Abstract
We describe a calorimetric assay for the detection of methicillin-resistant Staphylococcus aureus (MRSA) within 5 h. Microbial heat was calculated in culture with and without cefoxitin. Among 30 genetically distinct clinical isolates, 19/20 MRSA (95%) and 10/10 methicillin-susceptible Staphylococcus aureus (100%) were correctly identified. Microcalorimetry may be useful for rapid MRSA screening.
Methicillin-resistant Staphylococcus aureus (MRSA) is a frequent cause of health care- and community-associated infections. Its prevalence continues to increase in hospitals and in the outpatient setting. Infection control guidelines combine active surveillance with elaborate patient management, including screening for MRSA, contact isolation, and decolonization (12). Therefore, a rapid and accurate detection of MRSA is essential for both the efficient prevention of the spread of resistant bacteria and the initiation of an early targeted treatment of infections.
Various approaches are used to discriminate between methicillin-susceptible S. aureus (MSSA) and MRSA (2-4, 14). In staphylococci, resistance to oxacillin is usually screened by phenotypic tests, as recommended by the Clinical and Laboratory Standards Institute (1). In most clinical laboratories, culture methods using selective broth, disc diffusion, or chromogenic agar are widely used. The gold standard for the detection of MRSA includes the use of selective cultures in enriched salt-containing tryptic soy broth (TSB) followed by plating (8). The PCR amplification of the mecA gene from isolated colonies is one of the most accurate methods to differentiate MRSA from MSSA, but it is expensive and thus less appropriate for screening purposes.
Calorimetry is a highly sensitive and useful technique that allows the measurement of heat generated by biological processes in the living cell (9). Medically important microorganisms such as staphylococci replicate in an appropriate culture medium, resulting in an exponential increase of heat that can be recorded in real-time (i.e., heat-flow curve). Since antimicrobials inhibit bacterial growth, isothermal calorimetry can be used to determine antimicrobial susceptibility (6). This principle has been recently demonstrated with two laboratory strains of S. aureus (ATCC 25923 and ATCC 43300), cultured in the presence of oxacillin or cefoxitin (13).
We developed a calorimetric assay and evaluated its performance in terms of repeatability, validity, and robustness for the detection of methicillin resistance in S. aureus. First, we determined the assay repeatability using two well-characterized reference laboratory strains, MSSA ATCC 29213 and MRSA COL. Additionally, we tested MRSA ATCC 43300, a strain with delayed expression of methicillin resistance (5). Subsequently, we screened 30 genetically distinct clinical isolates of S. aureus (10 MSSA and 20 MRSA) and classified them in a blinded manner.
The clinical strains were collected from nonrelated patients admitted to our hospital between January 2005 and December 2007 and were isolated from intraoperative tissue specimens (n = 16), blood (n = 6), urine (n = 5), or respiratory aspirates (n = 3). The clinical isolates were characterized by pulsed-field gel electrophoresis pattern analysis with Pearson correlation using the Chef DR III system (Bio-Rad) for separating SmaI-digested genomic DNA, as previously described (10). All isolates displayed a Pearson correlation of <75%, except for two MRSA isolates (S1771 and T4545) that showed a correlation of 98.2% (see Fig. S1 in the supplemental material). For classification as MSSA or MRSA, the clinical isolates were screened for susceptibility to oxacillin using a microdilution broth procedure (Merlin Diagnostika, Bornheim-Hersel, Germany), interpreted in accordance with Clinical and Laboratory Standards Institute guidelines (1). Isolates showing an oxacillin MIC of ≥4 μg/ml were further evaluated with a slide latex agglutination test (Denka Seiken, Tokyo, Japan) detecting PBP2a and classified as MRSA. The oxacillin MIC of all 10 MSSA isolates was ≤1 μg/ml.
A 48-channel batch calorimeter (thermal activity monitor, model 3102 TAM III; TA Instruments, New Castle, DE) was used to measure the heat flow at 37°C controlled at ±0.0001°C and a sensitivity of ±0.2 μW. Heat was measured continuously in 4-ml glass ampoules and expressed as heat flow over time (in watts [W]). Total heat (in joules [J]) was determined by the integration of the area below the heat flow-time curve. Data analysis was accomplished using the manufacturer's software (TAM Assistant; TA Instruments) and Prism 4.0a (GraphPad Software, La Jolla, CA).
