Abstract
Approximately 5-30% of the ovarian cancers are metastatic malignancies. The prevalence of metastatic ovarian tumors varies with the incidence rates and spread patterns of primary malignancies. We evaluated the prevalence, pre- and postoperative characteristics of metastatic ovarian cancer in Korean women. We reviewed the records for 821 ovarian malignancies with pathological consultation from 1996-2006 and recorded patient demographical, radiological, histopathological, and survival data. The study included 112 cases of histologically confirmed metastatic ovarian cancer. Metastatic ovarian cancer accounted for 13.6% of all ovarian malignancy, primarily arising from the gastrointestinal tract. The preoperative detection rate with imaging was 75%, and none of the radiological or serological features were useful for differential diagnosis. In multivariate analysis for prognostic variables, the only significant factor was the primary tumor site (p=0.004). Furthermore, extensive resection increased survival for some patients. The differential diagnosis of metastatic ovarian cancer can be problematic, so multiple diagnostic approaches are necessary. The extent of cytoreductive surgery for this type of tumor must be decided on a case-by-case basis.
Keywords: Metastasis, Ovary, Prevalence, Diagnostic Imaging, Surgical Procedures
INTRODUCTION
The ovary is a common site of metastases from other primary malignancies, and 5-30% of ovarian cancers are metastatic malignancies. The most common primary origins are the breast, colon, and stomach (1). The incidence rate of metastatic ovarian cancer appears to be somewhat higher in Asia compared to Western countries (2-7). The prevalence of metastatic ovarian tumors appears to be associated with the incidence rates and spread patterns of primary malignancies.
When gynecologists are confronted with tumors metastasized to the ovaries, the pre- and intraoperative distinction of the tumors from primary ovarian malignancies is often difficult. A history of malignancy can provide a useful clue for diagnosing metastatic ovarian tumor, but symptoms due to the tumor can mask the primary neoplasm. Occasionally, metastatic ovarian tumors mimic a primary ovarian neoplasm morphologically and clinically. Furthermore, the radiological features of metastatic ovarian cancer show considerable variability (8-11).
Because metastatic ovarian cancer is not a target of typical chemo- and radiotherapy, there are few clinical reports on tumor management or prognostic factors. Cytoreductive surgery in metastatic ovarian cancer may be beneficial for the initial diagnosis or symptom relief, but the survival benefit of surgery remains controversial (3, 5, 12-16). In the present study, we evaluated the prevalence of metastatic ovarian cancer and preoperative characteristics, including diagnostic imaging findings, origin of primary malignancy, age of patient, mass size, bilaterality, and serum markers. We also analyzed the clinical effects of cytoreductive surgery in treating metastatic ovarian cancer.
MATERIALS AND METHODS
We identified patients from the records of the Department of Pathology at the Catholic University of Korea, retrieved through searches of the pathology report database and the medical informatics cancer registry at the Catholic Medical Center. The records of 821 patients with ovarian malignancies between 1 January 1996 and 31 December 2006 were screened, according to International Classification of Disease (ICD)-10 codes. For inclusion in this study, ovarian metastasis confirmed by histological and immunologic stains (i.e., cytokeratins 7 or 20) and pathological or clinical confirmation of the primary tumor were required. We included data from patients with metastatic tumor from other genital tract (i.e., uterus, cervix and fallopian tube, only except ovary). Two cases of primary ovarian cancer and one case with uncertain information were excluded based on the advice of a participating pathologist (A.W.L.). A total of 112 patients with histologically confirmed metastatic ovarian cancer were selected.
We recorded patient information, including age, menopause status, origin of the primary malignancy, the sequence and interval of diagnosing the primary and secondary tumors, cell type, radiology findings, preoperative serum cancer antigen (CA)-125 concentration, ascites, mass size, bilaterality, cytoreductive surgery results, and survival. Not all clinical data were available for all cases. Therefore, we calculated percentages based on the number of cases for which appropriate data were available. Surgical treatment was categorized as (1) extensive (e.g., optimal mass removal by primary tumor resection plus total hysterectomy with unilateral or bilateral salpingo-oophorectomy and/or pelvic lymphadenectomy, omentectomy, or other excisional mass removal procedures) or (2) minimal (e.g., palliative debulking, including incomplete removal of primary tumor and salpingo-oophorectomy, wedge resection or biopsy of ovarian masses). Surgery was defined as optimal when the largest residual tumor mass was <2 cm.
