Abstract
Type 5 adenovirus (Ad5) is a human pathogen that has been widely developed for therapeutic uses, with only limited success to date. We report here the novel finding that human erythrocytes present Coxsackie virus-adenovirus receptor (CAR) providing an Ad5 sequestration mechanism that protects against systemic infection. Interestingly, erythrocytes from neither mice nor rhesus macaques present CAR. Excess Ad5 fiber protein or anti-CAR antibody inhibits the binding of Ad5 to human erythrocytes and cryo-electron microscopy shows attachment via the fiber protein of Ad5, leading to close juxtaposition with the erythrocyte membrane. Human, but not murine, erythrocytes also present complement receptor (CR1), which binds Ad5 in the presence of antibodies and complement. Transplantation of human erythrocytes into nonobese diabetic/severe combined immunodeficiency mice extends blood circulation of intravenous Ad5 but decreases its extravasation into human xenograft tumors. Ad5 also shows extended circulation in transgenic mice presenting CAR on their erythrocytes, although it clears rapidly in transgenic mice presenting erythrocyte CR1. Hepatic infection is inhibited in both transgenic models. Erythrocytes may therefore restrict Ad5 infection (natural and therapeutic) in humans, independent of antibody status, presenting a formidable challenge to Ad5 therapeutics. “Stealthing” of Ad5 using hydrophilic polymers may enable circumvention of these natural virus traps.
Introduction
Adenovirus is a respiratory and intestinal pathogen that causes diseases ranging from pharyngitis to childhood pneumonia, readily transmitted between persons by coughs and sneezes.1 In severely immune suppressed patients, adenovirus can cause fatal systemic viremia, although in most persons it mediates relatively mild clinical pathologies. The recent observation2–4 that type 5 adenovirus (Ad5) binds coagulation factors to enable entry into hepatocytes suggests the virus may have evolved toward systemic infection, although the advantage of hepatic infection for a virus that is normally transmitted by respiratory droplets is unclear. Here we report the unexpected presence of high affinity Ad5 receptors on human erythrocytes, which may function as decoys to protect against systemic virus infection, perhaps representing an evolutionary response to the challenge of widespread adenovirus pathology.
There are at least 51 serotypes of human adenovirus, and Ad5 has been particularly widely studied in biology and medicine. Recombinant Ad5 has been used in studies of cancer gene therapy and virotherapy, and 2 Ad5-based products have now been licensed in China for treatment of cancer by direct injection.5 Ad5 infects cells via at least 4 distinct cell surface receptor-binding pathways. These include binding of Ad5 fiber protein to Coxsackie virus-adenovirus receptor (CAR) and/or heparin sulfate proteoglycans (HSPG), binding of penton-base protein to integrins6 as well as the recently identified pathway mediated by binding of coagulation factors to hexon protein.3,4
Here we define the mechanism of Ad5 binding to human erythrocytes and document presentation of CAR and complement receptor 1 (CR1), which efficiently sequester Ad5 in the absence and presence of anti-Ad5 antibodies, respectively. We demonstrate that erythrocyte binding alters the blood circulation profile of intravenously administered Ad5 and dramatically reduces its extravasation and infectivity. These findings suggest that human, but not murine, erythrocytes may have a function in preventing systemic Ad5 infection, acting as circulating “virus traps.” They also raise important questions over the use of murine models to predict clinical behavior of systemic Ad5 and suggest that, in humans, intravenous Ad5 therapeutics probably don't infect target disease tissues.
Methods
Cells and viruses
A431, A549, SKOV-3, and HT29 carcinoma cells were obtained from ATCC (Manassas, VA). E1, E3-deleted Ad5-expressing cytomegalovirus immediate early (IE) promoter-driven luciferase was purchased from Hybrid Systems (Oxford, United Kingdom) and is denoted Ad5 throughout.
Phlebotomy and preparation of blood samples
Human blood was taken by venipuncture into vacutainers with the anticoagulants citrate-phosphate-dextrose, acid citrate dextrose, ethylenediaminetetraacetic acid, or heparin (Greiner Bio-One, Frickenhausen, Germany) or into tubes containing hirudin (18 μg/mL final concentration, ZO510; Nanos, Hamburg, Germany), or without anticoagulant and allowed to clot. After centrifugation (2000g, 5 minutes), plasma and cells were separated and the plasma placed on ice or subjected to heat treatment (56°C, 30 minutes) or cobra venom factor (CVF) treatment (25 units/mL plasma; Quidel, San Diego, CA). The leukocytes layer were removed and the erythrocytes washed (4 times with a 10× volume of phosphate-buffered saline [PBS]). Erythrocytes were resuspended in PBS or experimental plasma at physiologic concentrations (5 × 109 cells/mL) and 2 × 108 Ad5 particles per milliliter were added. Incubation was performed at 37°C for 30 minutes. Inhibition studies used polyclonal rabbit anti–human C1q IgG, Fab fragment (Medical Research Council Immunochemistry Unit, Oxford, United Kingdom) at 600 ng/mL, rabbit anti–human CR1 IgG antibody (R223), or isotype control rabbit IgG (Vector Laboratories, Burlingame, CA) at 250 μg/mL. Fiber and hexon were isolated from soluble antigen as described by Carlisle et al7 and used at 50 μg/mL and 90 μg/mL, respectively. For studies using whole blood, fractionation after exposure to Ad5 was achieved using CPT vacutainers (code 362760; BD Biosciences, San Jose, CA). This allowed the plasma, platelets, monocyte/lymphocyte, and erythrocyte/neutrophil fractions to be isolated from 4 mL of whole blood incubated with 8 × 108 copies of Ad5. The erythrocyte/neutrophil fraction was separated by performing erythrocyte lysis (2× wash in 5× excess of 10 mM NH4Cl, 150 mM NaHCO3, 0.1 mM ethylenediaminetetraacetic acid). Fractions were then analyzed for Ad5 genome content by quantitative polymerase chain reaction (PCR). All blood donors signed an Oxford University–approved consent form, and informed consent was obtained in accordance with the Declaration of Helsinki.
Preparation of erythrocyte ghosts
Ghosts were formed from human and murine erythrocytes and used for Western blotting or cryo-electron microscopy within 24 hours. Erythrocytes were washed in PBS (as described in “Phlebotomy and preparation of blood samples”), before washing in buffer A (5 × vol/vol; 5 mM Tris-HCl, pH 7.4, 140 mM NaCl, 1 mM ethylenediaminetetraacetic acid). After pelleting by centrifugation (2000g, 5 minutes), erythrocytes were hemolysed by the addition of a 2.85 times (vol/vol) excess of buffer B (5 mM Tris-HCl, pH 7.4, 7 mM NaCl, 1 mM ethylenediaminetetraacetic acid). After centrifugation (13 000g, 30 minutes, 4°C), supernatant was removed and 3 washes in 10× volume of buffer B performed. The ghost layer was then removed to fresh tubes, and 4 washes in a 10× volume of buffer C performed (5 mM Tris-Cl, pH 7.4, 7 mM NaCl). A ghost diameter of 100 to 200 nm was achieved by 2 × 10-minute exposures in a sonicating water bath followed by vigorous vortexing (1 minute) and verified using photon correlation spectroscopy (Malvern Instruments, Malvern, United Kingdom).
