Abstract
During a large mumps virus (MuV) outbreak which occurred in the Palestinian refugee camps of the West Bank, 68.1% (2,636/3,871) of the cases were vaccinated with one dose of trivalent measles, mumps, and rubella (MMR) vaccine. Attack rates by camp ranged from less than 1 case per 1,000 people in the population to 43/1,000 (overall, 11/1,000). The outbreak lasted from December 2003 to June 2005, with two peaks, one from April to May 2004 and the other from March to April 2005. To control the outbreak, a mass MMR vaccination campaign was conducted in May 2005. Evaluation of the immune status of cases (n = 59) and healthy controls (n = 51) revealed high levels of mumps immunoglobulin G (IgG) and a low MuV-specific IgM in clinical cases indicative of a booster immune response. This suggested a secondary rather than a primary infection due to the insufficient protection conferred by the single vaccine dose included in the vaccination program. This prediction was further confirmed by the low seroprevalence (68.6%) found in the healthy control group, which was below the threshold level required for MuV herd immunity. Mumps diagnosis was established mainly by reverse transcription-PCR in clinical samples obtained within 48 h from the onset of disease. Of the parotid fluids and nasopharyngeal aspirates analyzed, 92% were positive for MuV RNA, while only 33% of the urine samples were positive. Phylogenetic analysis of the MuV SH gene identified the outbreak strain as the H genotype, which has been in circulation worldwide at least since 1989.
Mumps, a vaccine-preventable disease, is a highly contagious self-limiting childhood infection that presents mainly as bilateral parotitis. Mumps complications include orchitis, pancreatitis, epididymitis, and meningitis (20, 36). Death due to mumps is exceedingly rare and caused mostly by mumps encephalitis (10). Mumps virus (MuV)-specific immunoglobulin M (IgM) response usually precedes the IgG response early in the infection and wanes within the first 2 to 6 months (20). MuV is present in the saliva of infected individuals for several days before the onset of clinical disease and for up to 5 days afterwards (9, 27). The virus can also be detected in urine for several weeks after the onset of mumps (33).
Although monotypic, MuV isolates segregate into several genotypes (A to L) based on nucleotide sequence analysis of the highly variable small hydrophobic (SH) gene (17). Mumps genotypes are defined based on nucleotide variation of 2 to 4% within and 8 to 18% between genotypes (18).
Mumps vaccination has been widely in use since the triple measles, mumps, and rubella (MMR) vaccine was introduced in the 1980s. MMR single-dose vaccination was introduced by the United Nations Relief and Works Agency (UNRWA) in the West Bank refugee camps in 1988 and is administered at 15 months of age. In 2003, MMR vaccine coverage in the refugee camps was 94% (evaluated through rapid assessment technique), and consistently with other areas, the incidence of mumps had dropped since 1988 to four cases per 100,000 people in the population (22).
Sporadic mumps outbreaks in vaccinated populations have been attributed mainly to primary vaccine failure in individuals who had received one dose of MMR vaccine (30, 35). More recently, the CDC reported a mumps outbreak in 18- to 24-year-old individuals vaccinated with two MMR vaccine doses in the United States (7, 11). In addition, the CDC reported another outbreak in a similar age group in individuals vaccinated with one MMR vaccine dose in the United Kingdom (8). Park et al. also reported a mumps outbreak in a highly vaccinated 17- to 18-year-old Korean school population (26). The relative contribution of waning immunity to vaccine failure is still controversial (6, 13, 37).
The current MuV genotyping system is based primarily on the entire sequence of the viral SH gene. It was first developed in 1999 by Jin et al. (16), who also first identified the H genotype and found an isolate dating back to 1989 which belonged to this genotype. Since then, this genotype has been identified worldwide (4, 16, 19, 32), but an outbreak as large as that described in our current report has never been associated with this genotype.
In this report, we describe the epidemiology of a large mumps outbreak (3,871 cases), the laboratory diagnosis of a small subset of the outbreak population, and an evaluation of the immune status of the clinical cases and a matching cohort of healthy controls. We also describe the molecular characterization and phylogenetic analysis of the MuV outbreak strain.
MATERIALS AND METHODS
Case definition and data collection.
The definition of parotitis during the outbreak was as described by the WHO as follows: an acute onset of unilateral or bilateral tender, self-limited swelling of the parotid or other salivary gland lasting 2 or more days and without other apparent cause. The medical officer in charge of each of UNRWA health center was responsible for clinically diagnosing the parotitis cases, while the nurses were involved in performing line listing. Line listing included obtaining the following information from each patient: date of birth, gender, residence, occupation, date of disease onset, date of disease diagnosis, signs and symptoms, immunization status, complications if any, and reinfections. Mapping of parotitis cases was performed according to the refugee camp where the patient resided and the date of the onset of symptoms. Data were reported on a weekly basis from the health centers of affected camps to the main office where descriptive epidemiological methods were used to process, evaluate, and analyze all available patients' data.
