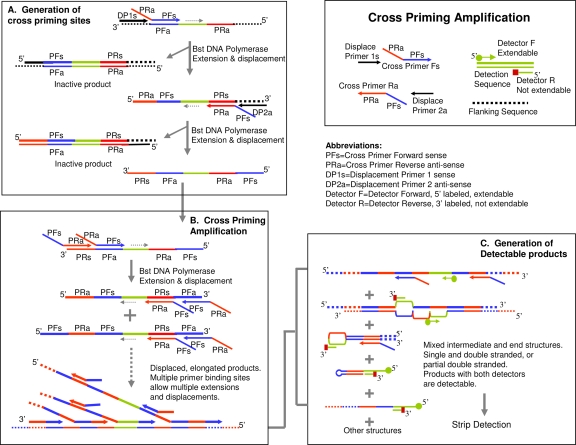
Cross-priming amplification (CPA) technology is a recent invention from an isothermal DNA amplification system by Ustar Biotechnologies Co., Ltd. Using multiple cross-linked primers (six to eight primers), a DNA target sequence can be amplified at a constant temperature (Fig. 1). The detection of amplified products is performed on a lateral flow strip housed in an enclosed, sealed plastic device to prevent the leakage of amplicons (4, 7). In this study, we evaluated the CPA isothermal amplification and detection kit (Ustar Biotech, Hangzhou, China) to determine whether it could accurately detect M. tuberculosis in clinical sputum specimens from a variety of different types of patients. Sputum specimens from 180 persons who were suspected of having TB, based on their clinical symptoms and presentation, were obtained from three hospitals in Shanghai. Sputum specimens were also collected from 98 non-TB patients (with lung cancer, lung edema, and other pulmonary illnesses) presenting at the same hospitals. For comparison with the CPA assay results, all of the clinical specimens in our study were also processed for AFB smear microscopy, L-J solid medium, and BacT/Alert 3D liquid culture following standard protocols (11). Bacterial genomic DNA was extracted by boiling, as previously described (9). The CPA method was performed according to the manufacturer's instructions, using primers targeting the gyrB gene of M. tuberculosis (Fig. 2A). The result of each CPA assay was determined by observing the presence (positive result) or absence (negative result) of visible bands on the test strips (Fig. 2B).
FIG. 1.
CPA scheme. Under an appropriate constant temperature (65°C), the denaturing and annealing of DNA molecules occur dynamically. Using multiple primers, PCR products are generated as interval tandem repeats of the target region.
FIG. 2.
(A) Primers were designed that target the gyrB gene of M. tuberculosis, based on H37Rv sequence (BX842572.1, NT 5582-5789). (B) Representative results of the CPA assay for M. tuberculosis, with a 60-min incubation time. Sample 1, M. tuberculosis strain H37Rv; sample 2, a clinical strain of M. tuberculosis; sample 3, a negative control. Samples 1 and 2 had two bands and showed positive results; sample 3 had one control band and showed a negative CPA result.
The detection rates of mycobacteria in the 180 sputum specimens used were 50.6% (91/180) by the CPA method, 36.1% (65/180) by the AFB smear microscopy, 38.9% (70/180) by L-J culture, and 53.9% (97/180) by the BacT/Alert 3D liquid culture. The 3D liquid culture is currently the “gold standard” for diagnosis of TB. Compared to the 3D liquid culture, the sensitivity and specificity of the CPA method were 92.8% (90/97) and 98.8% (82/83), respectively (Table 1). The AFB smear test is the most commonly used diagnostic method for M. tuberculosis in the world; however, it is limited by its low sensitivity and specificity. Therefore, the sensitivity of the new CPA method in smear-negative, liquid culture-positive samples was determined. Our results showed that the sensitivity of CPA was 87.5% (28/32) in smear-negative, 3D-positive specimens and 96.9% (63/65) in smear-positive, 3D-positive specimens. Therefore, the sensitivity of CPA for TB diagnosis is higher than that of AFB and L-J culture and slightly lower than that of 3D liquid culture, and CPA is very effective at detecting AFB-negative, 3D-positive specimens (Table 1).
