Abstract
Campylobacter rectus was isolated under routine anaerobic conditions (no additional hydrogen gas in the atmosphere) from an oral, nonperiodontal abscess from a patient with gastroesophageal adenocarcinoma. We report the first case of a palate abscess caused by C. rectus and review the literature and atmospheric requirements of this organism.
CASE REPORT
A 66-year-old male veteran presented to the Veterans Affairs Puget Sound Health Care System in Seattle, WA, in February, 2008, with a 6-month history of dysphagia. An esophagogastroduodenoscopy showed a 4-cm fungating mass that caused partial obstruction at the distal gastroesophageal junction, proximal to the stomach. A biopsy of the mass revealed gastroesophageal adenocarcinoma. He had smoked approximately one and one-half packs of cigarettes per day for the past 30 years and had poor dental care, although there was no evidence of periodontal disease or other active dental problems. The patient was admitted in March for initiation of neoadjuvant chemoradiation prior to surgical resection of the mass. On admission, the patient reported that he had intermittent drainage of purulent material from the roof of his mouth. A computed tomography scan showed that the patient had a left hard palate soft tissue abscess. The patient denied fever, nasal congestion, rhinorrhea, pain, bleeding, facial numbness, and weakness.
A complete blood count from the day of admission had the following laboratory values: a normal white blood cell count (5.3 × 109 cells/liter; normal range, 4.3 to 10 × 109/liter), low hemoglobin (11.6 g/dl; normal range, 13 to 18 g/dl), a low hematocrit (34.0%; normal range, 38 to 50%), and a normal platelet count (208,000 platelets/liter; normal range, 150,000 to 400,000/liter). A fine-needle aspiration was performed on the left palatal lesion. A sample was sent to Anatomic Pathology, and neoplastic cells were not observed. In addition, approximately 2 ml of purulent fluid from the left hard palate abscess was sent to a clinical microbiology laboratory for analysis. The patient was given a course of amoxicillin (amoxicilline)-clavulanate, and the mass healed after therapy and drainage.
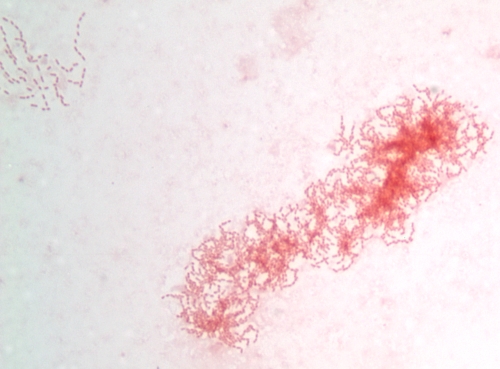
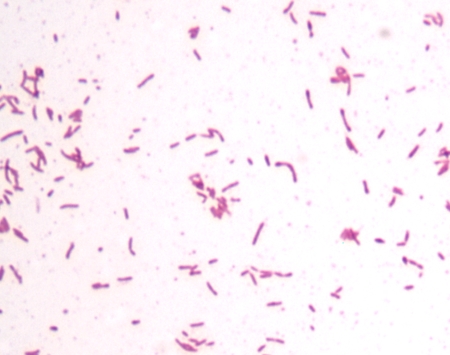
A Gram stain of the purulent fluid from the left hard palate abscess aspirate revealed many white blood cells and many small pleomorphic gram-negative rods and coccobacilli, some in chains, which were remarkable in that they were predominantly arranged only in packets of organisms (Fig. 1). Aerobic and anaerobic cultures of the palate abscess specimen were performed. After 2 days, two colonies of an alpha-hemolytic streptococcus (not Streptococcus pneumoniae) and a nonhemolytic streptococcus were isolated and not definitively identified from the aerobic culture. No gram-negative rods were isolated from the aerobic culture; however, on day 7, over 100 tiny gray colonies were observed from the anaerobic culture. The colonies were less than 0.1 mm wide and round. A Gram stain of the colonies revealed small, pleomorphic gram-negative rods. The bacteria were easier to visualize when counterstained with carbolfuchsin (Fig. 2). The isolate was oxidase positive and catalase negative. The RapID ANA system (Remel, Lenexa, KS) gave no identification (all tests were negative; biotype identification number 000000).