Statistical analyses included ANOVA, Fisher's exact test, Wilcoxon rank sum test and Kruskal-Wallis test as appropriate. We computed survival curves using the Kaplan-Meier's method, and we analyzed the prognostic variables with the log-rank test (univariate) and the Cox proportional hazards model (multivariate). Results were considered statistically significant if p<0.05. The study was approved by the appropriate institutional review board at the Catholic Medical Center.
RESULTS
Metastatic ovarian malignancy accounted for 112 (13.6%) of the 821 ovarian malignancies. More than 70% of those were metastases from the gastrointestinal tract. Colorectal cancer was the most common primary tumor followed by stomach cancer. Patient characteristics are summarized in Table 1. The mean age of patients was 46.8±11.0 yr, and 39 (34.8%) of them were postmenopausal. Primary malignancies were detected first in 48 (42.9%) patients and simultaneously with ovarian metastases in 61 (54.5%) patients. One hundred eight (94%) of the primary malignancies were classified as adenocarcinomas. Adjuvant therapy (postoperative chemotherapy±radiotherapy) was done in most of patients (98.2%).
Table 1.
Patient characteristics in metastatic ovarian cancer
*, first-degree relatives with breast, gastrointestinal, or gynecologic malignancies; †, interval between diagnoses of primary and secondary malignancies; ‡, postoperative chemotherapy±radiotherapy.
SD, standard deviation.
The number of patients with metastatic ovarian cancers that did not originate in the gastrointestinal or gynecologic organs was relatively small, and further classification of data was required for effective statistical analyses. Therefore, we re-categorized the data into four subgroups, according to primary origin: 1) colorectal cancer, 2) stomach cancer, 3) gynecologic cancer, and 4) others sites, including gallbladder, pancreas, breast, lung, lymphoma, and unknown origin.
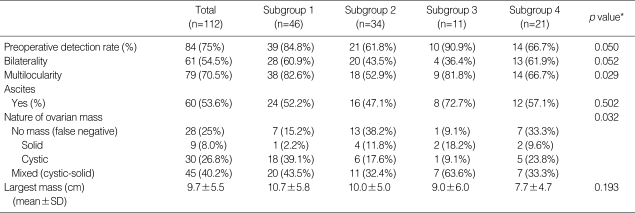
Preoperative findings classified by those subgroups are summarized in Table 2. Eighty-four (75%) cases were preoperatively diagnosed by ultrasonography, computed tomography, or magnetic resonance imaging. The mean size of the largest masses was 9.7±5.5 cm, and 54.5% of the cases had bilateral ovarian involvement. There were no statistically significant differences in the largest mass size or bilateral ovarian involvement among the subgroups. We considered preoperative mass detection by imaging successful if provided any suspicion or suggestion of the ovarian metastasis or malignant mass. Accordingly, the preoperative detection rate with imaging was 75%, and it appeared to be higher for ovarian metastases from colorectal and gynecologic origins (p=0.050). Multilocularity was a typical pattern in ovarian metastases from colorectal or gynecologic origins (p=0.028). A large number of metastatic ovarian cancers showed multilocular and mixed (cystic-solid) patterns with or without ascites, suggesting primary ovarian cancer.
Table 2.
Preoperative detection of metastatic ovarian cancer
*, p value from ANOVA and Fisher's exact test.
SD, standard deviation; Subgroup 1, from colorectal cancer; Subgroup 2, from stomach cancer; Subgroup 3, from gynecologic cancer; Subgroup 4, from gallbladder, appendix, breast, lung, lymphoma, and unknown origin.
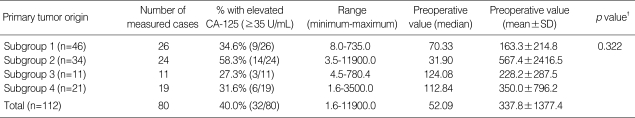
Preoperative CA-125 concentration was measured in 80 (71.4%) patients. Thirty-two (40.0%) of those had elevated serum CA-125 concentrations (≥35 U/mL), and the mean values of preoperative serum CA-125, according to the primary tumor site, were not significantly different among the subgroups (p=0.322) (Table 3).
Table 3.
Preoperative serum concentration of CA-125 in metastatic ovarian cancer*
SD, standard deviation; Subgroup 1, from colorectal cancer; Subgroup 2, from stomach cancer; Subgroup 3, from gynecologic cancer; Subgroup 4, from cancer of gallbladder, appendix, breast, lung, lymphoma, and unknown origin.
*, measured in 80 patients; †, p values from Kruskal-Wallis test.
