Abstract
Toxocariasis is one of the causes of pulmonary eosinophilic infiltrate that is increasing in Korea. This study was designed to identify the prevalence of toxocara seropositivity in patients with unexplained pulmonary patchy infiltrate and to evaluate associated factors. We evaluated 102 patients with unexplained pulmonary patchy infiltrate on chest computed tomography (CT) scan. As a control set, 116 subjects with normal chest CT were also evaluated. History of allergic disease, drug use, parasitic disease and raw cow liver intake were taken. Blood eosinophil count and total IgE level were measured. Specific serum IgG antibody to Toxocara canis larval antigen and specific IgG antibodies to 4 other parasites were measured by enzyme-linked immunosorbent assay (ELISA). In the infiltrate group, 66.7% subjects were toxocara seropositive whereas 22.4% of the control group were seropositive (p<0.001). In the infiltrate group, patients with a history of eating raw cow liver (odds ratio [OR], 7.8) and patients with eosinophilia (OR, 5.2) had a higher incidence of toxocara seropositivity. Thirty-five percent of toxocara seropositive patients with infiltrate exhibited migrating infiltrate and 48% had decreased infiltrate on the follow-up CT. We recommend that toxocara ELISA should be performed in patients with unexplained pulmonary patchy infiltrate, and that the eating of raw cow liver should be actively discouraged.
Keywords: Toxocariasis, Eosinophilia, Pulmonary Infiltrate, Raw Cow Liver
INTRODUCTION
Recent advances in chest computed tomograhy (CT) scan screening have led to an increase in the detection of unexplained multiple pulmonary patchy infiltrate (1, 2). Various kinds of diseases, such as infections, allergic diseases and malignancies, should be considered to be causes of unexplained pulmonary infiltrate. Parasitic infection is one of these causes, especially in combination with eosinophilia. A recent report on toxocariasis in Korea presents that 32 of 141 toxocariasis patients had pulmonary involvement (3). Toxocariasis is a zoonosis caused by ascarids from dogs or cats, Toxocara canis or Toxocara catis, in which animals they live as adults within the lumen of the small intestine. Human infection is aberrant and usually asymptomatic, but can cause visceral larva migrans to spread to specific target organs such as the liver, lung, eye, and central nerve systems. Visceral larva migrans may result in patients who present with fever, hepatosplenomegaly, lower respiratory symptoms such as bronchospasm, eosinophilia sometimes approaching 70%, and hypergammaglobulinemia of immunoglobulin M, G, and E.
Definitive diagnosis of visceral larva migrans in the lung can be made by tissue biopsy, but this is difficult to perform in the clinical setting. Therefore, the diagnosis of toxocariasis is primarily reached immunologically. The enzyme-linked immunosorbent assay (ELISA) using antigens secreted by second stage larva is the best indirect test for diagnosing this infection (4). The seroprevalence of toxocara is high in tropical areas and developing countries, but relatively low in developed countries. The seroprevalence among adult populations is 2.4% in the U.S.A. and 4.0% in Switzerland, but 20.5% in Brazil and 30.4% in Nigeria (5). The toxocara seroprevalence is closely related to the practice of eating raw liver (6). The seroprevalence among Korean rural adults is reported to be 5% (female: 6.1%, male: 4.0%) (7).
Toxocara larva migrans in lung is usually asymptomatic or elicits only nonspecific mild symptoms, although it can also cause pulmonary infiltrate due to a hypersensitive reaction to larva. However, the prevalence and clinical importance of toxocariasis among patients with unexplained pulmonary patchy infiltrate is unknown, and would be expected to vary along with the eating habits of the population.
Recently, many patients with unexplained pulmonary infiltrate have been referred to the Respiratory Center of Seoul National University Bundang Hospital from among the total patients screened by low dose chest CT scan at the Health Care Promotion Center of Seoul National University Bundang Hospital. Many of these patients displayed transient or migratory infiltrate on follow-up chest CT scan that were suggested to be of a transient immunologic or inflammatory nature. Therefore, we designed this study to identify the prevalence of toxocariasis in unexplained multiple pulmonary patchy infiltrate and to evaluate the clinical and laboratory factors associated with toxocariasis.