TABLE 1.
Sensitivity and specificity of the CPA assay and L-J culture medium for clinical specimens by AFB smear and 3D liquid culture resultsa
| Method | Smear+, 3D+ sensitivity
|
Smear−, 3D+ sensitivity
|
Smear−, 3D− specificity
|
|||
|---|---|---|---|---|---|---|
| No. of samples with result/total (%) | 95% CI | No. of samples with result/total (%) | 95% CI | No. of samples with result/total | 95% CI | |
| L-Jb | 62/65 (95.4) | 87.1-99.0 | 9/32 (28.1) | 13.8-46.7 | 81/83 (97.6) | 91.6-99.7 |
| CPA | 63/65 (96.9) | 89.3-99.6 | 28/32 (87.5) | 71.0-96.5 | 82/83 (98.8) | 93.5-100.0 |
Smear+ and Smear−, AFB smear microscopy positive and negative, respectively. 3D+ and 3D−, positive and negative results, respectively, for liquid culture with the BacT/Alert 3D system. 95% CI, 95% confidence interval.
Solid or liquid culture.
We further evaluated the specificity of the CPA method for the detection of M. tuberculosis using sputum specimens from 98 patients that did not have clinical symptoms and presentation of TB. All of the 98 specimens tested negative for AFB and had negative results for M. tuberculosis by L-J culture, 3D liquid culture, and the CPA assay. We also evaluated the specificity of the CPA method by testing strains from 13 mycobacteria other than M. tuberculosis, including M. marinum, M. gordonae, M. simiae, M. scrofulaceum, M. avium, M. intracellulare, M. kansasii, M. phlei, M. smegmatis, M. vaccae, M. fortuitum, M. chelonae subsp. abscessus, and M. chelonae subsp. chelonae (provided by China National Institute for the Control of Pharmaceutical and Biological Products). None of the 13 non-M. tuberculosis Mycobacterium species was positive by the CPA method.
Several other methods for the rapid diagnosis of TB have been described. Loop-mediated isothermal amplification (LAMP) is a new and promising low-cost method for detecting M. tuberculosis (3, 6). However, in one study less than half of the AFB-negative, L-J-positive cases were positively detected by LAMP (3). In the present study, the CPA method detected 87.5% of the AFB-negative, 3-D-positive cases of TB, whereas only 28.1% of those cases were detected by L-J culture. The CPA assay can detect M. tuberculosis in AFB-negative cases and has the potential to replace time-consuming methods such as L-J culture and 3D liquid culture. The CPA method also has many of the same advantages that make LAMP ideal for resource-limited settings, including rapid turnover time to results, simple operation and equipment (only needs a water bath), easy readout of the assay's results, and a closed device which reduces the chance of specimen contamination. However, the CPA method has a fairly complicated sputum processing step which needs to be performed in a biological safety cabinet. A simplified sputum specimen processing method that shortens the overall time the assay requires, minimizes biological risk, and decreases the risk of sample contamination, is being developed.
In conclusion, our study demonstrated that the CPA method has great advantages for the detection of M. tuberculosis in clinical sputum specimens, especially from AFB-negative patients. This rapid detection method does not require expensive equipment. In addition, the sealing device of CPA avoids cross-contamination between different specimens, and the results are easy to read. With its greater sensitivity and specificity for the identification of M. tuberculosis, the CPA method could be performed in primary care facilities and clinical laboratories in developing countries.
Acknowledgments
We thank Samuel Pickerill (Northwestern University) for modification of the manuscript.
This work was supported by the Key Project of Chinese National Programs (2008ZX100/03-010), the Chinese National Programs 973 (2005CB523102) and 863 (2006AA02Z423), and National Institutes of Health grant D43 TW007887.