FIG. 1.
Gram stain of purulent material from the oral abscess of our patient counterstained with safranin. Nearly all of the organisms seen in all fields were arranged in chains and packets, as seen in this image. Although the individual gram-negative rods are compatible with the appearance of C. rectus from culture, it is also possible that they are noncultivable organisms.
FIG. 2.
Gram stain of the C. rectus colonies counterstained with carbolfuchsin. While many of the cells are straight rods, some are slightly curved or pleomorphic. Most of the cells are 1 to 2 μm long.
The identity of the isolate was determined by 16S rRNA gene sequencing. Primers (synthesized by TIB Molbiol, Adelphia, NJ) VAB1 (5′-TGGAGAGTTTGATCCTGGCTCAG-3′) and VAB2 (5′-GTATTACCGCGGCTGCTGG-3′) were used to generate a 543-bp amplicon of the 16S rRNA gene. PCR was performed in a GeneAmp PCR system (model 9700; Applied Biosystems, Foster City, CA) with a 50-μl sample volume. Final reagent concentrations for the PCR were 4 mM MgCl2, 200 μM of each deoxynucleoside triphosphate, 0.5 μM of each primer, and 1.25 U of AmpliTaq DNA polymerase in Buffer II (without magnesium) (all reagents were obtained from Applied Biosystems). Five microliters of template DNA was added to the reaction mixture. Thermal cycling conditions were as follows: initial denaturation at 95°C for 3 min, followed by 35 cycles of 95°C for 30 s, 60°C for 30 s, and 72°C for 45 s. After a final hold at 72°C for 10 min, 10 μl of the sample was separated by agarose gel electrophoresis to ensure that an amplicon of the right size was present. The remaining 40 μl of the amplicon was purified using the Microcon PCR centrifugal filter device kit (Millipore, Bedford, MA). Eight-microliter samples of the amplicon were mixed in separate tubes with primer VAB1 or VAB2; the Core DNA Sequencing Facility at the University of Washington added BigDyes and separated the fragments. The generated sequences were analyzed using the Microseq database, and the neighbor-joining method was used to generate a dendrogram to determine strain relatedness. The obtained sequence of the isolate matched the C. rectus type strain from the Microseq database at all bases except at position 194 of the Escherichia coli 16S rRNA gene sequence.
C. rectus is a difficult organism to culture and identify. C. rectus requires an anaerobic atmosphere for optimal isolation, although the composition of gases necessary for cultivation varies according to the literature. When C. rectus was first named and identified in 1981 as Wolinella recta, the authors used an anaerobic atmosphere consisting of 10% H2, 10% CO2, and 80% N2 for isolation and maintenance (19). More-recent reviews of the genus Campylobacter describe that C. rectus requires either an anaerobic atmosphere (specific gas composition not mentioned) (20) or an atmosphere of 6% H2, 10% CO2, with the balance being N2 (6). Our isolate of C. rectus grew in an anaerobic atmosphere consisting of 18 to 20% CO2, with the balance being N2 (generated with the AnaeroPack System [Mitsubishi Gas Chemical Co., Inc., New York, NY]), on both blood (Columbia agar base) and chocolate agars after about 7 days of incubation. Because of the anaerobic-atmosphere differences described in the literature, we compared the levels of growth of our C. rectus isolate under several different atmospheric conditions after 7 days of incubation at 30°C, 35°C, and 42°C. Growth was observed at all three temperatures under both the 18-to-20% CO2 (with N2 as the balance) and the 10% H2, 10% CO2 (with N2 as the balance) atmospheric conditions. It did not grow in air with increased CO2 or in a conventional microaerophilic atmosphere (5% O2, 10% CO2, and 85% N2). In addition, under anaerobic conditions (18 to 20% CO2, with N2 as the balance), our isolate did not grow on selective Campylobacter agar (PML Microbiologicals, Wilsonville, OR). In the literature, C. rectus is reported as oxidase positive, catalase variable, urease negative, H2S negative when inoculated into a triple-sugar iron agar slant, hippurate hydrolysis negative, aryl sulfatase positive, selenite reduction negative, and able to grow in 1% glycine (20). Our isolate differed only in being confirmed catalase negative. Commercial identification systems do not have C. rectus in the database; our isolate could not be identified with the RapID ANA II panel. From the original Gram stain from clinical material, our isolate was a small, faintly staining, gram-negative rod or coccobacillus arranged in packets. The Gram stain from the colonies showed straight, curved, and pleomorphic bacilli.