Patients' samples.
Several patient sample types were collected by health care providers who had received training in specimen collection, storage, and transport. Serum samples were stored at −20°C, while all other samples were stored at −70°C until analyzed.
Serum samples from 59 MMR-vaccinated patients presenting with parotitis were collected for serological diagnosis within 48 h of the patient's visit to the clinics. In addition, 51 samples were obtained from healthy individuals, 10 of whom resided in the outbreak area and the rest resided in refugee camps with no reported cases of parotitis. Of the 51 healthy individuals, 3 were adults who had not been immunized with MMR vaccine.
Specimens collected for virus isolation and molecular diagnosis were seven parotid fluids collected on sterile swabs after milking the parotid gland, six fine-needle aspirates (FNA) from enlarged parotid glands, two cerebrospinal fluid (CSF) samples from patients with aseptic meningitis, six nasopharyngeal aspirates (NPA), and 10 urine samples.
Determination of MuV IgG and IgM.
The VIDAS MuV IgG assay (bioMérieux, Marcy-I'Etoile, France) was used to determine MuV IgG levels, while the Enzygnost anti-parotitis virus IgM kit (Dade-Behring, Marburg, Germany) was used to determine MuV IgM status. Both assays were performed according to the manufacturer's instructions. A patient was reported IgG positive when the IgG test value (TV) was greater than 0.5, equivocal between 0.35 and 0.49, and negative when the TV was less than 0.35. For MuV IgM, patients were reported positive with an optical density (OD) result of greater than 0.2, equivocal between 0.1 and 0.19 after a second confirmation, and negative with an OD result of less than 0.1.
Virus isolation.
Virus isolation was attempted from a variety of patient samples (parotid fluid, parotid FNA, CSF, NPA, and urine) using Vero and B95 cell lines (2). Cell monolayers in roller tubes were maintained in Dulbecco's modified Eagle's medium and supplemented with 10% fetal bovine serum, 200 μg/ml streptomycin, 100 U/ml penicillin, and 121.5 U/ml nystatin in a 37°C incubator. Patient samples were homogenized and vortexed for 30 s before 200 μl was used to inoculate cell monolayers in duplicate. Inoculated cells were maintained in Dulbecco's modified Eagle's medium supplemented with 200 μg/ml streptomycin, 100 U/ml penicillin, and 121.5 U/ml nystatin and incubated at 35°C. Cell cultures were observed daily for 6 to 8 days for cytopathic effects. Two blind passages were performed on all negative tissue cultures before the sample was scored negative.
RNA extraction and RT-PCR.
Viral genomic RNA was extracted from 140 μl of homogenized samples using the QIAamp viral RNA extraction kit (Qiagen GmbH, Hilden, Germany) following the manufacturer's suggested protocol. A 5-μl aliquot was used for the one-step reverse transcription-PCR (RT-PCR), and the remaining RNA was stored at −70°C. The primers reported by Jin et al. (16) which encompassed a region containing the complete MuV SH gene were used to amplify a 676-bp PCR product. These primers were also used for sequencing the amplicon from both directions. RT-PCR was performed using a Qiagen one-step RT-PCR kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer's instructions. Amplicons were visualized following electrophoresis on 2% agarose gel containing ethidium bromide.
Phylogenetic analysis.
RT-PCR products containing the complete SH gene were gel purified or purified directly from the RT-PCR mix using a Qiagen QIAquick gel extraction or Qiagen QIAquick PCR purification kit, respectively (Qiagen GmbH, Hilden, Germany) according to the manufacturer's recommendations. A total of 316 bp were sequenced using an ABI Prism dye deoxy-terminator cycle sequencing kit (Applied Biosystems, Foster City, CA). Reaction mixtures were analyzed on Applied Biosystems model 373 DNA automatic sequencing systems. The Sequencher gene analysis program (Gene Codes Corporation, Anne Arbor, MI) was used to compare nucleotide sequences. Phylogenetic trees were prepared by nearest neighbor analysis using ClustalX with 1,000 bootstraps. Trees were visualized using NJplot.
Statistical analysis.
Data was entered on customized Excel spreadsheets. SPSS software was used for descriptive and analytical statistics using the nonparametric Mann-Whitney test and the Fisher exact test. P values of <0.05 were considered statistically significant.
Nucleotide sequence accession numbers.
GenBank accession numbers for the complete SH genes described in this report are AM293337 (CSF), AM293335 (parotid FNA), and AM293341 (CSF).
RESULTS
Epidemic curve.
