Abstract
Since current microbiology methods are not suitable to detect Clostridium perfringens in formalin-fixed, paraffin-embedded tissue samples, we developed a PCR assay to detect toxin-encoding genes and the 16S rRNA gene of C. perfringens. We successfully detected and genotyped C. perfringens in tissue sections from two autopsy cases.
Clostridium perfringens causes several forms of enteric diseases, including food poisoning and fatal enterotoxemia (15, 17). Based on the presence of four major lethal toxins (alpha-, beta-, epsilon-, and iota-toxins), C. perfringens is classified into five toxigenic types (A through E), and each type can cause different diseases. The most commonly encountered type A (alpha-toxin) strain causes gas gangrene (myonecrosis), diarrhea, and food-borne illness in humans (4). Type B (alpha-, beta-, and epsilon-toxins) and type D (alpha- and epsilon-toxins) strains are the causative agents of fatal enterotoxemia in domestic animals and occasionally humans (16). The type C (alpha- and beta-toxins) strain causes severe necrotic enteritis in humans and is called Darmbrand in Germany and pig-bel in New Guinea (12). The pathogenicity of type E (alpha- and iota-toxins) strains is unclear and has rarely been isolated in humans (16). Similar toxins can be found in other Clostridium species, including C. novyi, C. septicum, and C. histolyticum (4).
While the presumptive diagnosis of C. perfringens can be achieved by clinical and pathological findings, confirmation is routinely performed by conventional microbiological isolation and characterization methods including bacterial culture, biochemical analysis, and enzyme-linked immunosorbent assay (6, 8). Conventional culture procedures are expensive and time-consuming and detect only live microorganisms. Therefore, current conventional detection techniques are not applicable for the detection of nonviable bacteria, such as those found in formalin-fixed tissue samples. PCR is a well-accepted, rapid, and sensitive technique for the detection of microbial pathogens, particularly in situations in which low bacterial copy numbers are present (17). PCR assays have been used to identify C. perfringens in animals (3, 6, 9, 13, 17). However, no studies describe methods to detect and differentiate the various toxigenic types in formalin-fixed, paraffin-embedded human tissue samples. Therefore, we developed a sensitive PCR-based method for the detection of C. perfringens strains and their associated toxin genes in formalin-fixed, paraffin-embedded tissue samples in situations when diagnosis of the infection by culture is unavailable.
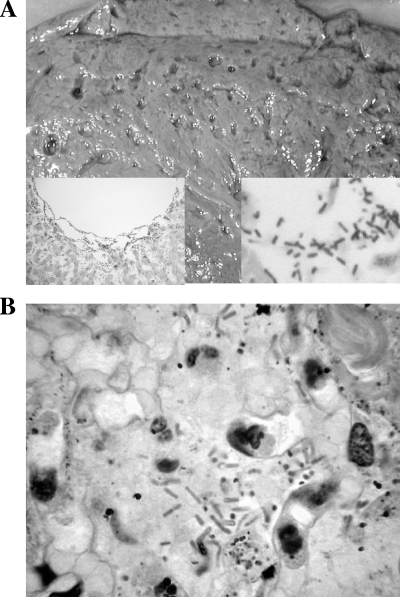
In order to confirm the sensitivity and specificity of the assay, four types of C. perfringens reference strains were obtained from American Type Culture Collection (ATCC; Manassas, VA): type A (ATCC 13124), type B (ATCC 3626), type C (ATCC 3628), and type D (ATCC 3629). Other bacterial strains, including Clostridium difficile (ATCC 9689), Clostridium sordellii (ATCC 9714), Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 27853), Proteus vulgaris (ATCC 13315), and Salmonella enterica serovar Typhimurium (ATCC 4924), were also obtained. We then studied two autopsy cases in which bacterial infection was clinically unsuspected as the cause of death. In case one, emphysematous gastritis involving the proximal half of the stomach and distal esophagus and focal necrosis of lungs were found. In case two, grossly visible air spaces (“Swiss cheese” appearance) (Fig. 1A) were found in the liver, brain, heart, kidneys, spleen, and intestine. Microscopic examination of the tissue sections from various organs of both cases revealed spore-forming, boxcar-shaped, gram-positive bacilli, morphologically consistent with C. perfringens (Fig. 1A and B). PCR was performed to confirm the diagnosis of C. perfringens and for further toxinogenic genotyping of the bacteria using formalin-fixed, paraffin-embedded tissue blocks of the stomach (case 1) and liver (case 2) in which gram-positive bacilli were identified.