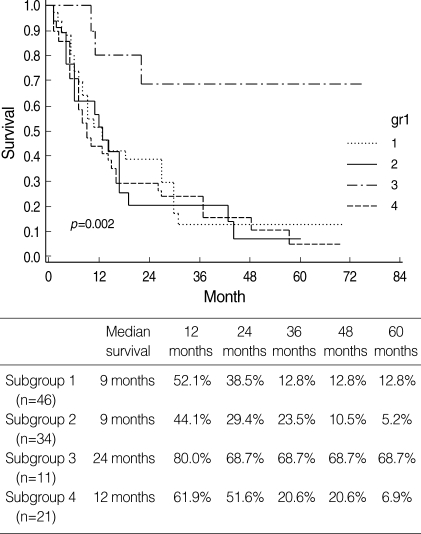
Patient survival rates are shown in Fig. 1, 2. The survival rate of subgroup 3 (gynecologic origin) was significantly better than that of other subgroups (p=0.002), and there were no significant differences in survival among subgroups 1, 2, and 4.
Fig. 1.
Survival rates of patients with metastatic ovarian cancer according to primary tumor origin.
Subgroup 1, from colorectal cancer; Subgroup 2, from stomach cancer; Subgroup 3, from gynecologic cancer; Subgroup 4, from cancers of gallbladder, appendix, breast, lung, lymphoma, and unknown origin.
Fig. 2.
Survival rates for patients according to extent of cytoreductive surgery.
Although we initially attempted curative and optimal cytoreduction for all patients, optimal resection was achieved by extensive surgery in 62 (55.4%). The median patient survival was 17 months after extensive cytoreduction and 9 months after minimal surgery. As shown in Fig. 2, extensive surgery improved the survival outcome of metastatic ovarian cancer (p=0.041).
We analyzed prognostic variables and estimated hazard ratios for patient death with a log-rank test and Cox proportional hazards model (Table 4). Metastasis from non-gynecologic malignancies and minimal resection were unfavorable prognostic factors in a univariate analysis. Ovarian metastasis from gynecologic origin was a significant favorable prognostic factor in multivariate analysis (p=0.004).
Table 4.
Hazard ratio for death
Statistically significant findings in bold.
HR, hazard ratio; CI, confidence interval.
DISCUSSION
In the present study, metastatic ovarian cancer accounted for 13.6% of all ovarian malignancies. The overall incidence of metastatic ovarian cancer in the Korean population is higher than that in Europe and North America. Colorectal and stomach cancer were major primary origins, and the proportion of breast cancer origin was much lower in the present study compared to data from Western countries (3-7). The global incidence of stomach cancer is concentrated in Eastern Asia and some South American, Southeastern European, and African countries. Regionally, stomach cancer is the most common cancer in Eastern Asia and the third most common cancer in South America and Southeastern Europe. Breast cancer is the second most common and colorectal cancer is the third most common cancer worldwide after lung and breast. The incidence rates of breast and colorectal cancer are highest in developed Western countries and lowest in Africa and Asia. However, Japan and Korea are exceptions, having age-standardized rates similar to Europe (20). According to the 2005 Korean Cancer Registry, stomach, breast, and colorectal cancer, in that order, were the most common cancers in Korean women. The prevalence of metastatic ovarian cancer seems to reflect the overall incidence and ethnic variances of primary cancer. In the present study, the mean age of patients with metastatic ovarian cancer was approximately 10 yr lower than that of patients with primary ovarian cancer (17).
Many metastatic adenocarcinomas involving the ovary show morphologically and clinically similar patterns. Therefore, multidisciplinary approaches, including radiological, serological, and pathological methods are required for diagnosis. A known history of a primary malignancy is useful for diagnosis, but many cases present symptoms related to an ovarian mass without a history of malignancy. In this study, more than half of the metastatic ovarian cancers were diagnosed simultaneously with primary malignancies, and the proportion of bilateral ovarian involvement was 54.5%. There were no significant differences in bilaterality and the largest mass size of metastatic ovarian malignancies based on the primary tumor site.
Preoperative detection of metastatic ovarian tumor was made in 75% of the present cases. As described in previous radiology studies, the nature of an ovarian mass and lesion size may be useful for diagnosis (8, 10). In the present study, multilocularity was a more frequent pattern in metastatic ovarian tumors from colorectal or gynecologic malignancies. However, differential diagnosis of ovarian masses based solely on radiology carries the risk of misinterpretation and may have adverse consequences for patients. As shown in Table 3, serum concentration of CA-125 was not useful for detection. In addition to morphological and clinical information, further evaluation, such as immunostains for cytokeratins 7 and 20 and other histopathological markers may be useful for differential diagnosis of metastatic ovarian cancers (2, 18, 19).