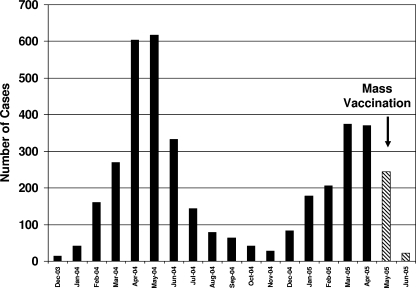
The exact date of disease onset was available for all 3,871 patients who presented with parotitis and resided in the refugee camps. The outbreak started in December 2003 and peaked in April and May 2004 (Fig. 1). A smaller peak was observed in March and April 2005 when mass MMR vaccination was initiated using the L-Zagreb strain. The biphasic curve could be attributed in part to the overcrowding that is present in the refugee camps, in particular during the winter season. Patients who developed parotitis or aseptic meningitis within 30 days of the MMR vaccination were not included in the case count because the infection might be attributed to the L-Zagreb vaccine strain. Including these cases could have led to an overestimation of the case count and consequently of the attack rate. Even though there is no clear consensus on the definition of post-MMR vaccine aseptic meningitis (5), the time span of 30 days was chosen to entirely encompass the incubation period of 15 to 35 days, proposed by Miller et al. (21). Few residual mumps cases were reported until December 2007, when the number of parotitis cases, mainly in school-age children, went back to its endemic level, 4/100,000 (22).
FIG. 1.
Monthly distribution of patients clinically diagnosed with mumps. Mumps cases that occurred within 1 month post the mass MMR vaccination (15 May to 15 June 2005) were considered vaccine complication cases and were not included in the final case count.
Mumps cases were distributed mainly in the northern and central camps of the West Bank with the highest incidence in the northern camps. Attack rates by camp ranged from less than 1 case per 1,000 people in the population to 43/1,000 (overall, 11/1,000). During the months of April and May 2004, 31.5% of the cases occurred in the northern camps of the West Bank, the Nablus area (Askar and Balta camps). The second wave of parotitis cases occurred during the months of March and April 2005, during which 15% of the cases occurred also in the Nablus area (Tul-Karem and Jenin camps).
Supplementary mass immunization campaign.
An MMR supplementary immunization campaign using the L-Zagreb strain was carried out in May 2005, in collaboration with the Palestinian Authority Ministry of Health and the United Nations Children's Fund (UNICEF) in all UNRWA health centers and schools of the West Bank. The target groups were all school children from the 1st to the 12th grades and college students. The immunization campaign was conducted during the second epidemic peak as shown in Fig. 1. Of the 58,561 MMR vaccine doses administered in the refugee camps, complications were reported in 1,037 (1.8%) individuals. These included 1,006 (1.72%) parotitis and 31 (0.05%) aseptic meningitis vaccine complications. The L-Zagreb vaccine strain was confirmed by genotyping from three/three parotid fluid samples collected from patients with parotitis. Moreover, urine samples obtained from two of these patients were also positive for the L-Zagreb strain. The L-Zagreb strain was also identified in one CSF sample from a patient presenting with aseptic meningitis post-MMR vaccination.
Patient characteristics.
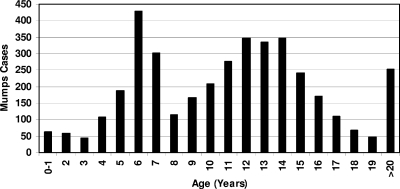
Of the 3,871 clinically diagnosed mumps cases, 2,169 (56%) were male and 1,702 (44%) were female (male-to-female ratio of 1.3:1). The overall mumps incidence was 11.6%. The age of infected patients ranged between 6 months and 68 years of age (mean, 11.65 ± 47 years old; median, 11 years old). A total of 76.3% of the patients were in the 5- to 15-year-old age group (Fig. 2). Once the data were stratified by year, the most affected age groups were the 6-year-olds in 2004 and the 7-year-olds in 2005, suggesting a cohort effect for patients vaccinated in 1999. A similar shift appears in the 12- to 14-year-old age group. Interestingly, a very low complication rate was noted during the outbreak. Only 1.1% of the patients presented with mumps complication, due mainly to orchitis. The low mumps complication rate during this outbreak could be a result of the majority of patients being previously vaccinated.
FIG. 2.
Age distribution of patients clinically diagnosed with mumps.
Immunization status of the affected population.
A total of 2,636 patients (68.1%) had been vaccinated with a single dose of MMR vaccine, while 147 patients (3.8%) had no records in the health facilities about their vaccination status and were considered not vaccinated.