FIG. 1.
Identification of C. perfringens by macroscopic and microscopic examination. (A) Cut surface of liver (case 2) exhibiting multiple cystic air spaces (“Swiss cheese” appearance) lined by bacteria (left and right insets, magnifications of ×10 and ×40, respectively; both insets stained with hematoxylin and eosin). (B) Section of stomach (case 1) demonstrating colonization of gastric epithelium by numerous boxcar-shaped, gram-positive organisms.
ATCC bacterial strains were cultivated in a thioglycolate broth at 37°C for 48 to 72 h in an anaerobic or aerobic atmosphere. A 200-μl aliquot of each culture was centrifuged (14,000 rpm), and the resultant pellets were used to extract total DNA. Formalin-fixed, paraffin-embedded tissue blocks were sectioned using a microtome with a disposable blade to a thickness of 10 μm, and five sections were placed in a 1.5-ml microcentrifuge tube. Precautions were taken to avoid cross-contamination. The blades were changed, and the holder was cleaned with a 10% sodium hypochlorite solution between each tissue block (7, 10). The sections were deparaffinized twice in 1 ml xylene at 60°C for 30 min and washed in 1 ml of absolute ethanol once. Total DNA was extracted using the DNeasy tissue kit (Qiagen, Valencia, CA) according to the manufacturer's protocols. Briefly, 340 μl of buffer ATL and 60 μl of proteinase K (20 mg/ml) were added to the each tube, and the tubes were incubated at 56°C overnight. The supernatants were removed after a brief centrifuge, 400 μl buffer AL was added to each tube, and the tubes were incubated at 70°C for an additional 20 min. At the end of incubation, 400 μl of ethanol was added to the reaction mixture, and the mixture was passed through a QIAamp spin column. DNA was eluted from the column with 100 μl of buffer AE and stored at −20°C for later use.
Specific primers corresponding to the 16S rRNA gene (GenBank accession no. Y12669) (15) and to the alpha-toxin gene or cpa (GenBank accession no. X13608) (8), beta-toxin gene or cpb (GenBank accession no. X83275) (8), and epsilon-toxin gene or etx (GenBank accession no. X60694) (8) of C. perfringens were designed by using the sequence data obtained from GenBank (National Institute of Health, Bethesda, MD) and synthesized by Gene Link (Hawthorne, NY). Primers were designed for alpha-, beta-, and epsilon-toxin genes of C. perfringens, as these toxins are the major cause of human diseases (12, 16). Furthermore, detection of these genes allows for the molecular genotyping of C. perfringens (13). Primer sequences, locations of the primers, and sizes of the products are shown in Table 1.
TABLE 1.
PCR primers used in this study
| Genea | Primer sequence (5′ to 3′) | Primer positions | Product size (bp) | Reference |
|---|---|---|---|---|
| 16S rRNA gene | AAAGATGGCATCATCATTCAAC | 184-205 | 279 | 17 |
| TACCGTCATTATCTTCCCCAAA | 441-462 | |||
| cpa | GCTAATGTTACTGCCGTTGACC | 1280-1300 | 324 | 9 |
| TCTGATACATCGTGTAAG | 1585-1602 | |||
| cpb | GCGAATATGCTGAATCATCTA | 737-757 | 196 | 9 |
| GCAGGAACATTAGTATATCTTC | 911-932 | |||
| etx | GCGGTGATATCCATCTATTC | 258-277 | 665 | 9 |
| CCACTTACTTGTCCTACTAAC | 893-913 |
cpa, cpb, and etx are the alpha-, beta-, and epsilon-toxin genes of C. perfringens, respectively.