Flow cytometry
CAR was labeled for flow cytometry using the primary antibody RmcB8 and isotype control W6/32 (raised against human major histocompatibility complex I [MHCI]), with a pycoerythrin-linked, secondary antibody RO480 (Dako Denmark, Glostrup, Denmark). Erythrocytes (105) were washed and resuspended in 200 μL PBS at 4°C. A volume of 10 μL primary antibody or isotype control was added; and after 30 minutes at 4°C, erythrocytes were centrifuged (1000g, 5 minutes) and supernatant removed. After washing in 1 mL of 4°C PBS followed by centrifugation (1000g, 5 minutes) and removal of supernatant, erythrocytes were then resuspended in 200 μL of 4°C PBS before addition of 2 μL of secondary antibody. After 30 minutes at 4°C, supernatant was removed and erythrocytes were washed as described previously. Erythrocyte fluorescence was analyzed using a Becton Dickinson FACSCalibur flow cytometer with CellQuestPro analysis (BD Biosciences). For CR1 staining, the same protocol was followed, but rabbit polyclonal anti-CR1 IgG (antibody R223; Medical Research Council Immunochemistry Unit, Oxford, United Kingdom) was used as the primary, Q0414 (Vector Laboratories) as the isotype control, and 4050-02 (Southern Biotechnology, Birmingham, AL) as the secondary.
Quantitation of Ad5 binding to erythrocytes by real-time (quantitative) PCR
After exposure to Ad5 (2 × 108/mL) for 30 minutes at 37°C with constant agitation, erythrocytes (5 × 109/mL) were centrifuged (1000g, 5 minutes), and the PBS or plasma supernatant removed for processing and measurement of Ad5 content. The erythrocyte pellet was then resupended in a 10× volume of PBS and centrifuged (1000g, 5 minutes) to wash. Supernatant was discarded and an erythrocyte sample removed for processing and measurement of Ad5 content. All samples tested for Ad5 genome content were first digested and DNA extracted using the Genelute mammalian DNA extraction kit according to the manufacturer's instructions (G1N-350; Sigma-Aldrich, St Louis, MO). Quantitative PCR was performed by sampling 5 μL of extracted sample using the primers, probe, and equipment outlined by Green et al.9
Western blotting
Interference created by high hemoglobin concentrations prevented polyacrylamide gel electrophoresis (PAGE) analysis of neat erythrocyte lysates; therefore, erythrocyte ghosts were analyzed for CAR expression. Ghosts were mixed with reducing sample buffer; and after boiling for 5 minutes, approximately 100 ng of protein was loaded per well on a 10% sodium dodecyl sulfate (SDS)–PAGE. Positive control derived from mouse liver (Santa Cruz Biotechnology, Santa Cruz, CA) and a negative control of lysate from A9 cells were also included. After separation (90 minutes, 150 V), overnight transfer (30 V) to nitrocellulose was performed and then blocking (5% milk PBS/Tween 0.1%, 2 hours, 20°C). Primary antibody addition (1:400, anti-CAR 15405; Santa Cruz Biotechnology, 5% milk PBS/Tween 0.1%, 1.5 hours, 20°C) was followed by washing (PBS/Tween 0.1%, 3 × 10 minutes) and secondary antibody addition (anti-rabbit, IgG, horseradish peroxidase conjugate 1 in 3000, W4011; Promega, Madison, WI; 5% milk PBS/Tween 0.1%, 1 hour, 20°C). After further washing (PBS/Tween 0.1%, 3 × 10 minutes), enhanced chemiluminescence reagent RPN2109 (GE Healthcare, Little Chalfont, United Kingdom) was added and visualization achieved using an Alpha Innotech 8000 (San Leandro, CA). To investigate Ad-C3 covalent adduct formation, Ad5 (7 × 1010) was incubated with 1 μL undiluted heparin plasma, ethylenediaminetetraacetic acid plasma (3 hours, 37°C), before the addition of reducing sample buffer and separation on 7.5% SDS-PAGE (2 hours at 170 V). Electrophoretic transfer (75 V, 3 hours) onto nitrocellulose was followed by overnight blocking (5% milk/PBS) and 1-hour incubation with a 1:1000 dilution anti-C3 antibody (Medical Research Council Immunochemistry Unit, Oxford, United Kingdom). Washing, secondary antibody addition, image development, and capture were then performed as stated for Western blotting of CAR.
Monolayer infections
A549 cells were plated (96-well plate, 104 per well); and 24 hours later, Ad5 (500 copies/A549 cell) mixed with neat or 5-fold serial dilutions of washed erythrocytes in PBS was added. After 90 minutes of infection, media were removed and 3 washes in 150 μL PBS performed to remove all erythrocytes, before the addition of fresh media. At 24 hours later, media were removed washing in PBS performed, lysis solution was added, and then luciferase levels measured using the luciferase reporter system E151A (Promega) and a luminometer (LB9507; Berthold Technologies, Bad Wildbad, Germany) and protein levels measured using the BCA assay (B9643; Sigma-Aldrich). SKOV-3 cells were plated at 104 per well; and 24 hours later, media were removed and replaced with serum-free media or serum-free media containing 8 μg/mL factor X (FX; Cambridge BioScience, United Kingdom). Ad5 (500 copies/SKOV cell) mixed with neat or 5-fold serial dilutions of washed erythrocytes in PBS was added, and then the experiment completed as with A549 cells; data were plotted as the fold increase in luciferase activity observed on FX addition for each serial erythrocyte dilution. For neutralization experiments with Ad5 versus polymer-coated epidermal growth factor–retargeted Ad5 (EGF-P-Ad5), dilutions of neutralizing rabbit antiserum (raised by exposure of rabbit to high levels of Ad5) were mixed with Ad5 or EGF-P-Ad5 before their addition to monolayers of A431 cells and luciferase assay as described. For comparison of Ad5 versus EGF-P-Ad5 infection activity in the presence of erythrocytes, an A431 monolayer was exposed to Ad5 or EGF-P-Ad5 mixed with erythrocytes (diluted 5-fold in PBS or plasma).
In vivo studies using NOD-SCID mice and CR1 and CAR transgenics
For in vivo investigation of the effect of human erythrocytes on Ad5 circulation kinetics, nonobese diabetic/severe combined immunodeficiency (NOD-SCID) mice were injected with Ad5 (1010) in 100 μL of PBS or Ad5 (1010) mixed with 100 μL of freshly isolated and thoroughly washed human erythrocytes in PBS (5 × 109 cells/mL). Blood samples (20 μL) were taken at defined time points and immediately separated into cell and plasma fractions; and after washing of the cell fraction in PBS, both fractions were assayed for Ad5 genome content by quantitative PCR. All animal experiments were performed with the approval of the University of Oxford Review Board and according to Home Office legal requirements and guidelines.
For in vivo studies of the effect of human erythrocytes on extravasation, HT29 cells (106 cells/mouse) were injected subcutaneously into female NOD-SCID mice (5-6 weeks old) and allowed to establish (diameter 5-10 mm). Animals were transplanted intravenously with washed human erythrocytes (10% mouse blood volume, 5 × 109 cells/mL) in PBS and Ad5 (1010/mouse). In studies using transgenic mice, Ad5 (1010/0.1 mL per mouse) was injected intravenously into wild-type (WT) C57BL/6, GATA1 CAR,10 or GATA1 CR1 mice11 and blood sampled after 10, 30, 360, and 1440 minutes.