Vaccination coverage was above 85% in all infected individuals born after the year 1994. The vaccine strain introduced was not constant throughout the years. Refugees were vaccinated with the Jeryl-Lynn strain from 1989 until 1994, with either the L-Zagreb or Urabe 9AM vaccine from 1995 until 1998, and with the Urabe 9AM vaccine from 2000 to 2005. The usage of the poorly immunogenic Rubini strain vaccine between 1999 and 2000 could not be ruled out (F. Averhoff, personal communication). A total of 1.7% of the refugee children had been vaccinated by other health care providers of host countries (Jordan, the Kingdom of Saudi Arabia, United Arab Emirates, and Australia). Almost half (49.5%) of all previously vaccinated mumps patients received the MMR vaccine either during 1999 and 2000 (29.8%) or during 1993 and 1994 (19.7%).
Serological profile of children presenting with parotitis.
Of the 59 serum samples evaluated for MuV IgM, 36 (61.1%) were negative, 12 (20.3%) were equivocal, and 11 (18.6%) were positive. Moreover, of the 11 positive samples (OD > 0.2), 9 were just above the cutoff level (low positive), while only 2 samples were highly positive. Since these two highly positive samples were from vaccinated patients, their infections appear to have been a result of primary vaccine failure. Of the four patients that were retested 30 days from clinical presentation, three were equivocal and one was low positive. These results are not typical of primary mumps infection, which is characterized by high titers of MuV IgM in the acute phase.
In contrast, 54 (91.5%) of the infected patients were MuV IgG positive, 3 (5.1%) were IgG equivocal, and only 2 (3.4%) were IgG negative. Furthermore, 80% of the IgG-positive samples had very high MuV IgG TV readings, six to 13 times higher than the cutoff TV of 0.5.
Stratification of MuV-infected patients by age group is shown in Table 1. The age groups in which most cases occurred (5- to 9-year-olds and 10- to 15-year-olds) had high IgM seronegativity rates and the highest IgG average TV. However, the difference in the results of the various age groups was not statistically significant (P = 0.174; Fisher's exact test). These results suggest that most of the patients had preexisting immunity to MuV, which also confirms that the lack of IgM response observed was not due to early sample collection.
TABLE 1.
Percent IgM seronegatives and the average enzyme-linked immunosorbent assay IgG TV of the different mumps patients by age group
| Age group (yr) | No. of patients tested | % Negative for IgM | Avg IgG TV |
|---|---|---|---|
| 0-4 | 6 | 50 | 3.5 |
| 5-9 | 13 | 46.2 | 4.5 |
| 10-15 | 33 | 69.7 | 4.2 |
| >15 | 7 | 57.1 | 2.6 |
Vaccination coverage and seropositivity.
Fifty-one serum samples from 48 healthy children less than 15 years of age, of whom 25 (55%) were between 6 and 15 years old, and three healthy adults (average age, 23.7 years) were tested for MuV IgG antibodies. All of the healthy children tested (n = 48) were vaccinated (vaccination cards physically examined), while the healthy adults (n = 3) were not vaccinated. Of the 51 individuals tested, 35 (68.6%) were IgG positive, 6 (11.8%) were IgG equivocal, and 10 (19.6%) were IgG negative. The difference between the mean MuV IgG TVs of the infected and healthy individuals (4.1 and 1.48, respectively) was statistically significant (P < 0.0001) using the nonparametric Mann-Whitney test. Stratifying by age, MuV immunity appears to deteriorate with time as shown in Table 2. The three individuals in the age group of >15 years who were not vaccinated were positive for MuV IgG, indicating previous natural infection.
TABLE 2.
Age-specific prevalence rates of MuV antibodies in the healthy control group
| Age group (yr) | No. of individuals | Vaccination status | % Positive for MuV IgG | Avg MuV IgG TV |
|---|---|---|---|---|
| 0-4 | 13 | Vaccinated | 92.3 | 1.9 |
| 5-9 | 19 | Vaccinated | 68.4 | 1.4 |
| 10-15 | 16 | Vaccinated | 43.8 | 1.0 |
| >15 | 3 | Nonvaccinated | 100.0 | 2.7 |
Virus isolation.
MuV isolation was attempted from several patient samples (parotid fluid, parotid FNA, CSF, NPA, and urine). Two viral isolates were obtained from specimens collected from two unvaccinated patients who were not part of the study cohort of 59 patients. One MuV was isolated from a parotid FNA specimen 2 days after the first blind passage, while the other MuV was isolated from a CSF sample on day six after primary inoculation.
Molecular detection of MuV genome.
RT-PCR analysis of the two isolates confirmed that they were both MuV. All additional samples collected and tested for MuV RNA were from the study cohort of the 59 patients who were MuV IgG positive and IgM negative. RT-PCR for MuV RNA was performed directly on the patients' specimens, and positive results were obtained from six of seven parotid fluids (85.7%), all five parotid FNA and NPA samples (100%), and 3 of 10 (33%) urine samples. The two CSF samples from patients with aseptic meningitis were also positive for MuV RNA by direct RT-PCR.