The multivariate analysis to evaluate prognostic factors for metastatic ovarian cancer showed that the primary tumor site was the most important factor. Ovarian metastases of gynecologic origin had a much better prognosis than those from non-gynecologic organs. Compared with metastases from non-gynecologic malignancy, carcinomatosis of advanced gynecologic cancer is relatively confined to the intraabdominal cavity without distant metastasis and shows superficial spreading patterns (21, 22). From these reasons, the metastatic ovarian cancer from gynecologic malignancy might show longer survival than those from non-gynecologic organs. Metastatic cancer of colorectal origin showed favorable prognosis among other non-gynecologic cancers, but there was no significant prognostic difference in non-gynecologic metastatic cancers based on the primary tumor site. Extensive resection may prolong survival. As described in previous studies, surgical resection should be considered as indicated by a patient's condition (5, 12-15).
The present study has some limits. First, our sample was not representative of the general population of Korean women. Second, information regarding diagnosis and treatment outcome was limited due to the study's retrospective design. However, it is difficult to execute a well-designed, prospective trial on this topic. Continued follow-up and large-scale data collection will provide accurate, long-term information about metastatic ovarian cancer and the benefits of a secondary tumor screening program. In addition to studies that evaluate outcomes of surgical resection, studies of chemotherapy outcomes are necessary.
In conclusion, the incidence of metastatic ovarian cancer appears to be higher in Korean women compared to the incidence in Western countries. Colorectal and stomach cancer were the major primary origins of metastatic ovarian tumors. The differential diagnosis of metastatic ovarian cancer is problematic, and multiple diagnostic approaches are required to enhance detection accuracy. Surgical intervention may be appropriate in some cases, and the extent and type of surgery should be decided on a case-to-case basis.
References
- 1.Young RH, Scully RE. Metastatic tumors of the ovary. In: Kurman RJ, editor. Blaustein's pathology of the female genital tract. 5th ed. New York: Springer; 2002. pp. 1063–1101. [Google Scholar]
- 2.McCluggage WG, Wilkinson N. Metastatic neoplasms involving the ovary: a review with an emphasis on morphological and immunohistochemical features. Histopathology. 2005;47:231–247. doi: 10.1111/j.1365-2559.2005.02194.x. [DOI] [PubMed] [Google Scholar]
- 3.Yada-Hashimoto N, Yamamoto T, Kamiura S, Seino H, Ohira H, Sawai K, Kimura T, Saji F. Metastatic ovarian tumors: a review of 64 cases. Gynecol Oncol. 2003;89:314–317. doi: 10.1016/s0090-8258(03)00075-1. [DOI] [PubMed] [Google Scholar]
- 4.Moore RG, Chung M, Granai CO, Gajewski W, Steinhoff MM. Incidence of metastasis to the ovaries from nongenital tract primary tumors. Gynecol Oncol. 2004;93:87–91. doi: 10.1016/j.ygyno.2003.12.039. [DOI] [PubMed] [Google Scholar]
- 5.Ayhan A, Guvenal T, Salman MC, Ozyuncu O, Sakinci M, Basaran M. The role of cytoreductive surgery in nongenital cancers metastatic to the ovaries. Gynecol Oncol. 2005;98:235–241. doi: 10.1016/j.ygyno.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 6.Khunamornpong S, Suprasert P, Chiangmai WN, Siriaunkgul S. Metastatic tumors to the ovaries: a study of 170 cases in northern Thailand. Int J Gynecol Cancer. 2006;16(Suppl 1):132–138. doi: 10.1111/j.1525-1438.2006.00302.x. [DOI] [PubMed] [Google Scholar]
- 7.Antila R, Jalkanen J, Heikinheimo O. Comparison of secondary and primary ovarian malignancies reveals differences in their pre- and perioperative characteristics. Gynecol Oncol. 2006;101:97–101. doi: 10.1016/j.ygyno.2005.09.046. [DOI] [PubMed] [Google Scholar]