MATERIALS AND METHODS
Study subjects
This is a frequency-matched case-control study. From January 2006 to February 2007, we prospectively enrolled patients with unexplained pulmonary patchy infiltrate on chest CT scan from the Health Promotion Center or the Respiratory Center of Seoul National University Bundang Hospital. Unexplained pulmonary patchy infiltrate was defined as single or multiple lung focal infiltrate without evidences of infection, malignancy or any other known disease. The enrolled patients were questioned as to whether they had any specific respiratory symptoms, allergic symptoms or signs, medication within the previous 6 months, and history of ingesting raw cow liver, meat, or fresh water fish within the previous year. Address of the enrolled subjects was also recorded to provide information.
Complete blood cell counts with eosinophil count, stool parasite exam, and other parasite ELISA tests for paragonimiasis, clonorchiasis, cysticercosis, and sparganosis, were evaluated. If a certain disease was suspected, we performed specific examinations such as transthoracic biopsy, bronchoscopic biopsy, bronchoalveolar lavage, and so on to rule out other known diseases. Patients were reevaluated with high resolution CT (HRCT) scan after 3 months to observe any changes.
As a control group, we prospectively enrolled patients with normal chest CT scan on routine check at Health Promotion Center of Seoul National University Bundang Hospital. We questioned them as to their history of allergic diseases, medications, parasite infection, and ingestion of any raw fish or raw cow liver within the previous year. Complete blood cell counts with eosinophil count and stool parasite exam were evaluated. The sera of the enrolled patients in both groups were collected and stored at -20℃ until use.
This protocol was reviewed and approved by the Institutional Review Board of Seoul National University Bundang Hospital with informed consent.
Diagnostic methods
Serologic diagnosis of toxocara infection was performed with a specific IgG antibody to T. canis with a toxocara ELISA kit (Bordier Affinity Products, Crissier, Switzerland). T. canis excreatory/secretory larval antigens were coated on the microtiter plates with antihuman IgG-alkaline phosphate conjugate. Measurement was performed by the protocol and seropositivity of toxocara was determined by positive criteria of the kit.
Specific IgG antibodies to parasites (paragonimiasis, cysticercosis, sparganum, and clonorchis) were analyzed at the Department of Parasitology of Seoul National University College of Medicine. Stool parasite exam was performed.
Statistical analysis
We compared the age, sex, intake of raw cow liver, eosinophil count, and toxocara seropositivity among the patients with unexplained pulmonary infiltrate and those with normal chest CT scan by using the chi-square test or Fisher's exact test for categorical variables, and the Student t test or Mann-Whitney test for continuous variables. Multivariable analysis was also performed by using a logistic regression model including toxocara seropositivity and other significant variables in univariable analysis. Among the patients with the pulmonary infiltrate, we compared the age, sex, intake of raw cow liver, eosinophil count, and total IgE levels according to toxocara seropositivity. We also performed univariable and multivariable analysis to evaluate the risk factors for toxocara seropositivity. A p value of less than 0.05 was regarded as statistically significant. The data were analyzed by SPSS version 12.0 K (SPSS inc., Chicago, IL, U.S.A.).
RESULTS
Demographic and baseline clinical characteristics
A total of 102 patients with unexplained pulmonary patchy infiltrate and 116 subjects without any infiltrate on chest CT scan as control were included. Demographic and baseline clinical characteristics of the patients are shown in Table 1. In the infiltrate group, 15 patients were excluded. Two patients were diagnosed as paragonimiasis with positive ELISA, eight patients were diagnosed as either atypical adenomatous hyperplasia or bronchoalveolar carcinoma by transthoracic biopsy, one patient was diagnosed as pulmonary tuberculosis by positive AFB smear and culture, and four patients were diagnosed as interstitial lung diseases by either transthoracic biopsy or bronchoscopic biopsy.
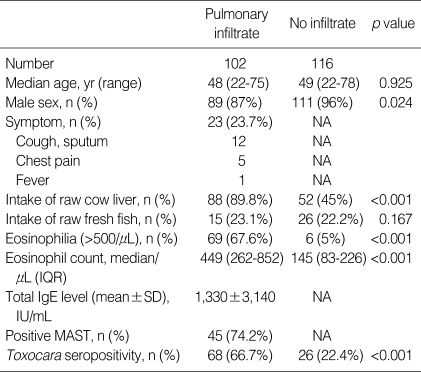
Table 1.