MuV phylogenetic analysis.
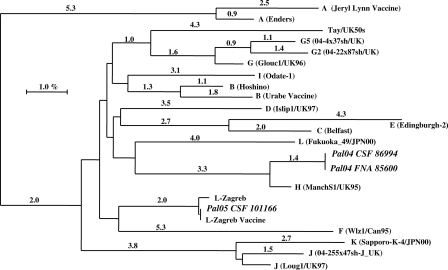
Phylogenetic analysis of a 316-nucleotide sequence that included the complete SH gene (171 bp) was performed as previously described (17). All outbreak MuV sequences were identical. Thus, only two samples, one CSF (Pal04 CSF 86994; accession no. AM293337) and one from parotid FNA (Pal04 FNA 85600; accession no. AM293335), were included in the phylogenetic tree (Fig. 3). The sequences of MuV RT-PCR products were aligned with the proposed standardized wild-type MuV strains (17). High homology (98.6%) was observed between MuV reference H genotype (ManchS1/UK 95; accession no. AF142771) and the outbreak virus strain. Detailed phylogenetic analysis of the complete SH gene of the circulating virus and the available H genotypes from NCBI/GenBank revealed that the outbreak virus belonged to the H2 clade (34).
FIG. 3.
Phylogenetic analysis of MuV SH gene nucleotides detected in samples from patients residing in the refugee camps of the West Bank. The ClustalX nearest neighbor-joining method (number of bootstraps, 1,000) was used to compare a 316-nucleotide sequence of the MuV SH gene with MuV reference sequences from the EMBL/GenBank database.
Sequence analysis from another CSF sample (Pal05 CSF 101166; accession no. AM293341) from an individual who developed aseptic meningitis during the mass vaccination campaign with the L-Zagreb strain revealed 100% homology to the L-Zagreb vaccine strain (Fig. 3).
DISCUSSION
The vaccination coverage with one dose of MMR vaccine among Palestinian refugees increased gradually from the time of its introduction in 1989 until 2003 when the coverage exceeded 95% (22). Even with high mumps vaccination coverage, mumps was still endemic in the Palestinian West Bank and in neighboring countries within the EMRO/WHO region and worldwide (12).
The occurrence of sporadic mumps outbreaks is well described even in populations with high vaccine coverage (7, 11, 31). This could be due in part to the accumulation of susceptible individuals in the population, which makes mumps outbreaks expected to occur 10 to 20 years after the introduction of routine immunization (12). However, such outbreaks are more likely to be seen among older age groups, especially those 15 to 30 years old, who are too old to have received vaccine and whose exposure to wild MuV was reduced by the herd effect of the vaccination program (7, 8, 38). However, in the current outbreak, the ages mostly affected ranged between 6 and 15 years.
Although there are no specific guidelines on mumps outbreak management, there is international consensus on the use of supplementary vaccination and patient isolation early in the course of the outbreak as tools to prevent the spread of infection to susceptible groups (38). In the West Bank outbreak, a supplementary vaccination campaign was conducted at the end of the second peak of the outbreak. The immunization probably contributed to controlling the outbreak by reducing the number of residual cases in the second half of 2005 and consequently decreasing virus circulation among susceptible groups. However, if the MMR mass vaccination campaign had been conducted during the first mumps peak (April to May 2004), it could have probably terminated the spread of the virus and prevented the second peak a year later.
The current study was initiated in order to report the epidemiology and to determine the causative agent of a large parotitis outbreak (3,871 cases) in recipients of a one-dose MMR vaccine. Patients presenting with parotitis appeared to manifest a booster immune response as evident from their serological profiles, negative or borderline MuV-specific IgM (81.4%) and high levels of MuV IgG (91.5%). A booster immune response was previously demonstrated in patients with mumps secondary vaccine failure by Narita et al. (24) and in patients with respiratory syncytial virus reinfection (25).
Whether mumps secondary vaccine failure (reinfection) occurs is controversial (13, 15, 26). Hersh et al. initially investigated a large mumps outbreak (n = 269) in Kansas and attributed the resurgence of mumps to primary vaccine failure or failure to vaccinate but did not rule out the possibility that secondary vaccine failure could have played a part in the outbreak (15). Mumps reinfection was also discussed by Gut et al. who reported 82 mumps patients with evidence of a booster immune response similar to that in the outbreak described in this paper (13). Our data suggest that waning immunity (secondary vaccine failure) was the main cause of the outbreak in the West Bank refugee camps and they underscore the need for a second vaccine dose as part of the standard immunization program.