Cryo-electron microscopy
Images of plunge-frozen microscope grids bearing erythrocyte ghosts and Ad5 were captured using an FEI F30 cryo-electron microscope operating under low-dose conditions. After scanning and correction of the contrast transfer function by phase flipping, the images were analyzed directly in the case of the peripheral fiber-mediated virus-membrane interaction or used for 3-dimensional reconstruction in the case of direct vertex-membrane binding (see Document S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Polymer synthesis and characterization and Ad5 polymer coating
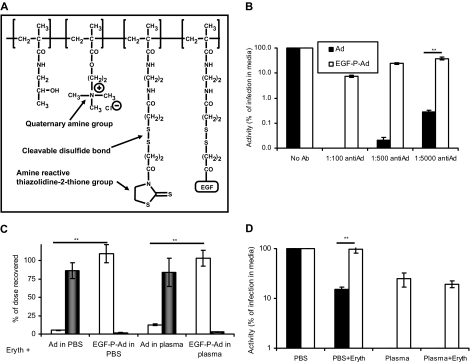
Copolymers (Figure 5A) based on N-(2-hydroxypropyl)methacrylamide (HPMA) containing monomers bearing quaternary ammonium groups (1.5 mol%) and disulphide-bearing side chains terminated in thiazolidine groups (3.4 mol% in product, for reaction with primary amines in virus coat proteins) and murine EGF (15.1 wt% in product, for retargeting via epidermal growth factor receptor) were synthesized and characterized as described elsewhere.12 It had weight average molecular weight 77 200 Da and number average molecular weight 32 200 Da. Coating was performed by mixing Ad5 with EGF-HPMA (1 hour, 20 mg/mL, pH 7.4) before purifying using S400 columns 27-5140-01 (GE Healthcare). Recoveries were calculated using a picogreen assay as previously reported for adeno-associated virus HPMA using Oligreen.13
Figure 5.
Polymer “stealthing” can prevent unwanted binding of Ad5 to human erythrocytes. (A) Representation of HPMA-EGF used to modify Ad5. (B) Comparison of normal and EGF-mediated infection in neutralizing plasma. Ad5 or EGF-P-Ad5 was incubated with dilutions of neutralizing antisera and then added to a monolayer of A431 cells; after 90 minutes, media was removed and washing performed in PBS; and after 24 hours, luciferase expression was analyzed. (C) Ad5 or EGF-P-Ad5 was incubated with washed erythrocytes suspended in PBS/1% BSA or whole fresh human plasma. After incubation, erythrocyte and liquid fractions were separated and assayed for Ad5 genome content as described in “Quantitation of Ad5 binding to erythrocytes by real-time (quantitative) PCR” (□ represents liquid fraction; ■, cell fraction). (D) Comparison of normal and EGF-mediated infection in presence of human erythrocytes. A431 cells were infected with Ad5 or EGF-P-Ad5 in the presence of a 1 in 5 dilution of erythrocytes suspended in PBS or plasma. After 90 minutes, media was removed and thorough washing in PBS performed; 24 hours later, luciferase expression was analyzed. ■ represent Ad5; □, EGF-P-Ad. (B-D) N = 4, SEM shown. **P < .005.
Statistical analysis
In vitro experiments were performed in quadruplet on at least 3 occasions. NOD-SCID in vivo experiments were performed with 6 animals per group; experiments in CAR and CR1 transgenics were performed with at least 3 per group. Results are representative of 2 repeat experiments. Throughout, P values were calculated by analysis of variance with the Tukey pairwise comparison, using Prism software (GraphPad, San Diego, CA).
Results
Ad5 binds to human erythrocytes via CAR
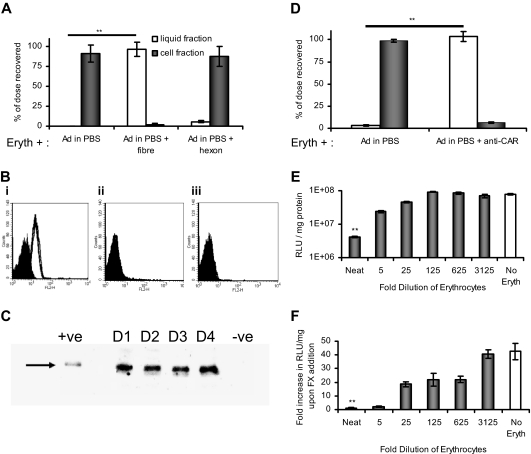
Recovery and distribution of Ad5 particles after exposure to blood components were quantified by real-time PCR. Ad5 bound extensively to human erythrocytes suspended in PBS (90% of input; Figure 1A) but was not internalized (Figure S1). No binding was observed to murine or macaque erythrocytes in PBS (Figure S2). Binding to human erythrocytes could be ablated using soluble fiber protein from Ad5 but not hexon (Figure 1A). The involvement of CAR was therefore assessed by flow cytometry. Washed human erythrocytes from several donors gave strong positive CAR staining, whereas macaque and murine erythrocytes were negative (Figure 1B). Western blotting showed human erythrocyte CAR is 46 kDa, as reported for full-length CAR8 (Figure 1C). This is the first documentation of CAR on human erythrocytes and represents an unexpected difference between mammalian species. Ad5 binding was ablated by preincubation of human erythrocytes with anti-CAR antibody, demonstrating that CAR binding is the primary binding interaction in PBS (Figure 1D). Studies with excess heparin have demonstrated an additional role for HSPGs (Figure S3).
Figure 1.
Ad5 is inactivated by binding to human erythrocytes via the CAR. (A) Fresh washed human erythrocytes were incubated with Ad5 in the presence or absence of fiber or hexon. Ad5 erythrocyte binding was assessed by separating the liquid (□) and erythrocyte (■) fractions by centrifugation and performing quantitative PCR specific for the Ad5 genome on each fraction. Data are represented as the percentage of the total input dose recovered; n = 4, SEM shown. **P < .005. (B) Human erythrocytes from 4 donors (i) or erythrocytes from C57BL/6 mice (ii) or rhesus macaques (iii) were analyzed for CAR presentation using the anti-CAR primary antibody RmcB,8 secondary antibody R0480 (Dako Denmark), and flow cytometric analysis. (i) Filled peak represents isotype control (antibody W6/32); empty peak, donors 1 to 4 with anti-CAR. (ii,iii) Filled peak represents isotype control; empty peak, + anti-CAR (data are superimposed). (C) Human erythrocytes from 4 donors (D1-D4) were treated to produce hemoglobin-free ghosts and then analyzed by SDS-PAGE/Western blotting using 100 ng/lane, primary antibody 15405 (Santa Cruz Biotechnology), and secondary antibody W4011 (Promega). Positive control (+ve) indicates mouse liver lysate (10 ng); negative control, mouse A9 cells (∼ 100 ng). (D) Human erythrocytes in PBS were incubated with anti-CAR antibody (RmcB) before Ad5 addition. After separation of the liquid (□) and cell fraction (■) by centrifugation, quantification of each fraction was achieved by quantitative PCR; n = 4, SEM shown. **P < .005. (E) A549 cells were incubated with Ad5 in the presence of serially diluted erythrocytes for 90 minutes at 37°C; after thorough washing in PBS and the addition of fresh media, the A549 cells were returned to the incubator and luciferase expression was measured 24 hours later; N = 4, SEM shown. **P < .005. (F) SKOV-3 cells were incubated with Ad5 in the presence or absence of physiologic FX levels (8 μg/mL) and serially diluted erythrocytes in PBS, for 90 minutes at 37°C; after thorough washing in PBS and the addition of fresh media, cells were returned to the incubator and luciferase expression was measured 24 hours later. The fold increase on FX addition was calculated by dividing the value achieved with FX by that achieved without FX, and plotted for each erythrocyte dilution; N = 4, SEM shown. **P < .005.