C. rectus was placed in the genus Campylobacter in 1991 (21). It was identified as a probable human periodontal pathogen in 1979 (17). Since then, C. rectus has been firmly established as a member of the oral flora of humans. In one large study, C. rectus was found in the saliva of 31.3% of tested healthy adults from southern Finland (8). In another large study from Buffalo, NY, C. rectus was found in subgingival plaque samples from 17.6% of postmenopausal women (2). C. rectus has been found in advanced numbers from samples from patients with periodontitis compared to numbers in samples from individuals without disease in several studies (9-12, 17, 18). There is evidence that C. rectus is involved in initial periodontitis (15, 16), in chronic periodontitis lesions (3), and in root canal infections (13). Other Campylobacter species that are found in the human oral cavity are C. gracilis, C. concisus, C. curvus, C. sputorum, and C. showae (5, 11, 17, 18). Of these, there is evidence that C. gracilis is involved in cases of periodontitis infections (10, 13), and C. showae may be involved in periodontitis infections (10).
In addition to causing dental infections in humans, C. rectus has been implicated as the causative agent in three extraoral infections. In one case, C. rectus was reported in large numbers from an actinomycotic chest wall mass along with large numbers of Actinomyces viscosus (14), but the identification was suspect as C. rectus was identified by phenotypic characteristics only. This patient had pulmonary symptoms along with shoulder and back pain. In another extraoral case, C. rectus was isolated and well characterized from a breast abscess along with a non-group A beta-hemolytic streptococcus (7). The infection in this case likely occurred after nipple piercing. C. rectus was isolated and identified by 16S rRNA gene sequencing in a third extraoral case from a patient with a vertebral abscess along with Actinomyces species and Eubacterium brachy infections (4). This patient had a history of meningoradiculitis (4).
There has been limited antimicrobial susceptibility data for C. rectus. One study showed sensitivity to imipenem, levofloxacin, amoxicillin-clavulanate, cefoxitin, clindamycin, and metronidazole; beta-lactamase activity was not detected (1). In our case, the patient recovered after drainage and treatment with amoxicillin-clavulanate and recovered. In the breast abscess case, the patient was treated with drainage, vancomycin, clindamycin, and aztreonam and recovered (7). The C. rectus reported from the actinomycotic chest wall mass was treated with drainage and penicillin; the isolate tested sensitive to penicillin in the study (14). The C. rectus isolated from the vertebral abscess was sensitive to clindamycin (4). In all of these cases, the patient recovered. All of these cases are summarized in Table 1.
TABLE 1.