Oral cavity fluid obtained after milking the parotid gland was successfully used to detect MuV genomic RNA (six of seven attempts) in patients with negative MuV-specific IgM and high levels of MuV-specific IgG. In addition, MuV RNA was detected in 100% (six of six) NPA samples collected from patients with no MuV-specific IgM. Our results demonstrate that such samples can be used to confirm the diagnosis of mumps in patients clinically presenting with parotitis but serologically negative for MuV IgM. Reid et al. also recommended the usage of oral fluid samples to detect MuV RNA in nonvaccinated and partially vaccinated individuals (28).
Urine samples have been well documented to contain MuV for several days post-primary mumps clinical presentation; however, a low positivity rate (33%) was observed in this outbreak, further indicating that in a vaccinated population, minimal MuV shedding occurs in the urine. Afzal et al. reported a similar observation from an investigation of an outbreak in Portugal in vaccinated individuals (1). Of the 31 urine samples evaluated by Afzal et al., only one was positive for MuV RNA (3%) (1).
Phylogenetic analysis of the amplified complete SH gene revealed that the MuV causing the outbreak belonged to the H genotype and had 96% homology to the Korean MuV H genotype (Yeoju1504; accession no. AY048996) outbreak strain (19). Interestingly, similar to patients in the Palestinian mumps outbreak, patients in the Korean outbreak had low IgM seropositivity rates (7.6%), which suggest that secondary vaccine failure was responsible for that outbreak, although the investigators did not raise this possibility. The Korean outbreak was also much smaller and included 736 suspected cases. Thus, an outbreak as large as the one described in our study due to genotype H has never been reported before.
The MuV IgG seropositivity rate in the healthy control group (68.6%) was lower than what is considered necessary for MuV herd immunity of 88 to 92% (3). Even if equivocal IgG results (11.8%) were considered positive as previously suggested (14, 29), the overall MuV IgG herd immunity (80.4%) would have been barely enough to prevent a MuV outbreak. These data further support our conclusion that waning immunity or secondary vaccine failure was the cause of this outbreak. Dayan et al. reported that minuscule waning immunity could potentiate a mumps outbreak in population with immunity near the herd threshold, particularly when combined with increased exposure (11). The low MuV IgG seroprevalence in Palestinian children aged 5 to 15 years may explain in part why the majority of infected patients were in this age group (76.3%). More recently, Muhsen et al. attributed a small mumps outbreak in Israeli individuals to low MuV IgG seroprevalence (75.3%) in the general population, which was below the herd immunity threshold, in addition to a trend of waning immunity between the first and second vaccine doses (23).
In view of the cumulative data regarding mumps reinfection in previously infected as well as previously vaccinated individuals, and the ability to demonstrate the MuV circulating lineages and genomic typing, the concept of life-long immunity to natural mumps infection should be revisited.
Acknowledgments
We thank all the UNRWA health care providers for their collaboration in investigating this outbreak. We also thank the members of Israel Central Virology Laboratory (ICVL), in particular Lily Ben-Yosef, for all their useful help in analyzing the samples. We also thank Karen Carroll, Johns Hopkins Medical Institutions, for her critical review of the manuscript.
Footnotes
Published ahead of print on 14 January 2009.
REFERENCES
- 1.Afzal, M. A., J. Buchanan, J. A. Dias, M. Cordeiro, M. L. Bentley, C. A. Shorrock, and P. D. Minor. 1997. RT-PCR based diagnosis and molecular characterisation of mumps viruses derived from clinical specimens collected during the 1996 mumps outbreak in Portugal. J. Med. Virol. 52349-353. [DOI] [PubMed] [Google Scholar]