To investigate whether CAR-mediated sequestration by erythrocytes would prevent Ad5 infection of other cells, cancer cell monolayers were incubated with Ad5 in the presence of washed human erythrocytes. When tested in A549 cells (which are infected mainly via CAR), undiluted human erythrocytes inhibited Ad5 infection 200-fold (Figure 1F), whereas FX-mediated Ad5 infection of SKOV-3 cells was totally ablated (Figure 1E).
In plasma Ad5 binds to human erythrocytes via CR1
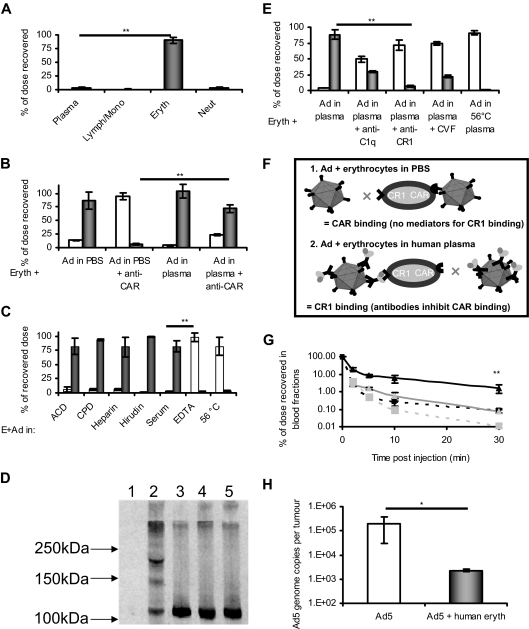
The effect of plasma components on erythrocyte binding of Ad5 was assessed using whole human blood with a high anti-Ad5 neutralizing titer. Blood components were fractionated after exposure to Ad5, and each was assayed by quantitative PCR. Figure 2A shows 90% of Ad5 associated with erythrocytes, and only 3.6% was recovered from plasma. Small amounts of Ad5 were associated with lymphocyte/monocyte (< 1%) and neutrophil fractions (< 4%), which do not express CAR. Although human platelets present CAR14 and their binding to Ad5 has vital repercussions regarding toxicity, such binding does not appear to account for a large percentage of the dose in whole blood.
Figure 2.
Human plasma mediates binding of Ad5 to erythrocytes via the complement cascade and CR1. (A) Ad5 was incubated with whole fresh blood and cell fractionation using CPT vacutainers (BD Biosciences) and erythrocyte lysis was performed; the amount of Ad5 associated with each fraction was then quantified by quantitative PCR; N = 4 separate donors, SEM shown. **P < .005. (B) Erythrocytes were isolated and washed before resuspension in either PBS or neutralizing plasma. Erythrocytes were preincubated or not with anti-CAR antibody (RmcB) and then incubated with Ad5; after separation of the liquid (□) and cell fraction (■) by centrifugation, quantification was achieved by quantitative PCR; n = 4, SEM shown, **P < .005. (C) Erythrocytes were thoroughly washed in PBS and resuspended in a variety of plasmas. Ad5 was added and after incubation, the liquid (□) and erythrocyte (■) fractions were separated by centrifugation and analyzed by quantitative PCR for Ad5 genome content (see “Quantitation of Ad5 binding to erythrocytes by real-time (quantitative) PCR”); N = 4, SEM shown. **P < .005. (D) Western blot analysis using anti-C3 antibody was performed to detect the formation of covalent C3-Ad5 adducts: lane 1 indicates Ad5; lane 2, Ad5 + heparin plasma; lane 3, Ad5 + ethylenediaminetetraacetic acid plasma; lane 4, heparin plasma; lane 5, ethylenediaminetetraacetic acid plasma. (E) In human plasma, inhibitors of the binding of complement to CR1 inhibit the association of Ad5 with erythrocytes. Ad5 was added to erythrocytes in plasma in the presence or absence of antibodies against CR1 or C1q or to plasma that had been pretreated with CVF or heat treated to 56°C for 30 minutes. After fractionation of erythrocytes and plasma, Ad5 genome content of each fraction (□ represents plasma; ■, cells) was quantified by quantitative PCR; N = 4, SEM shown. **P < .005. (F) Schematic representing the binding of Ad5 to erythrocytes in PBS or plasma. In PBS, Ad5 binds erythrocytes via CAR but cannot bind via CR1 because of an absence of complement. In neutralizing plasma, Ad5 binds via CR1 but cannot bind to CAR because the epitopes of fiber protein responsible for such binding are covered by neutralizing antibodies. (G) The presence of human erythrocytes in NOD-SCID mice alters Ad5 circulation kinetics. Ad5 was administered intravenously to NOD-SCID mice transplanted with a 10% vol/vol total blood volume of washed human erythrocytes. At defined time points, blood was sampled, separated into plasma and cell fractions, and assayed for Ad5 content by quantitative PCR. Black line/triangle represents dose recovered from the cell fraction of mice treated with human erythrocytes; black dashed line/square, cell fraction from mice that did not receive human erythrocytes; gray line/triangle, plasma fraction from mice that received human erythrocytes; gray dashed line/square, plasma fraction from mice that did not receive human erythrocytes; N = 3, SD shown. **P < .005. (H) The presence of human erythrocytes in NOD-SCID mice inhibits deposition of an intravenously administered Ad5 dose within subcutaneous xenograft tumors. HT29 cells were implanted into NOD-SCID mice and allowed to establish. Mice were injected with Ad5 in the presence or absence of 10% (vol/vol) total blood volume of fresh washed human erythrocytes. After 24 hours, tumors were harvested and assessed for virus genome content. Grubbs outlier test was applied to remove statistical outliers, leaving N = 4, SD shown. *P < .05.
The binding of Ad5 to human erythrocytes in plasma was only partially antagonized using an anti-CAR antibody (< 25%; Figure 2B), suggesting that additional mechanisms of erythrocyte binding can occur in plasma.
To identify which plasma factors influence Ad5 binding, erythrocytes were removed to allow treatment of plasma in isolation, before being recombined before addition of Ad5 (Figure 2C). Ad5 bound to human erythrocytes in plasmas treated with most anticoagulants (acid citrate dextrose, citrate-phosphate-dextrose, heparin, or hirudin treated) or even in serum. Conversely, when ethylenediaminetetraacetic acid or 56°C/30 minutes heating15 was used, the Ad5 was predominantly (> 90%) free in the plasma, demonstrating that complement fixation is involved in Ad5 human erythrocyte binding.
In complement fixation studies, approximately 40% of Ad5 bound anti-C3b antibody-coated plates after incubation in whole human plasma but not in complement-inhibited/depleted plasma treated with ethylenediaminetetraacetic acid or CVF (Figure S4). Measurement of complement protein C3a, a byproduct of fixation, confirmed these observations (Figure S5). To determine which Ad5 capsid proteins formed C3 adducts, Ad5 was incubated with human plasma and assayed by Western blotting (Figure 2D). Staining with an anti-C3 antibody gave no signal for Ad5 alone (lane 1) but produced a strong signal at approximately 110 kDa in lanes 2 to 5, representing nonreacted C3α. This band was diminished in lane 2 but not lane 3, suggesting its depletion by complement fixation. Furthermore, only in lane 2 were signals observed at molecular weights of 170 to 180 and 220 to 230 kDa, corresponding to complement adducts16 of penton, fiber, and hexon. Blotting of these samples with an anti-Ad5 antibody confirmed the presence of adducts containing Ad5 proteins at these high molecular weights (Figure S6A).