Demographic and baseline clinical characteristics
IQR, interquartile range; NA, not available.
In the infiltrate group, patients with a history of intake of raw liver comprised 89.8% and median eosinophil count was 449/µL (interquartile range [IQR], 262-852). Five patients were positive on ELISA to cysticercosis and six patients were positive on ELISA to clonorchiasis. No patients had positive stool parasite. Patients having any sign of respiratory symptoms comprised 23.7% in the infiltrate group. Sixty eight patients had ground glass opacity (GGO), 14 nodules, 3 consolidations, and 17 mixed infiltrate on chest CT scan out of the 102 subjects with infiltrate. In the control group, a history of raw liver intake was relatively high (45%).
Seroprevalence of toxocara
Sixty eight of 102 patients (66.7%) in the pulmonary infiltrate group were toxocara seropositive, whereas 22.4% of the control group were seropositive (p<0.001). Toxocara seropositivity in the pulmonary infiltrate group was significantly higher compared to the control group after adjustment for eosinophilia (eosinophil count ≥500/µL) (p<0.001) (Table 1).
Clinical characteristics and risk factor of toxocara seropositivity in the infiltrate group
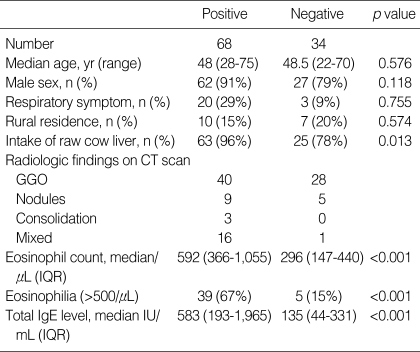
Clinical characteristics of the 68 toxocara positive patients with pulmonary infiltrate and the 34 negative patients among all of the patients with infiltrate are shown in Table 2. Peripheral eosinophil counts (median: 592, IQR 366-1,055 vs. 296, IQR 147-440, p<0.001) and total IgE levels (median, 583, IQR 193-1,965 vs. 135, IQR 44-331, p<0.001) were higher in the toxocara seropositive patients than in seronegative patients. Patients who had ingested raw cow liver within 1 yr comprised a higher percentage of toxocara seropositive patients (96% vs. 78%, p=0.013). The average duration between intake of raw cow liver and sampling of serum was 6 months. No significant difference was observed according to the intake of raw fresh fish (data not shown).
Table 2.
Differences according to toxocara seropositivity in the pulmonary infiltrate group (N=102)
GGO, ground glass opacity; IQR, interquartile range.
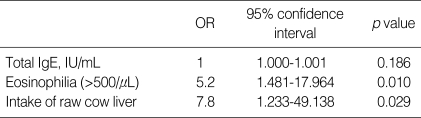
Multiple logistic regression analysis revealed that the history of eating raw cow liver (odds ratio [OR], 7.8, 95% confidence interval [CI], 1.23-49.14, p=0.029) and eosinophilia (OR, 5.2, 95% CI, 1.48-17.97, p=0.010) were independent risk factors for toxocara seropositivity after adjustment for the total IgE level, as shown in Table 3.
Table 3.
Multivariable analysis of risk factors for toxocara seropositivity
OR, odds ratio.