Footnotes
Published ahead of print on 30 December 2008.
REFERENCES
- 1.Abe, C., K. Hirano, M. Wada, Y. Kazumi, M. Takahashi, Y. Fukasawa, T. Yoshimura, C. Miyagi, and S. Goto. 1993. Detection of Mycobacterium tuberculosis in clinical specimens by polymerase chain reaction and Gen-Probe Amplified Mycobacterium Tuberculosis Direct Test. J. Clin. Microbiol. 313270-3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beavis, K. G., M. B. Lichty, D. L. Jungkind, and O. Giger. 1995. Evaluation of Amplicor PCR for direct detection of Mycobacterium tuberculosis from sputum specimens. J. Clin. Microbiol. 332582-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boehme, C. C., P. Nabeta, G. Henostroza, R. Raqib, Z. Rahim, M. Gerhardt, E. Sanga, M. Hoelscher, T. Notomi, T. Hase, and M. D. Perkins. 2007. Operational feasibility of using loop-mediated isothermal amplification for diagnosis of pulmonary tuberculosis in microscopy centers of developing countries. J. Clin. Microbiol. 451936-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chow, W. H. A., C. McCloskey, Y. Tong, L. Hu, Q. You, C. P. Kelly, H. Kong, Y.-W. Tang, and W. Tang. 2008. Application of isothermal helicase-dependent amplification with a disposable detection device in a simple sensitive stool test for toxigenic Clostridium difficile. J. Mol. Diagnostics 10453-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Down, J. A., M. A. O'Connell, M. S. Dey, A. H. Walters, D. R. Howard, M. C. Little, W. E. Keating, P. Zwadyk, Jr., P. D. Haaland, D. A. McLaurin III, and G. Cole. 1996. Detection of Mycobacterium tuberculosis in respiratory specimens by strand displacement amplification of DNA. J. Clin. Microbiol. 34860-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwamoto, T., T. Sonobe, and K. Hayashi. 2003. Loop-mediated isothermal amplification for direct detection of Mycobacterium tuberculosis complex, M. avium, and M. intracellulare in sputum samples. J. Clin. Microbiol. 412616-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kong, H., T. Ranalli, and B. Lemieux. 2007. New isothermal molecular diagnostics platforms. IVD Technol. 1335-43. [Google Scholar]
- 8.Lachnik, J., B. Ackermann, A. Bohrssen, S. Maass, C. Diephaus, A. Puncken, M. Stermann, and F.-C. Bange. 2002. Rapid-cycle PCR and fluorimetry for detection of mycobacteria. J. Clin. Microbiol. 403364-3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen, G. M., J. Zha, L. Xu, B. Sun, X. H. Gui, Y. F. Wang, J. Mei, and Q. Gao. 2005. Evaluation of the mycobacterial interspersed repetitive units typing as a practical approach in molecular epidemiology of Mycobacterium tuberculosis. Zhonghua Jiehe He Huxi Zazhi 28292-296. [PubMed] [Google Scholar]
- 10.Steingart, K. R., M. Henry, V. Ng, P. C. Hopewell, A. Ramsay, J. Cunningham, R. Urbanczik, M. Perkins, M. A. Aziz, and M. Pai. 2006. Fluorescence versus conventional sputum smear microscopy for tuberculosis: a systematic review. Lancet Infect. Dis. 6570-581. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. 1998. Laboratory services in TB control. Parts I, II, and III. WHO/tb/98.258. World Health Organization, Geneva, Switzerland. http://whqlibdoc.who.int/hq/1998/WHO_TB_98.258_(part1).pdf.
- 12.Yeboah-Manu, D., M. D. Yates, and S. M. Wilson. 2001. Application of a simple multiplex PCR to aid in routine work of the mycobacterium reference laboratory. J. Clin. Microbiol. 394166-4168. [DOI] [PMC free article] [PubMed] [Google Scholar]