- 8.Brown DL, Zou KH, Tempany CM, Frates MC, Silverman SG, McNeil BJ, Kurtz AB. Primary versus secondary ovarian malignancy: imaging findings of adnexal masses in the Radiology Diagnostic Oncology Group study. Radiology. 2001;219:213–218. doi: 10.1148/radiology.219.1.r01ap28213. [DOI] [PubMed] [Google Scholar]
- 9.Alcázar JL, Galán MJ, Ceamanos C, García-Manero M. Transvaginal gray scale and color Doppler sonography in primary ovarian cancer and metastatic ovarian tumors to the ovary. J Ultrasound Med. 2003;22:243–247. doi: 10.7863/jum.2003.22.3.243. [DOI] [PubMed] [Google Scholar]
- 10.Choi HJ, Lee JH, Seo SS, Lee S, Kim SK, Kim JY, Lee JS, Park SY, Kim YH. Computed tomography findings of ovarian metastases from colon cancer: comparison with primary malignant ovarian tumors. J Comput Assist Tomogr. 2005;29:69–73. doi: 10.1097/01.rct.0000149958.86165.ca. [DOI] [PubMed] [Google Scholar]
- 11.Koyama T, Mikami Y, Saga T, Tamai K, Togashi K. Secondary ovarian tumors: spectrum of CT and MR features with pathologic correlation. Abdom Imaging. 2007;32:784–795. doi: 10.1007/s00261-007-9186-4. [DOI] [PubMed] [Google Scholar]
- 12.Kim HK, Heo DS, Bang YJ, Kim NK. Prognostic factors of Krukenberg's tumor. Gynecol Oncol. 2001;82:105–109. doi: 10.1006/gyno.2001.6210. [DOI] [PubMed] [Google Scholar]
- 13.Kikkawa F, Ino K, Nomura S, Ishikawa H, Kuzuya K, Yamamuro O, Kobayashi I, Kawai M, Mizutani S. Prognostic factors of secondary ovarian carcinoma. Oncology. 2002;63:124–129. doi: 10.1159/000063805. [DOI] [PubMed] [Google Scholar]
- 14.Eitan R, Gemignani ML, Venkatraman ES, Barakat RR, Abu-Rustum NR. Breast cancer metastatic to abdomen and pelvis: role of surgical resection. Gynecol Oncol. 2003;90:397–401. doi: 10.1016/s0090-8258(03)00275-0. [DOI] [PubMed] [Google Scholar]
- 15.Cheong JH, Hyung WJ, Chen J, Kim J, Choi SH, Noh SH. Survival benefit of metastasectomy for Krukenberg tumors from gastric cancer. Gynecol Oncol. 2004;94:477–482. doi: 10.1016/j.ygyno.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 16.Tserkezoglou A, Kontou S, Hadjieleftheriou G, Apostolikas N, Vassilomanolakis M, Sikiotis K, Salamalekis E, Tseke P, Magiakos G. Primary and metastatic ovarian cancer in patients with prior breast carcinoma. Pre-operative markers and treatment results. Anticancer Res. 2006;26:2339–2344. [PubMed] [Google Scholar]
- 17.Stat bite: Age-specific incidence and mortality rates for ovarian cancer, 1998-2002. J Natl Cancer Inst. 2006;98:511. doi: 10.1093/jnci/djj179. [DOI] [PubMed] [Google Scholar]
- 18.Prat J. Ovarian carcinomas, including secondary tumors: diagnostically challenging areas. Mod Pathol. 2005;18:S99–S111. doi: 10.1038/modpathol.3800312. [DOI] [PubMed] [Google Scholar]
- 19.Dennis JL, Hvidsten TR, Wit EC, Komorowski J, Bell AK, Downie I, Mooney J, Verbeke C, Bellamy C, Keith WN, Oien KA. Markers of adenocarcinoma characteristic of the site of origin: development of a diagnostic algorithm. Clin Cancer Res. 2005;11:3766–3772. doi: 10.1158/1078-0432.CCR-04-2236. [DOI] [PubMed] [Google Scholar]
- 20.GLOBOCAN 2002 cancer incidence. Mortality and prevalence worldwide. IARC CancerBase; 2002. [Google Scholar]
- 21.Bae JH, Lee JM, Ryu KS, Lee YS, Park YG, Hur SY, Ahn WS, Namkoong SE. Treatment of ovarian cancer with paclitaxel- or carboplatin-based intraperitoneal hyperthermic chemotherapy during secondary surgery. Gynecol Oncol. 2007;106:193–200. doi: 10.1016/j.ygyno.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 22.Tong SY, Lee YS, Park JS, Bae SN, Lee JM, Namkoong SE. Clinical analysis of synchronous primary neoplasms of the female reproductive tract. Eur J Obstet Gynecol Reprod Biol. 2008;136:78–82. doi: 10.1016/j.ejogrb.2006.09.010. [DOI] [PubMed] [Google Scholar]