Bacterial inocula for the calorimetry samples were prepared from discrete colonies of S. aureus freshly grown on Columbia 5% sheep blood agar and resuspended in 0.85% sterile saline to a McFarland turbidity of 5. The high bacterial inocula were chosen to allow early heat measurements and to increase the chances of including methicillin-resistant colonies when testing strains heterogeneous in the expression of resistance (2, 7). Aliquots of 300 μl were inoculated into sterile calorimetry ampoules prefilled with 2.7 ml TSB with and without cefoxitin (Sigma, Buchs, Switzerland), a strong inducer of mecA expression in both high-level and low-level methicillin-resistant staphylococci (3, 11). Twofold dilutions of cefoxitin were tested against the laboratory strains. The lowest concentration of cefoxitin that inhibited the heat production of MSSA was 4 μg/ml (data not shown), which was used for the subsequent calorimetric studies. The cefoxitin MIC in TSB using a macrodilution broth assay was 2 μg/ml (for MSSA ATCC 29213), 16 μg/ml (for MRSA COL), and 8 μg/ml (for MRSA ATCC 43300).
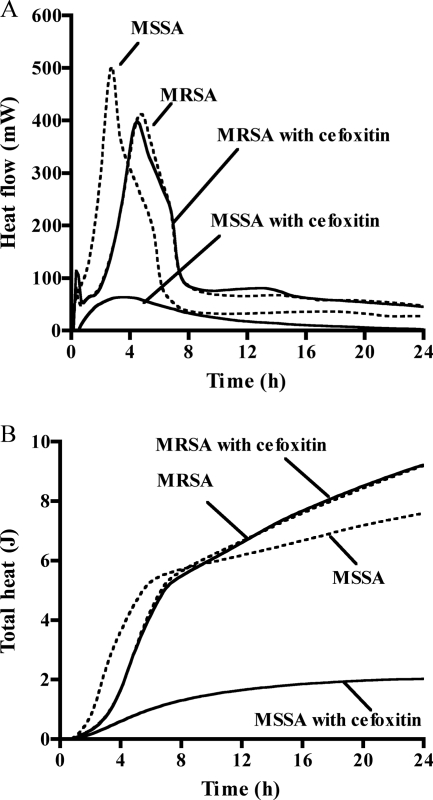
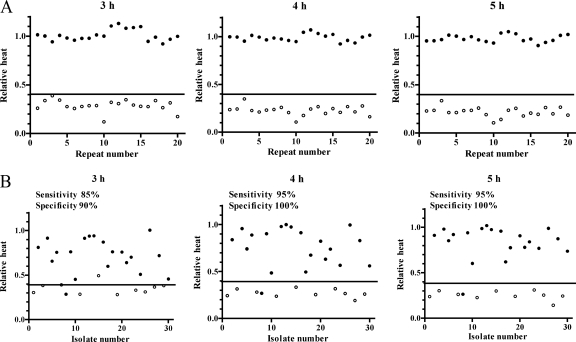
For laboratory strains, the calorimetric measurement was performed in 20 replicates on consecutive days in order to calculate the interday variation. Figure 1 shows the heat-flow and total heat curves in the presence and absence of cefoxitin (4 μg/ml of a representative measurement). Cefoxitin inhibited the heat production of the MSSA ATCC 29213 strain for 24 h, whereas the heat production of the MRSA COL strain was only insignificantly affected. The relative heat was calculated as the ratio between the total heat measured in the presence and absence (growth control) of cefoxitin at different time points of incubation. Figure 2A shows the relative heat of 20 repeated measurements at 3 h, 4 h, and 5 h. The mean relative heat at 5 h was significantly lower for MSSA than for MRSA reference strains (0.22 versus 1.00; P < 0.001) (Table 1). Based on the relative heat distribution at 5 h, a cutoff value was generated for the discrimination of MSSA (<0.4) and MRSA (≥0.4).
FIG. 1.
Heat-flow (A) and total heat (B) of both reference MSSA ATCC 29213 and MRSA COL strains cultured in the presence of cefoxitin at 4 μg/ml (solid lines) or without antibiotic (dashed lines).
FIG. 2.
Relative heat distribution in 20 repeated measurements of the reference strains MSSA ATCC 29213 and MRSA COL (A); and 30 clinical isolates, 10 different MSSA and 20 different MRSA strains (B). Relative heat is calculated as the ratio between the total heat in the presence and absence of 4 μg/ml cefoxitin after 3 h, 4 h, and 5 h of incubation. Open circles indicate MSSA and closed circles MRSA; the horizontal line indicates the cutoff value of relative heat (0.4) used for the discrimination of MSSA and MRSA.