Clinical course and treatment
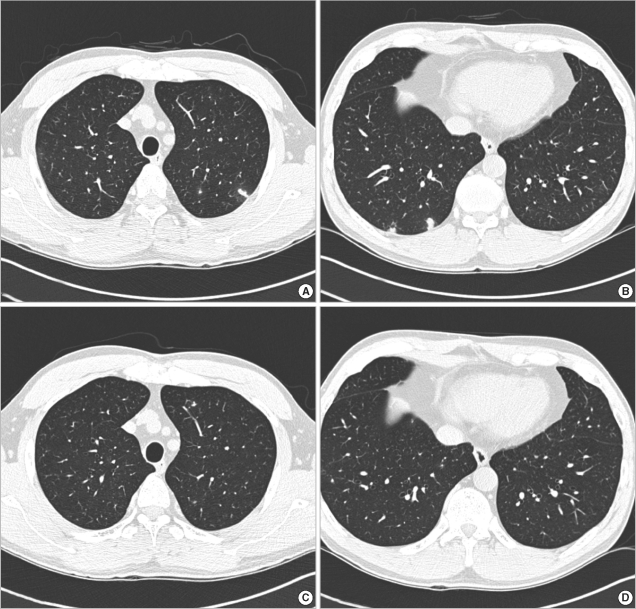
Follow-up CT scan was performed in 83 patients after 3 months. Pulmonary infiltrate were shown to have decreased or disappeared in 48% (28 patients) migrated in 35% (20 patients), increased in 5% and remained unchanged in 12% of 58 toxocara positive patients. Among 25 toxocara seronegative patients, pulmonary infiltrate was shown to have decreased or disappeared in 60%, migrated in 8%, increased in 0% and remained unchanged in 32%. Among the 68 toxocara, seropositive 7 patients were treated. All patients had severe respiratory symptoms such as cough and dyspnea and more than 10 percent of eosinophilia. Three patients were treated with albendazole only and the other four were treated with albendazole along with a steroid. All of them had improved clinically and/or radiologically on follow-up chest CT scan. Fig. 1 shows a typical example of radiologic improvement in one of the patients treated with albendazole. Untreated patients were followed up with HRCT scan.
Fig. 1.
High-resolution CT scan findings before treatment and after treatment. The figures on the top (A and B) show multiple ground glass opacities with peripheral halo in both lower lobes before treatment. The figures on the bottom (C and D) show the disappearance of ground glass opacities 3 months after treatment with albendazole. The patient was a 53-yr-old man and ingested raw cow liver frequently.
Seroprevalence of toxocara in patients with eosinophilia
Among the 69 patients with eosinophilia, 52 patients were toxocara seropositive and 17 patients were negative (p=0.013).
DISCUSSION
Toxocarial infection in humans can occur by the ingestion of viable, embryonated eggs from contaminated soil or hands (8, 9). Therefore, the seroprevalence of toxocara is high in younger children and low in adults. The risk factors of toxocariasis in children are local animal population, high prevalence of toxocara species in dogs and cats, soil contamination by infective toxocara eggs, low socioeconomic condition, and high frequency of pet ownership (10). However, a major risk factor for toxocariasis in adults is the ingestion of viable eggs from the uncooked internal organs or meat of parasitic hosts, especially raw cow liver (5, 6, 11-13). Visceral larva migrans to the liver, lung, eye and central nerve systems are largely dependent upon the death of larvae. Their death occurs due to both delayed and immediate hypersensitivity responses and results in an eosinophilic granulomatous reaction and pulmonary infiltrate in the lung (4).
In present study, a positive result on ELISA for toxocara was higher among patients with pulmonary infiltrate, compared with people with normal findings on chest CT scan, even after the adjustment for eosinophilia and intake of raw cow liver.
We showed GGO, GGO combined with consolidation, or GGO combined with nodules to be a common finding in the multiple pulmonary patchy infiltrate. Kim et al. have reported the most common finding on chest CT scan in patients with toxocariasis to be randomly distributed GGO (3). Also in toxocara seropositive patients, the peripheral eosinophil count is reported to be higher than in seronegative patients. This finding is correlated with the finding that toxocariasis is one of the causes of pulmonary infiltrate with eosinophilia (14).
In Korea, there are dietary habits that include eating of raw meat, including raw animal liver, especially cow liver, for the promotion of health, among adults. According to the responses to our questionnaire, 88 of the 102 patients with unexplained pulmonary infiltrate and 52 of 116 in the control group had a history of intake of raw cow liver that explained the relatively high seropositivity. This study shows that intake of raw cow liver and eosinophilia are major predictors for toxocara seropositivity among patients with unexplained pulmonary patchy infiltrate. These results indicate that physicians should consider testing for toxocariasis in patients with unexplained pulmonary infiltrate and a history of intake of raw cow liver, especially with peripheral blood eosinophilia.