PCR was carried out in a 50-μl reaction mixture composed of 10 mM Tris-HCl (pH 8.3), 2 mM MgCl2, 50 mM KCl, 0.2 mM deoxynucleoside triphosphate, 0.5 μM of each primer, 15 ng of DNA, and 4 units of AmpliTaq Gold DNA polymerase (Roche, Indianapolis, IN). The reaction was performed in a thermal cycler (GeneAmp 9600 PCR system; Perkin Elmer, Boston, MA) for 35 cycles (1 cycle consists of 1 min at 94°C, 1 min at 53°C, and 1 min at 72°C) for the 16S rRNA and cpb genes and 35 cycles (1 cycle consists of 1 min at 94°C, 1 min at 50°C, and 1 min at 72°C) for the cpa and etx genes. Fifteen microliters of PCR products was examined by gel electrophoresis through a 2.0% agarose gel and visualized after staining with 0.5 μg/ml of ethidium bromide.
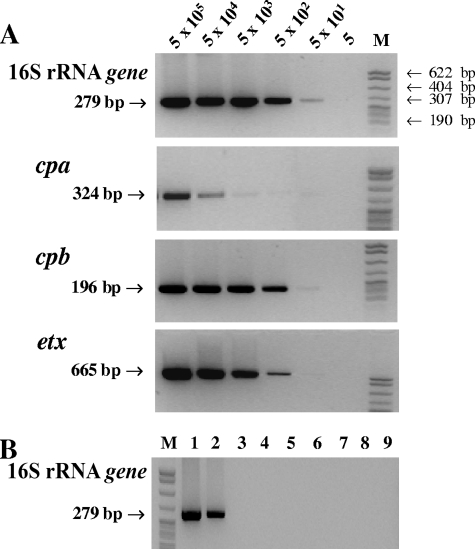
PCR amplification yielded the expected products for the 16S rRNA (279-bp), cpa (324-bp), cpb (196-bp), and etx (665-bp) genes (Fig. 2A). The assay sensitivity was determined using a serial 10-fold dilution of genomic DNA isolated from cultured C. perfringens. Since the cpb and etx genes are on plasmids, the exact copy numbers of these plasmids in these strains are unknown. Therefore, the copy number of genomic DNA was used to determine analytical sensitivity. The analytical sensitivity of the PCR assay for the 16S rRNA gene was 50 copies of genomic DNA and ranged from 50 (cpb and etx) to 500 (cpa) copies for the other genes (Fig. 2A). The lower sensitivity for cpa (500 copies) may be due to sequence variation of this gene in different strains or insufficient sensitivity of the primer (14).
FIG. 2.
Detection of 16S rRNA and toxin genes of C. perfringens by PCR. (A) Sensitivity of the PCR assay for the detection of 16S rRNA and toxin genes (cpa, cpb, and etx) of C. perfringens. Genomic DNA was serially diluted 10-fold from 5 × 105 to 5 copies. The results showed that the sensitivity of detecting each gene was between 50 and 500 copies. (B) Specific amplification of the 16S rRNA gene of C. perfringens (279 bp) by PCR. Lane 1, C. perfringens type B (5 × 105 copies); lane 2, C. perfringens type B (5 × 102 copies); lane 3, blank (no target); lane 4, C. difficile; lane 5, C. sordelli; lane 6, P. aeruginosa; lane 7, E. coli; lane 8, S. enterica serovar Typhimurium; lane 9, P. vulgaris. Lanes M in panels A and B contain DNA size markers (pBR322 DNA MspI digest). Genomic DNA (5 × 105 copies) was tested for bacteria other than C. perfringens.
The 16S rRNA gene primer set was specific for C. perfringens, and no cross-reactivity with other bacterial species was observed (Fig. 2B). This is consistent with a previous report that tested 11 other Clostridium isolates including C. butyricum, C. difficile, C. sporogenes, and C. novyi as well as 38 other clinically important species of common bacteria, such as Listeria and Bacillus spp. (15). Therefore, this primer set can be used for primary screening of clostridial microorganisms.