TABLE 1.
Relative heat of two reference strains (1 MSSA and 1 MRSA) and 30 clinical isolates of S. aureus (10 MSSA and 20 MRSA) measured after 3 h, 4 h, and 5 h of incubation
| Strain | Relative heata at different incubation times
|
||
|---|---|---|---|
| 3 h | 4 h | 5 h | |
| Reference strains | |||
| MSSA (ATCC 29213) | 0.29 ± 0.06 | 0.23 ± 0.05 | 0.22 ± 0.05 |
| MRSA (COL) | 1.00 ± 0.08 | 0.97 ± 0.08 | 0.97 ± 0.05 |
| Clinical isolates | |||
| MSSA (n = 10) | 0.35 ± 0.06 | 0.27 ± 0.04 | 0.25 ± 0.05 |
| MRSA (n = 20) | 0.76 ± 0.15 | 0.80 ± 0.16 | 0.84 ± 0.11 |
Values are means ± standard deviations of 20 repeated measurements (for both reference strains) and of 30 different clinical isolates (10 MSSA and 20 MRSA).
The heterogeneous MRSA ATCC 43300 showed a delayed peak of the heat-flow curve in the presence of cefoxitin compared to the growth control (data not shown). Therefore, the calorimetric measurement for this strain was prolonged. The relative heat (mean ± standard deviation) increased from 0.48 ± 0.14 (at 5 h) to 0.60 ± 0.18 (at 6 h) and 0.80 ± 0.15 (at 8 h) (P value for trend, <0.05). Of 20 MRSA ATCC 43300 replicates, the relative heat of ≥0.4 was measured in 15, 17, and 20 replicates after 5 h, 6 h, and 8 h of incubation, respectively. Thus, the prolonged incubation time of 8 h was necessary to detect the MRSA strain ATCC 43300 with 100% accuracy.
Figure 2B shows the relative heat of the 30 clinical isolates at 3 h, 4 h, and 5 h of incubation. The isolates were tested in duplicate. Cefoxitin at 4 μg/ml inhibited the heat production of all MSSA clinical isolates but none of the MRSA isolates. After 24 h, all growth controls and all of the MRSA cultures with cefoxitin showed turbidity, while all of the MSSA cultures with cefoxitin remained clear. Table 1 summarizes the relative heat of the clinical isolates at 3 h, 4 h, and 5 h of incubation. Based on the relative heat cutoff of 0.4, 17 (85%) of 20 MRSA clinical isolates were correctly identified after 3 h of incubation. When the incubation was prolonged to 5 h, 19 (95%) of 20 MRSA isolates were correctly identified. The relative heat of all MSSA strains remained at <0.4 after 4 h and 5 h of incubation. At 5 h of incubation, the sensitivity, specificity, and positive and negative predictive values (and their 95% confidence intervals) for the detection of methicillin resistance were 95% (89% to 100%), 100% (92% to 100%), 100% (91% to 100%), and 91% (85% to 97%), respectively.
One clinical isolate of MRSA (T3011) gave a discrepant result. The relative heat value remained at <0.4 up to 5 h, and the heat-flow curve showed no peak during 24 h. This clinical isolate had a low growth rate in the presence of cefoxitin, despite the fact that conventional tests demonstrated resistance to methicillin (oxacillin MIC, ≥4 μg/ml; positive PBP2a). The cefoxitin MIC in TSB, further evaluated with the macrodilution broth method, was 4 μg/ml and thus lower than the one for MRSA COL (16 μg/ml). The low cefoxitin MIC may explain the slow growth and the low heat production in the cefoxitin cultures. One MSSA isolate (T 448-1) displayed a relative heat between 0.4 and 0.5 after 3 h of incubation, which decreased below 0.4 after 4 h and 5 h. The unusually high relative heat at 3 h was due to a prolonged lag phase of the isolate.