The role of complement in the binding of Ad5 to human erythrocytes in human plasma was confirmed using antibodies against the complement component C1q or complement receptor CR1 to inhibit binding (Figure 2E). We hypothesize that binding of Ad5 to human erythrocytes follows the schematic outlined in Figure 2F. Use of vKH3,17 an Ad5 modified to remove CAR binding capacity, confirmed that engineering Ad5 to remove CAR binding can prevent erythrocyte binding in PBS but does not alter binding to erythrocytes in human plasma (Figure S6B).
Erythrocyte-binding influences Ad5 bloodstream kinetics and extravasation
Ad5 is being developed as a systemic gene delivery agent. Accordingly, we tested the influence of human erythrocytes on Ad5 systemic pharmacokinetics by injecting it intravenously into NOD-SCID mice transplanted with washed human erythrocytes. Animals receiving human erythrocytes showed extended circulation of Ad5 (22% remaining at 2 minutes), predominantly associated with blood cells (Figure 2G). Animals without human erythrocytes showed more rapid clearance (2% remaining at 2 minutes), the majority of which was present in the plasma. This differential was maintained for up to 30 minutes, with more than 20-fold more Ad5 in the bloodstream of mice with human erythrocytes than in those without.
Binding of Ad5 to human erythrocytes extends its blood circulation in NOD-SCID mice but might decrease its access to extravascular target tissues; accordingly, we assessed extravasation by measuring Ad5 accumulation within human HT29 xenograft tumors (Figure 2H). Without human erythrocytes, accumulation reached nearly 2 × 105 Ad5/mg tumor; however, in mice treated with human erythrocytes, accumulation decreased approximately 100-fold. These data suggest that, although the binding of Ad5 to human erythrocytes may prolong its circulation in the clinical setting, access to extravascular targets will be poor.
CR1 or CAR on erythrocytes changes Ad5 kinetics and prevents hepatic infection in transgenic mice
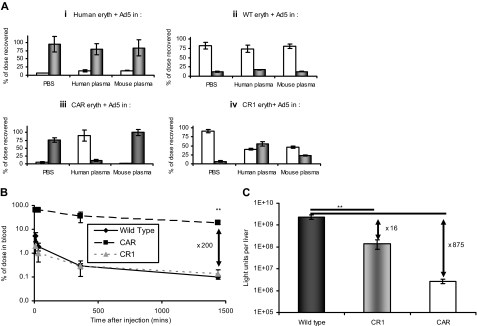
CAR and CR1 were both absent on erythrocytes from WT mice but present on erythrocytes from transgenic CAR10 or CR111 mice (Figure S7). Binding of Ad5 to these erythrocytes was compared ex vivo with binding to human and WT murine erythrocytes. Ad5 showed human erythrocytes association in both PBS and neutralizing human plasma (Figure 3Ai) and also bound human erythrocytes in non-neutralizing mouse plasma, reflecting either binding to CAR or complement-mediated binding to CR1. Conversely, WT mice erythrocytes showed minimal Ad5 binding (∼ 10% under all conditions; Figure 3Aii). CAR-expressing erythrocytes from CAR transgenics showed strong Ad5 binding in PBS and murine plasma but not in neutralizing human plasma, confirming antibody blockade of CAR-binding epitopes (Figure 3Aiii). Erythrocytes from CR1 transgenics bound Ad5 in murine and human plasma, but not in PBS (Figure 3Aiv). Whereas binding in human plasma probably involves anti-Ad5 antibodies, binding in murine plasma may be mediated by low-affinity “natural” IgMs recognizing Ad5.18
Figure 3.
Erythrocyte binding, circulation kinetics, and infection profiles are altered in CAR and CR1 transgenic mice. (A) Erythrocytes were isolated from human donors (i), WT mice (ii), CAR mice (iii), or CR1 mice (iv), and after washing were resuspended in PBS, human plasma, or mouse plasma. Ad5 was added and after incubation, liquid (□) and erythrocyte (■) fractions were separated and assayed for Ad5 genome content; N = 4, SEM shown. (B) The circulation kinetics of Ad5 are altered in CAR but not CR1 transgenic mice. Ad5 was injected intravenously into WT, CAR, or CR1 mice and blood sampled at 10, 30, 360, or 1440 minutes. Samples were assayed for Ad5 genome content by quantitative PCR; N = 3, SD shown. **P < .005. (C) The livers from the mice injected in panel B were harvested at 24 hours and after homogenization were assayed for reporter gene expression; N = 3, SD shown. **P < .005.
We then tested whether erythrocyte binding influences Ad5 circulation kinetics. In WT mice, intravenous Ad5 showed a bloodstream half-life less than 2 minutes, characteristic of first-pass hepatic clearance (Figure 3B). CR1 transgenics showed a similar clearance profile. In contrast, CAR transgenics showed extended circulation, with approximately 70% of Ad5 detectable in circulation after 6 hours. Because Ad5 is associated with erythrocytes in both transgenic models, this suggests that the nature of binding is crucial, with CAR-bound Ad5 remaining in the bloodstream, whereas CR1-bound Ad5 is targeted for rapid clearance. This agrees with the reported mechanism of processing of CR1-bound antigen, which involves deposition in hepatic and splenic macrophages.19
The modified circulation profile in the transgenic mice was reflected in substantial differences in liver transduction (Figure 3C), which was decreased more than 800-fold in CAR mice compared with WT. Ad5 bound to erythrocytes via CAR is therefore incapable of infecting hepatocytes in vivo, presumably independent of the presence or absence of anti-Ad5 antibodies. Similarly, CR1 transgenics showed 16-fold less hepatic transduction than WT mice, compatible with the role of CR1 sequestration in accelerating delivery to macrophages for degradation rather than allowing hepatocyte infection.
Cryo-electron microscopy of Ad5 binding to erythrocytes
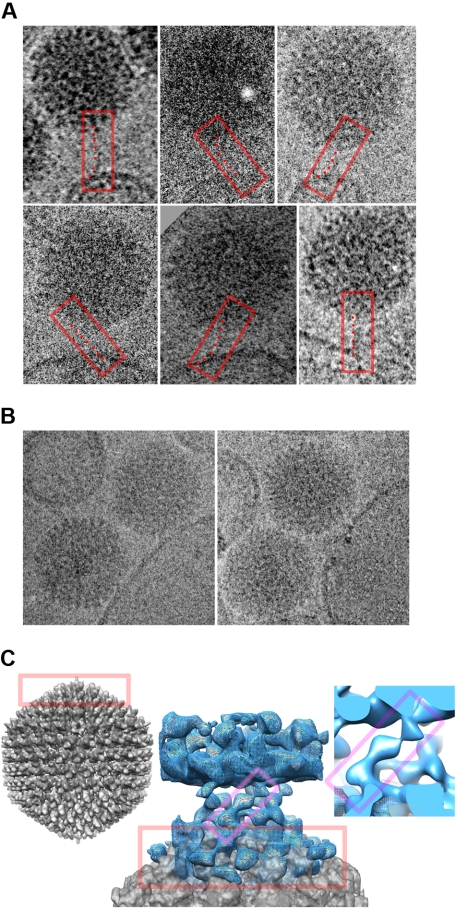
The interaction of Ad5 with human and murine erythrocytes was characterized using cryo-electron microscopy. Erythrocyte ghosts presenting CAR and CR1 (as confirmed by flow cytometry; Figure S8) were mixed with Ad5 for 30 minutes before cryo-electron microscopy imaging. Ad5 associated with the membrane of human ghosts in both PBS and human plasma. In PBS (but not in plasma), some Ad5 were bound via their fiber proteins (Figure 4A). These are the first cryo-electron microscopy images demonstrating the Ad5 fiber interacting with a CAR-positive membrane and the predicted flexibility of fiber can be seen.20 Furthermore, in both PBS (Figure 4B) and plasma (Figure S9), Ad5 bound closely to the membrane, although no association was evident with erythrocyte ghosts from WT mice under any conditions. Ad5 was also closely associated with ghosts from CAR transgenics in PBS (Figure S9) and with ghosts from CR1 transgenics in the presence of plasma. To better characterize the binding interaction, regions of micrographs of human erythrocyte ghosts in PBS showing Ad5 and membrane in close association were subjected to image analysis using an existing cryo-electron microscopy reconstruction of Ad5.21 This gave an image of the Ad5 in direct contact with the membrane (Figure 4C). Thus, microscopy of the Ad5-membrane interaction supports the existence of a CAR-dependent initial interaction, succeeded by a closer association via a secondary receptor22 with 5-fold symmetry, perhaps located in the penton base. Ad5 penton base could contain epitopes capable of binding HSPG, as recently documented for penton base from type 3 adenovirus.23 Inhibition studies using heparin support this theory (Figure S3).