Characteristics of four different cases of C. rectus abscess infections
| Case | Site of infection | Organisms recovered | Age of patient (yr) | Underlying disease or risk | Treatment |
|---|---|---|---|---|---|
| 1a | Chest wall abscess | C. rectus and Actinomyces viscosus | 62 | No significant past medical history; poor oral hygiene; patient drank several cans of beer a day | Drainage, penicillin |
| 2b | Breast abscess | C. rectus and non-group A beta-hemolytic streptococci | 32 | Lymphoma, leucopenia, nipple piercing | Drainage, vancomycin, clindamycin, aztreonam |
| 3c | Vertebral abscess | C. rectus, an Actinomyces species, and Eubacterium brachy | 24 | Meningoradiculitis | Hemilaminectomy, clindamycin |
| 4d | Buccal-mucosa abscess | C. rectus and alpha-hemolytic streptococci | 66 | Gastroesophageal adenocarcinoma, long history of cigarette smoking | Drainage, amoxicillin-clavulanate |
In summary, C. rectus is an oral anaerobe that is capable of causing human disease. The organism is known to reside in the human oral cavity and is a known causative agent of periodontitis. In addition, it has been found in perhaps two extraoral abscesses. We report the first case of C. rectus from an oral abscess that is likely not related to periodontal disease and for the first time document the occurrence of the organism as packets or clusters in the original specimen, and we remind clinical microbiologists of its straight and curved-rod morphology. We also document that C. rectus will grow under anaerobic conditions routinely used in clinical microbiology laboratories and that it does not require additional hydrogen gas.
Nucleotide sequence accession number.
The obtained sequence of the Campylobacter rectus isolate was deposited as GenBank accession number EU743946.
Acknowledgments
We thank Valeria Potigaib and Edwardo Mendez for an excellent specimen and patient history. We thank the Seattle VA clinical microbiology laboratory and especially Bao Pham, Geri Nowak, and Sally Mizuki for superior work.
Footnotes
Published ahead of print on 24 December 2008.
REFERENCES
- 1.Blandino, G., I. Milazzo, D. Fazio, S. Puglisi, M. Pisano, A. Speciale, and S. Pappalardo. 2007. Antimicrobial susceptibility and beta-lactamase production of anaerobic and aerobic bacteria isolated from pus specimens from orofacial infections. J. Chemother. 19495-499. [DOI] [PubMed] [Google Scholar]
- 2.Brennan, R. M., R. J. Genco, G. E. Wilding, K. M. Hovey, M. Trevisan, and J. Wactawski-Wende. 2007. Bacterial species in subgingival plaque and oral bone loss in postmenopausal women. J. Periodontal. 781051-1061. [DOI] [PubMed] [Google Scholar]
- 3.Colombo, A. V., C. M. Silva, A. Haffajee, and A. P. Colombo. 2006. Identification of oral bacteria associated with crevicular epithelial cells from chronic periodontitis lesions. J. Med. Microbiol. 55609-615. [DOI] [PubMed] [Google Scholar]
- 4.de Vries, J. J. C., N. L. A. Arents, and W. L. Manson. 2008. Campylobacter species isolated from extra-oro-intestinal abscesses: a report of four cases and literature review. Eur. J. Clin. Microbiol. Infect. Dis. 271119-1123. [DOI] [PubMed] [Google Scholar]
- 5.Etoh, Y., F. E. Dewhirst, B. J. Paster, A. Yamamoto, and N. Goto. 1993. Campylobacter showae sp. nov., isolated from the human oral cavity. Int. J. Syst. Bacteriol. 43631-639. [DOI] [PubMed] [Google Scholar]
- 6.Fitzgerald, C., and I. Nachamkin. 2007. Campylobacter and Arcobacter, p. 933-946. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. L. Landry, and M. A. Pfaller (ed.), Manual of clinical microbiology, 9th ed. ASM Press, Washington, DC.