Most toxocariasis is self-limiting, but the treatment of choice is albendazole (10 mg/kg of body weight/day for 10-14 days) and/or corticosteroids. In the present study, the pulmonary infiltrate of patients (over 80%) with toxocara seropositivity improved with or without treatment. This strongly suggests that ELISA test for toxocariasis and follow-up chest CT scan at 3 month intervals might reduce unnecessary diagnostic workups for unexplained pulmonary patchy infiltrate.
There are limitations of this study which should be acknowledged. First, it was difficult to identify the exact causal relationship between seropositivity of toxocara and pulmonary toxocariasis. This problem is inevitable because the diagnosis of toxocariasis is dependent on an immunologic assay such as ELISA. A definitive laboratory diagnosis of human toxocarial infection can be achieved by pathology examination of involved organ specimens, including the liver, brain, lung or enucleated eye. However, such a direct parasitologic assessment is difficult because larva is mobile as well as invasive. Therefore, serologic methods are the mainstay for the diagnosis. Although previous studies have been rare (15), ELISA and eosinophil counts in bronchoscopic lavage fluid can be helpful. Second, toxocara IgG detected with ELISA method can persist several months after infection (16, 17). Therefore, in this study history of raw cow liver intake was restricted within previous one year. Third, the seroprevalence (22.4%) in the control group is higher than the reported seroprevalence (5%) in rural districts of Korea in a previous study (7). This difference could be explained by differences in the ELISA kits and also the different populations. Both studies used toxocara excretory-secretory antigen (TES). A previous study (7) defined the seroprevalence of toxocariasis as a positive reaction to a self-made ELISA kit against TES and a positive reaction on immunoblot to toxocara in case of positive patients with ELISA. However, the present study used the toxocara ELISA kit from Bordier Affinity products (Crissier), which is well standardized and has a reasonably high sensitivity (86%) and specificity (91%) (18). In another study on the prevalence of toxocariasis among patients with unknown eosinophilia in Korea using the same ELISA kit as used in this study, 68% of patients with unknown eosinophilia were diagnosed as having toxocariasis (11). Although there was an epidemiologic study using this kit in developed countries (18), further epidemiologic study using this kit is required in Korean population. Furthermore, the control group of this study specifically included the adult patients receiving low-dose chest CT scan for lung cancer screening in Seoul National University Bundang Hospital with a history of raw cow liver ingestion (45%). The male predominance of those who received low dose chest CT scan for lung cancer screening would be another likely factor, as men reportedly more frequently ingest raw cow liver than women do. Seropositive subjects with eosinophilia in control group may have toxocariasis without lung involvement.
In conclusion, the prevalence of toxocara seropositivity was high in patients with unexplained pulmonary multiple patchy infiltrate, and a strong relationship was found between toxocara seropositivity and raw cow liver intake. We recommend that toxocara ELISA should be performed in patients with unexplained multiple pulmonary patchy infiltrate with peripheral blood eosinophilia and the eating of raw cow liver should be actively discouraged.