Figure 4.
The binding of Ad5 to erythrocytes can be visualized by cryo-electron microscopy. Erythrocyte ghosts were exposed to Ad5 for 30 minutes before fixation and imaging. (A) Gallery of 6 images of Ad5 interacting peripherally with human erythrocyte ghost membranes in PBS. In each case, the tubular density visible for the pentagonal vertex fiber is boxed in red, with a series of points marking the flexible path of the fiber from the Ad5 surface above to the membrane below. (B) Images of Ad5 and ghost membranes in close association in PBS. (C) Central image shows a reconstruction of the close interaction of Ad5 with the human ghost membrane. The planar membrane (blue density mesh) forms the top part of the image, and the curved pentagonal vertex of the Ad5 (gray density surface) the bottom portion. They are linked by a crown of obliquely oriented features. The Ad5 portion of the image is superimposed with the equivalent portion of a whole-Ad5 reconstruction, for comparison.21 The whole Ad5 is shown to the left (gray density), in the same orientation as in the middle panel, with equivalent portions in the 2 boxed in red. A closeup of one connection is shown to the right, and equivalent portions of it and the central image are boxed in magenta.
Polymer “stealthing” of Ad5 to prevent binding to CAR and antibodies
Systemic delivery of Ad5 therapeutics would be extremely clinically valuable. However, genetic modification of Ad5 to avoid both CAR and CR1-mediated human erythrocyte binding requires modification of the CAR-binding domain and all B-cell epitopes on the capsid because approximately 90% of the population have anti-Ad5 antibodies. Even if this were possible, it would raise significant safety concerns. Physical “stealthing” of Ad5 provides a safer and more practical strategy, and we have shown previously that HPMA9,24,25 has great potential for coating adenovirus. In this study, we used a new generation of EGF-targeted, positively charged, HPMA polymer (EGF-P; Figure 5A). The ability of this polymer to coat Ad5 was verified by zeta potential analysis (Figure S10). In infection studies (Figure 5B), activity of Ad5 was decreased 105-fold by a 1 in 100 dilution of anti-Ad5 antiserum, whereas EGF-P-Ad5 showed only a 10-fold decrease in activity, suggesting good antibody protection. Indeed, in separate experiments, EGF-P-Ad still maintained 10% of its activity even when neat serum was used (Figure S11). We also assessed whether polymer coating would prevent sequestration onto human erythrocytes (Figure 5C) and found that EGF-P-Ad5 showed no binding to human erythrocytes in either PBS or human plasma (∼ 100% recovered in liquid fraction), indicating that CAR and antibody-binding epitopes were fully shielded. Finally, we assessed whether EGF-P-Ad5 could infect an EGF receptor–expressing cancer cell line in the presence of human erythrocytes (Figure 5D). Whereas CAR-mediated infection of Ad5 in PBS was decreased 10-fold by the inclusion of erythrocytes, EGF-P-Ad5 showed no inhibition, reflecting coverage of CAR-binding epitopes by the polymer and retargeting through EGF. In plasma, Ad5 mediated no detectable infection with or without erythrocytes. Conversely, EGF-P-Ad5 activity was reduced less than 10-fold even in the presence of both plasma and erythrocytes. These data provide evidence that the EGF-P-Ad5 is protected from binding of both neutralizing and non-neutralizing antibodies.
Discussion
Here we demonstrate that CAR is presented on human erythrocytes. This was unexpected because CAR has been previously described as a cell adhesion molecule involved in the formation of interepithelial contacts. Given its well-defined function in promoting integrity of epithelial tissues,26–31 it seems probable that it has a different (presently unclear) biologic role on human erythrocytes.
CAR detected on human erythrocytes is the full-length molecule previously characterized32 as the main high affinity receptor for Ad5 and group B Coxsackie virus. Although adenovirus is normally restricted to infections of the airway and pleura, except in severely immune-suppressed persons, 2 research groups3,4 recently documented an alternative pathway for Ad5 infection involving binding of clotting FX to Ad5 hexon. This uptake pathway is responsible for the high levels of hepatic infection seen in mice after intravenous administration of Ad5. This suggests that adenovirus has adapted to exploit cell entry mechanisms based on receptors for clotting factors and, accordingly, that its infectious niche includes exposure to plasma proteins. However, we show here that CAR and CR1 on human erythrocytes provide a formidable defense against blood-borne infection. Our studies transplanting washed human erythrocytes into NOD-SCID mice (with non–Ad5-neutralizing plasma) showed that human erythrocytes sequester Ad5 in the mouse bloodstream and abolish extravasation, thereby preventing access to extravascular targets. This compounds our observations that sequestration of Ad5 by washed human erythrocytes ablates both CAR and FX-mediated infection of tumor cell lines in vitro, indicating inhibition of infection even when the virus can make contact with the target cells. Similarly, after systemic administration of Ad5 in CAR transgenic mice, the virus is sequestered onto erythrocytes and fails to infect the liver, including by the FX-binding pathway.
CR1-bearing erythrocytes from CR1 transgenic mice also showed extensive Ad5 binding in human plasma and, to a lesser extent, in non-neutralizing murine plasma in vitro. As a consequence of such binding, liver infection after intravenous administration of Ad5 was significantly lower in CR1 mice than in WT mice. In human plasma, the CR1 interaction involves complement component C1q, suggesting involvement of the classic complement pathway and anti-Ad5 antibodies as previously reported,33 although in mouse plasma the mechanism is unclear. Direct binding of C3 (the alternative pathway) has been proposed,34 although Xu et al18 showed recently that murine plasma contains natural IgM that can mediate opsonization of Ad5 for phagocytosis. It is known that these innate antibodies are capable of activating complement. In either case, it seems probable that Ad5 will bind to CR1 on human erythrocytes when administered intravenously in all patients. Whether it binds also to CAR appears to depend on the presence or absence of antibodies capable of blocking the CAR-binding epitopes of the fiber.
Cryo-electron microscopy analysis showed that Ad5 binds human erythrocyte membranes in PBS both via fiber-CAR and also through a second receptor that mediates a closer interaction, apparently mediated through the penton base. Our data and the recent observation that structurally related Ad3 dodecamers bind cell surface HSPGs23 suggest that HSPGs are involved.