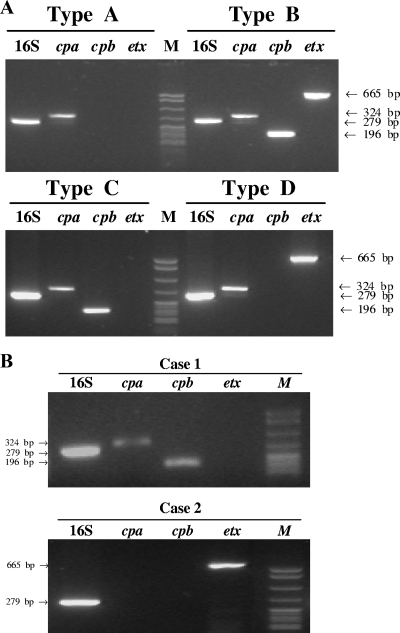
As expected, the 16S rRNA gene (279 bp) and alpha-toxin gene (324 bp) were present in all types of C. perfringens, the beta-toxin gene (196 bp) was present in types B and C, and the epsilon-toxin gene (665 bp) was present in types B and D (Fig. 3A). These results indicate that selected toxin gene primers can reliably categorize C. perfringens into specific subtypes (8, 13).
FIG. 3.
Genotyping of C. perfringens by PCR. The DNAs extracted from C. perfringens types A to D (ATCC strains) (A) or from tissue sections of case 1 (stomach) and case 2 (liver) (B) were amplified using primers specific for the 16S rRNA gene and each toxin gene (cpa, cpb, and etx) by PCR. Lanes M contain DNA size markers (pBR322 DNA MspI digest).
The PCR using 16S rRNA gene and toxin gene primers reliably detected C. perfringens in formalin-fixed, paraffin-embedded tissue blocks from two patients (stomach from case 1 and liver from case 2). An initial PCR assay using primers specific for the 16S rRNA gene confirmed adequate DNA for PCR analysis and confirmed the presence of C. perfringens in these tissues, negating the need for the use of a housekeeping gene to assess inhibition due to formalin cross-link formation (Fig. 3B). Furthermore, the PCR products of the 16S rRNA genes from both cases were sequenced, which matched with 100% concordance the C. perfringens sequence (GenBank accession no. Y12669) obtained from GenBank. PCR analysis with primers specific to cpa, cpb, and etx toxin genes detected a 324-bp (alpha-toxin) band and a 196-bp (beta-toxin) band in case one, consistent with C. perfringens type C. In case two, only a 665-bp (epsilon-toxin) PCR product was detected, suggesting the presence of either type B or type D, as only these types carry the etx gene (2, 5, 11) (Fig. 3A and B). The inability to detect the cpa and/or cpb genes in this bacterial species may be the result of high genetic variability in different strains (14) in which only the etx gene is present. Alternatively, primer selection may lack sufficient sensitivity for the detection of the cpa and cpb genes.
As the majority of cases of gas gangrene are caused by C. perfringens (1), this assay can be used to confirm the diagnosis of C. perfringens gas gangrene, particularly in a situation where the only material available for microbiological testing is formalin-fixed, paraffin-embedded tissue. However, it has to be acknowledged that a negative result with the described PCR assay does not rule out the involvement of other, non-C. perfringens, gas gangrene-causing Clostridium species, such as C. welchii, C. septicum, C. novyi, and C. histolyticum.
In conclusion, the described PCR assay could be used to detect C. perfringens and simultaneously detect the presence of its specific toxin genes in formalin-fixed, paraffin-embedded tissue samples. These primer sets hold promise as a means of detecting C. perfringens and the genes that encode its principal toxins, but further optimization and confirmatory studies are needed. More importantly, to improve the clinical value of the assay, a similar PCR assay that includes other gas gangrene-causing Clostridium species (e.g., C. welchii, C. septicum, C. novyi, C. histolyticum) as well can now be developed.
Footnotes
Published ahead of print on 24 December 2008.