References
- 1.Kaneko M, Eguchi K, Ohmatsu H, Kakinuma R, Naruke T, Suemasu K, Moriyama N. Peripheral lung cancer: screening and detection with low-dose spiral CT scan versus radiography. Radiology. 1996;201:798–802. doi: 10.1148/radiology.201.3.8939234. [DOI] [PubMed] [Google Scholar]
- 2.International Early Lung Cancer Action Program Investigators. Henschke CI, Yankelevitz DF, Libby DM, Pasmantier MW, Smith JP, Miettinen OS. Survival of patients with stage I lung cancer detected on CT scan screening. N Engl J Med. 2006;355:1763–1771. doi: 10.1056/NEJMoa060476. [DOI] [PubMed] [Google Scholar]
- 3.Kim YJ, Kyung SY, An CH, Lim YH, Park JW, Jeong SH, Lee SP, Choi DC, Jeong YB, Kang SY. The characteristics of eosinophilic lung diseases cause by toxocara canis larval infestation. Tuberc Respir Dis. 2007;62:19–26. [Google Scholar]
- 4.Despommier D. Toxocariasis: clinical aspects, epidemiology, medical ecology, and molecular aspects. Clin Microbiol Rev. 2003;16:265–272. doi: 10.1128/CMR.16.2.265-272.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan CK, Lan HS, Hung CC, Chung WC, Liao CW, Du WY, Su KE. Seroepidemiology of Toxocara canis infection among mountain aboriginal adults in Taiwan. Am J Trop Med Hyg. 2004;71:216–221. [PubMed] [Google Scholar]
- 6.Lee KT, Min HK, Chung PR, Chang JK. Studies on the inducing possibility of human visceral larva migrans associated with eating habit of raw liver of domestic animals. Kisaengchunghak Chapchi. 1976;14:51–60. doi: 10.3347/kjp.1976.14.1.51. [DOI] [PubMed] [Google Scholar]
- 7.Park HY, Lee SU, Huh S, Kong Y, Magnaval JF. A seroepidemiological survey for toxocariasis in apparently healthy residents in Gangwon-do, Korea. Korean J Parasitol. 2002;40:113–117. doi: 10.3347/kjp.2002.40.3.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glickman LT, Schantz PM. Epidemiology and pathogenesis of zoonotic toxocariasis. Epidemiol Rev. 1981;3:230–250. doi: 10.1093/oxfordjournals.epirev.a036235. [DOI] [PubMed] [Google Scholar]
- 9.Morris PD, Katerndahl DA. Human toxocariasis. Review with report of a probable case. Postgrad Med. 1987;81:263–267. doi: 10.1080/00325481.1987.11699681. [DOI] [PubMed] [Google Scholar]
- 10.Schantz PM. Toxocara larva migrans now. Am J Trop Med Hyg. 1989;41(3 Suppl):21–34. doi: 10.4269/ajtmh.1989.41.21. [DOI] [PubMed] [Google Scholar]
- 11.Kwon NH, Oh MJ, Lee SP, Lee BJ, Choi DC. The prevalence and diagnostic value of toxocariasis in unknown eosinophilia. Ann Hematol. 2006;85:233–238. doi: 10.1007/s00277-005-0069-x. [DOI] [PubMed] [Google Scholar]
- 12.Magnaval JF, Glickman LT, Dorchies P, Morassin B. Highlights of human toxocariasis. Korean J Parasitol. 2001;39:1–11. doi: 10.3347/kjp.2001.39.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taira K, Saeed I, Permin A, Kapel CM. Zoonotic risk of Toxocara canis infection through consumption of pig or poultry viscera. Vet Parasitol. 2004;121:115–124. doi: 10.1016/j.vetpar.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 14.Inoue K, Inoue Y, Arai T, Nawa Y, Kashiwa Y, Yamamoto S, Sakatani M. Chronic eosinophilic pneumonia due to visceral larva migrans. Intern Med. 2002;41:478–482. doi: 10.2169/internalmedicine.41.478. [DOI] [PubMed] [Google Scholar]
- 15.Morimatsu Y, Akao N, Akiyoshi H, Kawazu T, Okabe Y, Aizawa H. A familial case of visceral larva migrans after ingestion of raw chicken livers: appearance of specific antibody in bronchoalveolar lavage fluid of the patients. Am J Trop Med Hyg. 2006;75:303–306. [PubMed] [Google Scholar]
- 16.Elefant GR, Shimizu SH, Sanchez MC, Jacob CM, Ferreira AW. A serological follow-up of toxocariasis patients after chemotherapy based on the detection of IgG, IgA, and IgE antibodies by enzyme-linked immunosorbent assay. J Clin Lab Anal. 2006;20:164–172. doi: 10.1002/jcla.20126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malafiej E, Spiewak E. The significance of the level of antibodies in the evaluation of the effects of treatment of toxocariasis. Wiad Parazytol. 2001;47:805–810. [PubMed] [Google Scholar]
- 18.Jacquier P, Gottstein B, Stingelin Y, Eckert J. Immunodiagnosis of toxocarosis in humans: evaluation of a new enzyme-linked immunosorbent assay kit. J Clin Microbiol. 1991;29:1831–1835. doi: 10.1128/jcm.29.9.1831-1835.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]