The absence of CAR and CR1 on murine erythrocytes highlights differences in the cell biology and innate defense mechanisms of different mammals. It is possible that the need for erythrocyte CAR “decoys” is less important in mice because they are not normally subject to infectious pathology from CAR-infecting adenoviruses; nevertheless, mice are very sensitive to Coxsackie virus infection.35 Indeed, Asher et al showed that expression of CAR on transgenic murine erythrocytes provided effective protection against challenge with Coxsackie virus.10 The effect of adenovirus in immunosuppressed patients undergoing stem cell transplantion provides information regarding the protective effect of erythrocyte CAR in humans. In these patients, invasive adenovirus disease has an associated mortality of up to 50%.36 However, the transition from asymptomatic Ad5 viremia to symptomatic disseminated infection is typically preceded by a 10-fold increase in the level of circulating Ad5 and is also associated with reported serum levels of 107 to 108 Ad5 /mL (which corresponds to whole blood levels of 109 to 1010 Ad5/mL).37,38 Hence, it may be the saturation of erythrocyte CAR that allows progression of Ad5 disease.
In the context of gene and virotherapy, this battery of defenses against Ad5 raises concerns that infection of hepatocytes (or indeed any other cells) cannot occur after intravenous delivery of therapeutic Ad5 in humans, irrespective of neutralizing antibody status. The remarkable absence of hepatic toxicity in clinical trials, even after delivery of Ad5 via the hepatic artery, has already led others to question whether Ad5 infects the liver in humans as well as it does in preclinical models.39 The human erythrocyte-based sequestration mechanisms outlined here may accompany adaptive antibody responses to significantly restrict Ad5 infection irrespective of prior Ad5 exposure.
The use of physical coating strategies to protect therapeutic viruses from unwanted interactions with antibodies and cells provides a way forward. Whereas genetic modification of viruses to evade the immune system is a daunting challenge and could yield hazardous variants, physical coating provides a simple “cover-all” that is not inherited by progeny viruses. This approach may protect Ad5 against binding to antibodies and erythrocytes, improving extravasation and allowing access to disseminated disease targets, while ensuring that any progeny virus produced in vivo will be rapidly inactivated when it reenters the bloodstream. Therefore, the absence of erythrocyte binding we observed with Ad5 coated with the latest generation of HPMA polymers represents a significant step forward.
Expression of CR1 on human erythrocytes also represents an important species difference that impinges on “translation” of murine data using any microorganism for human medication because even low affinity antibodies will probably sequester the microorganism onto human, but not murine, erythrocytes. Conventional murine preclinical models are therefore doubtful to be useful for testing therapeutic microorganisms intended to contact the bloodstream, even if the animals are preimmunized to reflect human adaptive immune status. Identification of preclinical systems capable of accurately predicting performance of therapeutic microorganisms in humans is a rather underdeveloped area.
Viruses have a distinguished medical history as vaccines and are beginning to show therapeutic activity in other disciplines ranging from the use of retroviruses to express immune components in bone marrow precursor cells in congenital immune deficiencies,40 parvoviruses for retinal treatment of Leber congenital amaurosis,41 through to use of “oncolytic” viruses for experimental treatment of advanced cancer.42 These agents promise versatile treatment innovations; however, given the species-specific defenses observed here for Ad5, it seems wise that clinical translation of therapeutic viruses should maximize the use of human cells and tissues to predict clinical activity and toxicity, and not rely too heavily on preclinical animal models.
Acknowledgments
The authors thank Sam Illingworth for technical assistance, Richard Iggo for kind provision of vKH3, and Ned Jenkinson for provision of samples.
This work is supported by Cancer Research UK and the European Union (GIANT project LSHB-CT-2004-512087). R.J.C.G. is a Royal Society University Research Fellow. A.F.-P.S. holds a Wellcome Trust 4-year DPhil studentship.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: R.C.C. designed research and performed research, analyzed data, and wrote the paper; Y.D., A.M.C., A.F.-P.S., N.K.G., V.S., and R.J.C.G. performed research and analyzed data; R.B.S., K.U., K.D.F., and R.W.F. designed research; and L.W.S. designed research and wrote the paper.
Conflict-of-interest disclosure: L.W.S. and K.D.F. are founders and shareholders of Hybrid Systems Ltd. The remaining authors declare no competing financial interests.
Correspondence: Robert Carlisle, Department of Clinical Pharmacology, University of Oxford, Roosevelt Drive, Oxford OX3 7DQ, United Kingdom; e-mail: Robert.carlisle@clinpharm.ox.ac.uk.
References
- 1.Hayashi S, Hogg JC. Adenovirus infections and lung disease. Curr Opin Pharmacol. 2007;7:237–243. doi: 10.1016/j.coph.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parker AL, Waddington SN, Nicol CG, et al. Multiple vitamin K-dependent coagulation zymogens promote adenovirus-mediated gene delivery to hepatocytes. Blood. 2006;108:2554–2561. doi: 10.1182/blood-2006-04-008532. [DOI] [PubMed] [Google Scholar]
- 3.Kalyuzhniy O, Di Paolo NC, Silvestry M, et al. Adenovirus serotype 5 hexon is critical for virus infection of hepatocytes in vivo. Proc Natl Acad Sci U S A. 2008;105:5483–5488. doi: 10.1073/pnas.0711757105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waddington SN, McVey JH, Bhella D, et al. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell. 2008;132:397–409. doi: 10.1016/j.cell.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 5.Peng Z. Current status of gendicine in China: recombinant human Ad-p53 agent for treatment of cancers. Hum Gene Ther. 2005;16:1016–1027. doi: 10.1089/hum.2005.16.1016. [DOI] [PubMed] [Google Scholar]
- 6.Nakano MY, Boucke K, Suomalainen M, Stidwill RP, Greber UF. The first step of adenovirus type 2 disassembly occurs at the cell surface, independently of endocytosis and escape to the cytosol. J Virol. 2000;74:7085–7095. doi: 10.1128/jvi.74.15.7085-7095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlisle RC, Bettinger T, Ogris M, Hale S, Mautner V, Seymour LW. Adenovirus hexon protein enhances nuclear delivery and increases transgene expression of polyethylenimine/plasmid DNA vectors. Mol Ther. 2001;4:473–483. doi: 10.1006/mthe.2001.0472. [DOI] [PubMed] [Google Scholar]
- 8.Tomko RP, Xu R, Philipson L. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci U S A. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green NK, Herbert CW, Hale SJ, et al. Extended plasma circulation time and decreased toxicity of polymer-coated adenovirus. Gene Ther. 2004;11:1256–1263. doi: 10.1038/sj.gt.3302295. [DOI] [PubMed] [Google Scholar]