- 2.Afzal, M. A., V. Dussupt, P. D. Minor, P. A. Pipkin, R. Fleck, D. J. Hockley, and G. N. Stacey. 2005. Assessment of mumps virus growth on various continuous cell lines by virological, immunological, molecular and morphological investigations. J. Virol. Methods 126149-156. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, R. M., and R. M. May. 1985. Age-related changes in the rate of disease transmission: implications for the design of vaccination programmes. J. Hyg. 94365-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boga, J. A., M. de Ona, A. Fernandez-Verdugo, D. Gonzalez, A. Morilla, M. Arias, L. Barreiro, F. Hidalgo, and S. Melon. 2008. Molecular identification of two genotypes of mumps virus causing two regional outbreaks in Asturias, Spain. J. Clin. Virol. 42425-428. [DOI] [PubMed] [Google Scholar]
- 5.Bonnet, M. C., A. Dutta, C. Weinberger, and S. A. Plotkin. 2006. Mumps vaccine virus strains and aseptic meningitis. Vaccine 247037-7045. [DOI] [PubMed] [Google Scholar]
- 6.Briss, P. A., L. J. Fehrs, R. A. Parker, P. F. Wright, E. C. Sannella, R. H. Hutcheson, and W. Schaffner. 1994. Sustained transmission of mumps in a highly vaccinated population: assessment of primary vaccine failure and waning vaccine-induced immunity. J. Infect. Dis. 16977-82. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. 2006. Mumps epidemic—Iowa, 2006. MMWR Morb. Mortal. Wkly. Rep. 55366-368. [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. 2006. Mumps epidemic—United Kingdom, 2004-2005. MMWR Morb. Mortal. Wkly. Rep. 55173-175. [PubMed] [Google Scholar]
- 9.Chiba, Y., K. Horino, M. Umetsu, Y. Wataya, and S. Chiba. 1973. Virus excretion and antibody response in saliva in natural mumps. Tohoku J. Exp. Med. 111229-238. [DOI] [PubMed] [Google Scholar]
- 10.Cooper, E. 2004. Review of Hear the Silence: confusion has resulted from conflating two questions into one. BMJ 32850-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dayan, G. H., M. P. Quinlisk, A. A. Parker, A. E. Barskey, M. L. Harris, J. M. Schwartz, K. Hunt, C. G. Finley, D. P. Leschinsky, A. L. O'Keefe, J. Clayton, L. K. Kightlinger, E. G. Dietle, J. Berg, C. L. Kenyon, S. T. Goldstein, S. K. Stokley, S. B. Redd, P. A. Rota, J. Rota, D. Bi, S. W. Roush, C. B. Bridges, T. A. Santibanez, U. Parashar, W. J. Bellini, and J. F. Seward. 2008. Recent resurgence of mumps in the United States. N. Engl. J. Med. 3581580-1589. [DOI] [PubMed] [Google Scholar]
- 12.Galazka, A. M., S. E. Robertson, and A. Kraigher. 1999. Mumps and mumps vaccine: a global review. Bull. W. H. O. 773-14. [PMC free article] [PubMed] [Google Scholar]
- 13.Gut, J. P., C. Lablache, S. Behr, and A. Kirn. 1995. Symptomatic mumps virus reinfections. J. Med. Virol. 4517-23. [DOI] [PubMed] [Google Scholar]
- 14.Hatakeyama, S., K. Moriya, S. Itoyama, Y. Nukui, M. Uchida, Y. Shintani, Y. Morisawa, and S. Kimura. 2004. Prevalence of measles, rubella, mumps, and varicella antibodies among healthcare workers in Japan. Infect. Control Hosp. Epidemiol. 25591-594. [DOI] [PubMed] [Google Scholar]
- 15.Hersh, B. S., P. E. Fine, W. K. Kent, S. L. Cochi, L. H. Kahn, E. R. Zell, P. L. Hays, and C. L. Wood. 1991. Mumps outbreak in a highly vaccinated population. J. Pediatr. 119187-193. [DOI] [PubMed] [Google Scholar]
- 16.Jin, L., S. Beard, and D. W. Brown. 1999. Genetic heterogeneity of mumps virus in the United Kingdom: identification of two new genotypes. J. Infect. Dis. 180829-833. [DOI] [PubMed] [Google Scholar]
- 17.Jin, L., B. Rima, D. Brown, C. Orvell, T. Tecle, M. Afzal, K. Uchida, T. Nakayama, J. W. Song, C. Kang, P. A. Rota, W. Xu, and D. Featherstone. 2005. Proposal for genetic characterisation of wild-type mumps strains: preliminary standardisation of the nomenclature. Arch. Virol. 1501903-1909. [DOI] [PubMed] [Google Scholar]
- 18.Johansson, B., T. Tecle, and C. Orvell. 2002. Proposed criteria for classification of new genotypes of mumps virus. Scand. J. Infect. Dis. 34355-357. [DOI] [PubMed] [Google Scholar]
- 19.Lee, J. Y., B. K. Na, J. H. Kim, J. S. Lee, J. W. Park, G. C. Shin, H. W. Cho, H. D. Lee, U. Y. Gou, B. K. Yang, J. Kim, C. Kang, and W. J. Kim. 2004. Regional outbreak of mumps due to genotype H in Korea in 1999. J. Med. Virol. 7385-90. [DOI] [PubMed] [Google Scholar]
- 20.Leinikki, P. 1995. Mumps, 3rd ed. John Wiley & Sons Ltd., New York, NY.
- 21.Miller, E., M. Goldacre, S. Pugh, A. Colville, P. Farrington, A. Flower, J. Nash, L. MacFarlane, and R. Tettmar. 1993. Risk of aseptic meningitis after measles, mumps, and rubella vaccine in UK children. Lancet 341979-982. [DOI] [PubMed] [Google Scholar]
- 22.Mousa, F. A., and R. A. Cook. 2004. Disease prevention and control. Annual reports of UNRWA, 1999-2004. United Nations Relief and Works Agency, Department of Health, Amman, Jordan.