In this report, we described a calorimetric assay for the rapid and accurate discrimination of clinical MSSA and MRSA isolates, which is highly repeatable, easy to set up, and suitable for automation and computer-generated results. The hands-on preparation time is around 15 min, and the consumables are inexpensive (two disposable 4-ml glass ampoules with rubber-aluminum lids and 6 ml standard culture medium per test isolate). A prerequisite for a valid test result is the normal growth of the isolate in the absence of antibiotic (growth control). With the optimization of the calorimetric assay, the accuracy and speed of MRSA detection could be further increased and potentially extended to other organisms and antimicrobial substances. However, a validation study with additional clinical strains from different body sites is needed before introduction into the clinical routine.
Supplementary Material
Acknowledgments
We acknowledge the Swiss National Science Foundation (#3200B0-112547/1), Gebert Rüf Stiftung (GRS-070/06), and the Stanley Thomas Johnson Foundation for their financial support.
Footnotes
Published ahead of print on 21 January 2009.
Supplemental material for this article may be found at http://jcm.asm.org/.
REFERENCES
- 1.Amsterdam, D. 2005. Susceptibility testing of antimicrobials in liquid media, p. 61-143. In V. Lorian (ed.), Antibiotics in laboratory medicine, 5th ed. LWW Press, Philadelphia, PA.
- 2.Brown, D. F., D. I. Edwards, P. M. Hawkey, D. Morrison, G. L. Ridgway, K. J. Towner, and M. W. Wren. 2005. Guidelines for the laboratory diagnosis and susceptibility testing of methicillin-resistant Staphylococcus aureus (MRSA). J. Antimicrob. Chemother. 561000-1018. [DOI] [PubMed] [Google Scholar]
- 3.Fang, H., and G. Hedin. 2006. Use of cefoxitin-based selective broth for improved detection of methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 44592-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Felten, A., B. Grandry, P. H. Lagrange, and I. Casin. 2002. Evaluation of three techniques for detection of low-level methicillin-resistant Staphylococcus aureus (MRSA): a disk diffusion method with cefoxitin and moxalactam, the Vitek 2 system, and the MRSA-screen latex agglutination test. J. Clin. Microbiol. 402766-2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mackenzie, A. M., H. Richardson, R. Lannigan, and D. Wood. 1995. Evidence that the National Committee for Clinical Laboratory Standards disk test is less sensitive than the screen plate for detection of low-expression-class methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 331909-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mardh, P., T. Ripa, K. Andersson, and I. Wadso. 1976. Kinetics of the actions of tetracyclines on Escherichia coli as studied by microcalorimetry. Antimicrob. Agents Chemother. 10604-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niemeyer, D. M., M. J. Pucci, J. A. Thanassi, V. K. Sharma, and G. L. Archer. 1996. Role of mecA transcriptional regulation in the phenotypic expression of methicillin resistance in Staphylococcus aureus. J. Bacteriol. 1785464-5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Safdar, N., L. Narans, B. Gordon, and D. G. Maki. 2003. Comparison of culture screening methods for detection of nasal carriage of methicillin-resistant Staphylococcus aureus: a prospective study comparing 32 methods. J. Clin. Microbiol. 413163-3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spink, C., and I. Wadso. 1976. Calorimetry as an analytical tool in biochemistry and biology. Methods Biochem. Anal. 231-159. [DOI] [PubMed] [Google Scholar]
- 10.Stranden, A., R. Frei, and A. F. Widmer. 2003. Molecular typing of methicillin-resistant Staphylococcus aureus: can PCR replace pulsed-field gel electrophoresis? J. Clin. Microbiol. 413181-3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swenson, J. M., R. Skov, and J. Patel. 2007. The cefoxitin disk test—what a clinical microbiologist needs to know. Clin. Microbiol. Newsl. 2933-40. [Google Scholar]
- 12.Tomic, V., P. Svetina Sorli, D. Trinkaus, J. Sorli, A. F. Widmer, and A. Trampuz. 2004. Comprehensive strategy to prevent nosocomial spread of methicillin-resistant Staphylococcus aureus in a highly endemic setting. Arch. Intern. Med. 1642038-2043. [DOI] [PubMed] [Google Scholar]
- 13.Von Ah, U., D. Wirz, and A. U. Daniels. 2008. Rapid differentiation of methicillin-susceptible Staphylococcus aureus from methicillin-resistant S. aureus and MIC determinations by isothermal microcalorimetry. J. Clin. Microbiol. 462083-2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Von Eiff, C., D. Maas, G. Sander, A. W. Friedrich, G. Peters, and K. Becker. 2008. Microbiological evaluation of a new growth-based approach for rapid detection of methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 611277-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.