REFERENCES
- 1.Awad, M. M., A. E. Bryant, D. L. Stevens, and J. I. Rood. 1995. Virulence studies on chromosomal alpha-toxin and theta-toxin mutants constructed by allelic exchange provide genetic evidence for the essential role of alpha-toxin in Clostridium perfringens-mediated gas gangrene. Mol. Microbiol. 15191-202. [DOI] [PubMed] [Google Scholar]
- 2.Bentancor, A. B., M. R. Fermepin, L. D. Bentancor, and R. A. de Torres. 1999. Detection of the etx gene (epsilon-toxin inducer) in plasmids of high molecular weight in Clostridium perfringens type D. FEMS Immunol. Med. Microbiol. 24373-377. [DOI] [PubMed] [Google Scholar]
- 3.Gkiourtzidis, K., J. Frey, E. Bourtzi-Hatzopoulou, N. Iliadis, and K. Sarris. 2001. PCR detection and prevalence of alpha-, beta-, beta 2-, epsilon-, iota- and enterotoxin genes in Clostridium perfringens isolated from lambs with clostridial dysentery. Vet. Microbiol. 8239-43. [DOI] [PubMed] [Google Scholar]
- 4.Hatheway, C. L. 1990. Toxigenic clostridia. Clin. Microbiol. Rev. 366-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Havard, H. L., S. E. Hunter, and R. W. Titball. 1992. Comparison of the nucleotide sequence and development of a PCR test for the epsilon toxin gene of Clostridium perfringens type B and type D. FEMS Microbiol. Lett. 7677-81. [DOI] [PubMed] [Google Scholar]
- 6.Kadra, B., J. P. Guillou, M. Popoff, and P. Bourlioux. 1999. Typing of sheep clinical isolates and identification of enterotoxigenic Clostridium perfringens strains by classical methods and by polymerase chain reaction (PCR). FEMS Immunol. Med. Microbiol. 24259-266. [DOI] [PubMed] [Google Scholar]
- 7.Konomi, N., E. Lebwohl, and D. Zhang. 2002. Comparison of DNA and RNA extraction methods for mummified tissues. Mol. Cell. Probes 16445-451. [DOI] [PubMed] [Google Scholar]
- 8.Meer, R. R., and J. G. Songer. 1997. Multiplex polymerase chain reaction assay for genotyping Clostridium perfringens. Am. J. Vet. Res. 58702-705. [PubMed] [Google Scholar]
- 9.Moller, K., and P. Ahrens. 1996. Comparison of toxicity neutralization-, ELISA- and PCR tests for typing of Clostridium perfringens and detection of the enterotoxin gene by PCR. Anaerobe 2103-110. [Google Scholar]
- 10.Park, Y. N., K. Abe, H. Li, T. Hsuih, S. N. Thung, and D. Y. Zhang. 1996. Detection of hepatitis C virus RNA using ligation-dependent polymerase chain reaction in formalin-fixed, paraffin-embedded liver tissues. Am. J. Pathol. 1491485-1491. [PMC free article] [PubMed] [Google Scholar]
- 11.Petit, L., M. Gibert, and M. R. Popoff. 1999. Clostridium perfringens: toxinotype and genotype. Trends Microbiol. 7104-110. [DOI] [PubMed] [Google Scholar]
- 12.Petrillo, T. M., C. M. Beck-Sague, J. G. Songer, C. Abramowsky, J. D. Fortenberry, L. Meacham, A. G. Dean, H. Lee, D. M. Bueschel, and S. R. Nesheim. 2000. Enteritis necroticans (pigbel) in a diabetic child. N. Engl. J. Med. 3421250-1253. [DOI] [PubMed] [Google Scholar]
- 13.Songer, J. G., and R. R. Meer. 1996. Genotyping of Clostridium perfringens by polymerase chain reaction is a useful adjunct to diagnosis of clostridial enteric disease in animals. Anaerobe 2197-203. [Google Scholar]
- 14.Tsutsui, K., J. Minami, O. Matsushita, S. Katayama, Y. Taniguchi, S. Nakamura, M. Nishioka, and A. Okabe. 1995. Phylogenetic analysis of phospholipase C genes from Clostridium perfringens types A to E and Clostridium novyi. J. Bacteriol. 1777164-7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang, R. F., W. W. Cao, W. Franklin, W. Campbell, and C. E. Cerniglia. 1994. A 16S rDNA-based PCR method for rapid and specific detection of Clostridium perfringens in food. Mol. Cell. Probes 8131-137. [DOI] [PubMed] [Google Scholar]
- 16.Yamagishi, T., K. Sugitani, K. Tanishima, and S. Nakamura. 1997. Polymerase chain reaction test for differentiation of five toxin types of Clostridium perfringens. Microbiol. Immunol. 41295-299. [DOI] [PubMed] [Google Scholar]
- 17.Yoo, H. S., S. U. Lee, K. Y. Park, and Y. H. Park. 1997. Molecular typing and epidemiological survey of prevalence of Clostridium perfringens types by multiplex PCR. J. Clin. Microbiol. 35228-232. [DOI] [PMC free article] [PubMed] [Google Scholar]