- 23.Muhsen, K., Y. Aboudy, E. Mendelson, M. S. Green, and D. Cohen. 2008. Prevalence of mumps antibodies in the Israeli population in relation to mumps vaccination policy and incidence of disease. Epidemiol. Infect. 136688-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Narita, M., Y. Matsuzono, Y. Takekoshi, S. Yamada, O. Itakura, M. Kubota, H. Kikuta, and T. Togashi. 1998. Analysis of mumps vaccine failure by means of avidity testing for mumps virus-specific immunoglobulin G. Clin. Diagn. Lab. Immunol. 5799-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogra, P. L. 2004. Respiratory syncytial virus: the virus, the disease and the immune response. Paediatr. Respir. Rev. 5(Suppl. A)S119-S126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park, D. W., M. H. Nam, J. Y. Kim, H. J. Kim, J. W. Sohn, Y. Cho, K. J. Song, and M. J. Kim. 2007. Mumps outbreak in a highly vaccinated school population: assessment of secondary vaccine failure using IgG avidity measurements. Vaccine 254665-4670. [DOI] [PubMed] [Google Scholar]
- 27.Polgreen, P. M., L. C. Bohnett, J. E. Cavanaugh, S. B. Gingerich, L. E. Desjardin, M. L. Harris, M. P. Quinlisk, and M. A. Pentella. 2008. The duration of mumps virus shedding after the onset of symptoms. Clin. Infect. Dis. 461447-1449. [DOI] [PubMed] [Google Scholar]
- 28.Reid, F., J. Hassan, F. Irwin, A. Waters, W. Hall, and J. Connell. 2008. Epidemiologic and diagnostic evaluation of a recent mumps outbreak using oral fluid samples. J. Clin. Virol. 41134-137. [DOI] [PubMed] [Google Scholar]
- 29.Riddell, M. A., J. A. Leydon, A. Ugoni, and H. A. Kelly. 2001. A serosurvey evaluation of the school-based measles ‘catch-up’ immunisation campaign in Victorian school-aged children. Aust. N. Z. J. Public Health 25529-533. [DOI] [PubMed] [Google Scholar]
- 30.Schmid, D., H. Holzmann, C. Alfery, H. Wallenko, T. H. Popow-Kraupp, and F. Allerberger. 2008. Mumps outbreak in young adults following a festival in Austria, 2006. Eurosurveillance 138049. [DOI] [PubMed] [Google Scholar]
- 31.Tecle, T., B. Bottiger, C. Orvell, and B. Johansson. 2001. Characterization of two decades of temporal co-circulation of four mumps virus genotypes in Denmark: identification of a new genotype. J. Gen. Virol. 822675-2680. [DOI] [PubMed] [Google Scholar]
- 32.Uchida, K., M. Shinohara, S. Shimada, Y. Segawa, and Y. Hoshino. 2001. Characterization of mumps virus isolated in Saitama Prefecture, Japan, by sequence analysis of the SH gene. Microbiol. Immunol. 45851-855. [DOI] [PubMed] [Google Scholar]
- 33.Utz, J. P., C. F. Szwed, and J. A. Kasel. 1958. Clinical and laboratory studies of mumps. II. Detection and duration of excretion of virus in urine. Proc. Soc. Exp. Biol. Med. 99259-261. [DOI] [PubMed] [Google Scholar]
- 34.Utz, S., J. L. Richard, S. Capaul, H. C. Matter, M. G. Hrisoho, and K. Muhlemann. 2004. Phylogenetic analysis of clinical mumps virus isolates from vaccinated and non-vaccinated patients with mumps during an outbreak, Switzerland 1998-2000. J. Med. Virol. 7391-96. [DOI] [PubMed] [Google Scholar]
- 35.Vandermeulen, C., M. Roelants, M. Vermoere, K. Roseeuw, P. Goubau, and K. Hoppenbrouwers. 2004. Outbreak of mumps in a vaccinated child population: a question of vaccine failure? Vaccine 222713-2716. [DOI] [PubMed] [Google Scholar]
- 36.Wharton, I. P., A. H. Chaudhry, and M. E. French. 2006. A case of mumps epididymitis. Lancet 367702. [DOI] [PubMed] [Google Scholar]
- 37.Yoshida, N., M. Fujino, A. Miyata, T. Nagai, M. Kamada, H. Sakiyama, T. Ihara, T. Kumagai, T. Okafuji, T. Okafuji, and T. Nakayama. 2008. Mumps virus reinfection is not a rare event confirmed by reverse transcription loop-mediated isothermal amplification. J. Med. Virol. 80517-523. [DOI] [PubMed] [Google Scholar]
- 38.Zimmerman, L., S. Reef, and M. Wharton. 2002. Mumps, VPD surveillance manual, 3rd ed., vol. 7-12. Centers for Disease Control, Atlanta, GA.