- 7.Han, X. Y., J. J. Tarrand, and D. C. Rice. 2005. Oral Campylobacter species involved in extraoral abscess: a report of three cases. J. Clin. Microbiol. 432513-2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Könönen, E., S. Paju, P. J. Pussinen, M. Hyvönen, P. Di Tella, L. Suominen-Taipale, and M. Knuuttila. 2007. Population-based study of salivary carriage of periodontal pathogens in adults. J. Clin. Microbiol. 452446-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lai, C. H., K. Oshima, J. Slots, and M. A. Listgarten. 1992. Wolinella recta in adult gingivitis and periodontitis. J. Periodontal. Res. 278-14. [DOI] [PubMed] [Google Scholar]
- 10.Macuch, P. J., and A. C. R. Tanner. 2000. Campylobacter species in health, gingivitis, and periodontitis. J. Dent. Res. 79785-792. [DOI] [PubMed] [Google Scholar]
- 11.Moore, W. E. C., L. V. Holdeman, E. P. Cato, R. M. Smibert, J. A. Burmeister, K. G. Palcanis, and R. R. Ranney. 1985. Comparative bacteriology of juvenile periodontitis. Infect. Immun. 48507-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rams, T. E., D. Feik, and J. Slots. 1993. Campylobacter rectus in human periodontitis. Oral Microbiol. Immunol. 8230-235. [DOI] [PubMed] [Google Scholar]
- 13.Siqueira, J. F., Jr., and I. N. Rôcas. 2003. Campylobacter gracilis and Campylobacter rectus in primary endodontic infections. Int. Endod. J. 36174-180. [DOI] [PubMed] [Google Scholar]
- 14.Spiegel, C. A., and G. Telford. 1984. Isolation of Wolinella recta and Actinomyces viscosus from an actinomycotic chest wall mass. J. Clin. Microbiol. 201187-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanner, A., and H. Bouldin. 1989. The microbiota of early periodontitis lesions in adults. J. Clin. Periodontal. 16467-471. [DOI] [PubMed] [Google Scholar]
- 16.Tanner, A., M. F. Maiden, P. J. Macuch, L. L. Murray, and R. L. Kent, Jr. 1998. Microbiota of health, gingivitis, and initial periodontitis. J. Clin. Periodontal. 2585-98. [DOI] [PubMed] [Google Scholar]
- 17.Tanner, A. C., C. Haffer, G. T. Bratthall, R. A. Visconti, and S. S. Socransky. 1979. A study of the bacteria associated with advancing periodontitis in man. J. Clin. Periodontal. 6278-307. [DOI] [PubMed] [Google Scholar]
- 18.Tanner, A. C., S. S. Socransky, and J. M. Goodson. 1984. Microbiota of periodontal pockets losing crestal alveolar bone. J. Periodontal. Res. 19279-297. [DOI] [PubMed] [Google Scholar]
- 19.Tanner, A. C. R., S. Badger, C.-H. Lai, M. A. Listgarten, R. A. Visconti, and S. S. Socransky. 1981. Wolinella gen. nov., Wolinella succinogenes (Vibrio succinogenes Wolin et al.) comb. nov., and description of Bacteroides gracilis sp. nov., Wolinella recta sp. nov., Campylobacter concisus sp. nov., and Eikenella corrodens from humans with periodontal disease. Int. J. Syst. Bacteriol. 31432-445. [Google Scholar]
- 20.Vandamme, P., F. E. Dewhirst, B. J. Paster, and S. L. W. On. 2005. Genus I. Campylobacteraceae Sebald and Veron 1963, 907,AL emend. Vandamme, Falsen, Rossau, Hoste, Segers, Tytgat and De Ley 1991a, 98, p. 1147-1160. In D. J. Brenner, N. R. Krieg, and J. T. Staley (ed.), Bergey's manual of systematic bacteriology, 2nd ed., vol. 2, part C. Springer, New York, NY. [Google Scholar]
- 21.Vandamme, P., E. Falsen, R. Rossau, B. Hoste, P. Segers, R. Tytgat, and J. De Ley. 1991. Revision of Campylobacter, Helicobacter, and Wolinella taxonomy: emendation of generic descriptions and proposal of Arcobacter gen. nov. Int. J. Syst. Bacteriol. 4188-103. [DOI] [PubMed] [Google Scholar]