- 10.Asher DR, Cerny AM, Finberg RW. The erythrocyte viral trap: transgenic expression of viral receptor on erythrocytes attenuates coxsackievirus B infection. Proc Natl Acad Sci U S A. 2005;102:12897–12902. doi: 10.1073/pnas.0506211102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Repik A, Pincus SE, Ghiran I, et al. A transgenic mouse model for studying the clearance of blood-borne pathogens via human complement receptor 1 (CR1). Clin Exp Immunol. 2005;140:230–240. doi: 10.1111/j.1365-2249.2005.02764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Subr V, Kostka L, Selby-Milic T, et al. Coating of adenovirus type 5 with polymers containing quaternary amines prevents binding to blood components. J Control Release. 2009 doi: 10.1016/j.jconrel.2008.12.009. (in press). DOI: 10.1016/j.jconrel.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Carlisle RC, Benjamin R, Briggs SS, et al. Coating of adeno-associated virus with reactive polymers can ablate virus tropism, enable retargeting and provide resistance to neutralising antisera. J Gene Med. 2008;10:400–411. doi: 10.1002/jgm.1161. [DOI] [PubMed] [Google Scholar]
- 14.Othman M, Labelle A, Mazzetti I, Elbatarny HS, Lillicrap D. Adenovirus-induced thrombocytopenia: the role of von Willebrand factor and P-selectin in mediating accelerated platelet clearance. Blood. 2007;109:2832–2839. doi: 10.1182/blood-2006-06-032524. [DOI] [PubMed] [Google Scholar]
- 15.Jasin HE. Human heat labile opsonins: evidence for their mediation via the alternate pathway of complement activation. J Immunol. 1972;109:26–31. [PubMed] [Google Scholar]
- 16.Sahu A, Lambris JD. Structure and biology of complement protein C3, a connecting link between innate and acquired immunity. Immunol Rev. 2001;180:35–48. doi: 10.1034/j.1600-065x.2001.1800103.x. [DOI] [PubMed] [Google Scholar]
- 17.Homicsko K, Lukashev A, Iggo RD. RAD001 (everolimus) improves the efficacy of replicating adenoviruses that target colon cancer. Cancer Res. 2005;65:6882–6890. doi: 10.1158/0008-5472.CAN-05-0309. [DOI] [PubMed] [Google Scholar]
- 18.Xu Z, Smith JS, Tian J, Brynes AP. Clearance of adenovirus by Kupffer cells is mediated by natural antibodies, complement and scavenger receptors. Mol Ther. 2008;16(suppl):S93. doi: 10.1128/JVI.01320-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cornacoff JB, Hebert LA, Smead WL, VanAman ME, Birmingham DJ, Waxman FJ. Primate erythrocyte-immune complex-clearing mechanism. J Clin Invest. 1983;71:236–247. doi: 10.1172/JCI110764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu E, Pache L, Von Seggern DJ, et al. Flexibility of the adenovirus fiber is required for efficient receptor interaction. J Virol. 2003;77:7225–7235. doi: 10.1128/JVI.77.13.7225-7235.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fabry CM, Rosa-Calatrava M, Conway JF, et al. A quasi-atomic model of human adenovirus type 5 capsid. EMBO J. 2005;24:1645–1654. doi: 10.1038/sj.emboj.7600653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cusack S. Adenovirus complex structures. Curr Opin Struct Biol. 2005;15:237–243. doi: 10.1016/j.sbi.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Fender P, Schoehn G, Perron-Sierra F, Tucker GC, Lortat-Jacob H. Adenovirus dodecahedron cell attachment and entry are mediated by heparan sulfate and integrins and vary along the cell cycle. Virology. 2008;371:155–164. doi: 10.1016/j.virol.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 24.Fisher KD, Stallwood Y, Green NK, Ulbrich K, Mautner V, Seymour LW. Polymer-coated adenovirus permits efficient retargeting and evades neutralising antibodies. Gene Ther. 2001;8:341–348. doi: 10.1038/sj.gt.3301389. [DOI] [PubMed] [Google Scholar]
- 25.Morrison J, Briggs SS, Green N, et al. Virotherapy of ovarian cancer with polymer-cloaked adenovirus retargeted to the epidermal growth factor receptor. Mol Ther. 2008;16:244–251. doi: 10.1038/sj.mt.6300363. [DOI] [PubMed] [Google Scholar]
- 26.Cohen CJ, Shieh JT, Pickles RJ, Okegawa T, Hsieh JT, Bergelson JM. The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc Natl Acad Sci U S A. 2001;98:15191–15196. doi: 10.1073/pnas.261452898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Honda T, Saitoh H, Masuko M, et al. The coxsackievirus-adenovirus receptor protein as a cell adhesion molecule in the developing mouse brain. Brain Res Mol Brain Res. 2000;77:19–28. doi: 10.1016/s0169-328x(00)00036-x. [DOI] [PubMed] [Google Scholar]
- 28.Coyne CB, Voelker T, Pichla SL, Bergelson JM. The coxsackievirus and adenovirus receptor interacts with the multi-PDZ domain protein-1 (MUPP-1) within the tight junction. J Biol Chem. 2004;279:48079–48084. doi: 10.1074/jbc.M409061200. [DOI] [PubMed] [Google Scholar]
- 29.Raschperger E, Thyberg J, Pettersson S, Philipson L, Fuxe J, Pettersson RF. The coxsackie- and adenovirus receptor (CAR) is an in vivo marker for epithelial tight junctions, with a potential role in regulating permeability and tissue homeostasis. Exp Cell Res. 2006;312:1566–1580. doi: 10.1016/j.yexcr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 30.Yamashita M, Ino A, Kawabata K, Sakurai F, Mizuguchi H. Expression of coxsackie and adenovirus receptor reduces the lung metastatic potential of murine tumor cells. Int J Cancer. 2007;121:1690–1696. doi: 10.1002/ijc.22852. [DOI] [PubMed] [Google Scholar]
- 31.Martin TA, Watkins G, Jiang WG. The Coxsackie-adenovirus receptor has elevated expression in human breast cancer. Clin Exp Med. 2005;5:122–128. doi: 10.1007/s10238-005-0076-1. [DOI] [PubMed] [Google Scholar]
- 32.Bergelson JM, Cunningham JA, Droguett G, et al. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 33.Cichon G, Boeckh-Herwig S, Schmidt HH, et al. Complement activation by recombinant adenoviruses. Gene Ther. 2001;8:1794–1800. doi: 10.1038/sj.gt.3301611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang H, Wang Z, Serra D, Frank MM, Amalfitano A. Recombinant adenovirus vectors activate the alternative complement pathway, leading to the binding of human complement protein C3 independent of anti-ad antibodies. Mol Ther. 2004;10:1140–1142. doi: 10.1016/j.ymthe.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 35.Chen JW, Ghosh R, Finberg RW, Bergelson JM. Structure and chromosomal localization of the murine coxsackievirus and adenovirus receptor gene. DNA Cell Biol. 2003;22:253–259. doi: 10.1089/104454903321908647. [DOI] [PubMed] [Google Scholar]
- 36.Neofytos D, Ojha A, Mookerjee B, et al. Treatment of adenovirus disease in stem cell transplant recipients with cidofovir. Biol Blood Marrow Transplant. 2007;13:74–81. doi: 10.1016/j.bbmt.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 37.Seidemann K, Heim A, Pfister ED, et al. Monitoring of adenovirus infection in pediatric transplant recipients by quantitative PCR: report of six cases and review of the literature. Am J Transplant. 2004;4:2102–2108. doi: 10.1111/j.1600-6143.2004.00631.x. [DOI] [PubMed] [Google Scholar]
- 38.Lion T, Baumgartinger R, Watzinger F, et al. Molecular monitoring of adenovirus in peripheral blood after allogeneic bone marrow transplantation permits early diagnosis of disseminated disease. Blood. 2003;102:1114–1120. doi: 10.1182/blood-2002-07-2152. [DOI] [PubMed] [Google Scholar]
- 39.Au T, Thorne S, Korn WM, Sze D, Kirn D, Reid TR. Minimal hepatic toxicity of Onyx-015: spatial restriction of coxsackie-adenoviral receptor in normal liver. Cancer Gene Ther. 2007;14:139–150. doi: 10.1038/sj.cgt.7700988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gaspar HB, Parsley KL, Howe S, et al. Gene therapy of X-linked severe combined immunodeficiency by use of a pseudotyped gammaretroviral vector. Lancet. 2004;364:2181–2187. doi: 10.1016/S0140-6736(04)17590-9. [DOI] [PubMed] [Google Scholar]
- 41.Bainbridge JW, Smith AJ, Barker SS, et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 42.Liu TC, Galanis E, Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat Clin Pract Oncol. 2007;4:101–117. doi: 10.1038/ncponc0736. [DOI] [PubMed] [Google Scholar]