Abstract
We evaluated systemic alterations to the blood coagulation system that occur during a coronary thrombotic event. Peripheral blood coagulation in patients with acute coronary thrombosis was compared with that in people with stable coronary artery disease (CAD). Blood coagulation and platelet activation at the microvascular injury site were assessed using immunochemistry in 28 non-anticoagulated patients with acute myocardial infarction (AMI) versus 28 stable CAD patients matched for age, sex, risk factors, and medications. AMI was associated with increased maximum rates of thrombin-antithrombin complex generation (by 93.8%; P < .001), thrombin B-chain formation (by 57.1%; P < .001), prothrombin consumption (by 27.9%; P = .012), fibrinogen consumption (by 27.0%; P = .02), factor (f) Va light chain generation (by 44.2%; P = .003), and accelerated fVa inactivation (by 76.1%; P < .001), and with enhanced release of platelet-derived soluble CD40 ligand (by 44.4%; P < .001). FVa heavy chain availability was similar in both groups because of enhanced formation and activated protein C (APC)–mediated destruction. The velocity of coagulant reactions in AMI patients showed positive correlations with interleukin-6. Heparin treatment led to dampening of coagulant reactions with profiles similar to those for stable CAD. AMI-induced systemic activation of blood coagulation markedly modifies the pattern of coagulant reactions at the site of injury in peripheral vessels compared with that in stable CAD patients.
Introduction
After vascular injury, blood clotting is initiated when plasma factor (f) VIIa gains access to tissue factor (Tf). The resulting complex activates the plasma zymogens fIX and fX.1 Factor Xa activates small amounts of thrombin, which activates platelets, and the procofactors fV and fVIII to their respective active forms.2 These reactions result in the formation of the intrinsic fXase (fVIIIa-fIXa) and prothrombinase (fVa-fXa) on the activated platelet surface.3 The major bolus of thrombin formed by the prothrombinase complex is largely responsible for the ultimate hemostatic process.4 A potent, synergistic inhibitory system principally composed of tissue factor pathway inhibitor (TFPI), antithrombin (AT), and the thrombin-thrombomodulin (Tm)–catalyzed dynamic protein C (PC) system opposes thrombin generation.5,6
The formation of fVa and its regulation by activated protein C (APC) are key processes for maintaining blood homeostasis. The fV activation process involves sequential cleavages to first produce a heavy chain (1-709) and subsequently a light chain (1545-2196) resulting in the active cofactor.7 The PC system inactivates fVa in a kinetically controlled series of cleavage reactions in which APC cleaves the heavy chain of fVa at 2 locations (R506 and R306); the resulting fVai can no longer function in the coagulation system.8,9 The balance between activation and inactivation of fV is critical to the synergistic control of the coagulation process.10
The activation of the Tf coagulation pathway appears to be central in arterial and venous thrombosis.11 A major clinical manifestation of arterial thrombosis is represented by the acute coronary syndromes (ACS), which result from platelet-rich thrombus formation on the surface of ruptured or eroded atheromatosus plaque in the coronary artery.12 Typical procoagulant abnormalities in ACS are increased circulating thrombin marker levels, such as prothrombin fragment 1.2 (F1.2) or thrombin-antithrombin complexes (TAT), with the maximum values seen in subjects with ST-elevation myocardial infarction (STEMI) and markedly elevated cardiac troponin levels, a marker of myocardial necrosis.13–17 Moreover, enhanced thrombin activity toward fibrinogen within 12 hours of acute myocardial infarction (AMI) has been found to identify patients at an increased risk of cardiac mortality.18 Patients with ACS who developed in-hospital recurrent ischemia despite at least 72 hours of heparin infusion had significantly higher plasma levels of thrombin markers determined before the coronary event, and heparin was only partially able to antagonize thrombin activity.17,19
It is not known how acute coronary thrombosis on the damaged atheromatosus plaque may alter the kinetics of the systemic coagulant system and to what degree global coagulation alterations contribute to subsequent pathology. No consistent changes in coagulation factor levels in venous blood have been observed in ACS patients. Vaziri et al20 reported increased activity of fIX and decreased fII and fV in ACS patients. Elevated fVIII antigen and coagulant activity can also be detected in AMI patients.21 Recently, we reported increased fXIa in ACS patient plasma and predicted enhanced thrombin generation based upon blood coagulation factor composition.22 Furthermore, increased thrombin formation in ACS may be supported by impaired anticoagulant mechanisms. It has been shown that ACS is associated with reduced AT activity.15,20 Levels of free TFPI have been reported to be elevated in acute myocardial ischemia.23 In our numerical simulations, the collective contributions of AT, fII, and fVIII were most prominent.22
In this study, we have evaluated the potential for alteration of the systemic coagulation system in ACS by studying injury to the peripheral microcirculation in non-anticoagulated patients undergoing AMI and compared their response to a control cohort with stable coronary artery disease (CAD). The microvascular injury model used enables an in vivo evaluation of thrombin generation and platelet activation.24 It consists of performing standardized skin incisions by means of a bleeding-time device that cuts blood vessels 5 to 25 μm in diameter. Blood is collected at equal time intervals into canules, then passed into an anticoagulant solution; in the separated supernatant, several substances can be detected. Weiss and Lages25 showed that thrombin formation in this model depends on local Tf expression closely coupled with platelet activation. In previous studies with this model we have reported the influence of aspirin and statins on thrombin generation24–29 and platelet activation.28,30
Methods
Patients
We enrolled 28 patients, 22 men, aged 46 to 75 years (mean, 61 years), admitted to the hospital for AMI within the first 12 hours (mean, 5 hours) after onset of chest pain, and 28 patients with angiographically confirmed stable CAD (> 50% stenosis in at least one major epicardial artery). Inclusion criteria for AMI patients were typically chest pain and ST-segment elevation of at least 1 mm or ST depression in at least 2 contiguous leads on standard ECG, and elevated cardiac troponin I. All AMI patients were diagnosed with STEMI (n = 14) or non–ST-elevation MI (NSTEMI; n = 14). Exclusion criteria in AMI patients were as follows: cardiogenic shock, ACS within the previous 6 months, any acute illness, cancer, history of venous thromboembolism, oral anticoagulant, or heparin administration. Fifty percent of AMI patients (n = 14) had arterial hypertension, 4 had diabetes mellitus, and 10 patients were current smokers. All patients took aspirin (300 mg) 3 to 8 hours before the study. Fourteen AMI subjects were previously treated with low-dose aspirin, as were all subjects with stable CAD. No individuals received thienopyridines or thrombolytics prior to blood collection. Other cardiovascular drugs included β-blockers (n = 15), nitrates (n = 20), angiotensin-converting enzyme inhibitors (n = 14), statins (n = 9), and calcium antagonists (n = 9). Stable angina patients (Canadian Cardiovascular Society class II or III), 20 men and 8 women, were matched to the AMI patients for age, sex, coronary risk factors, and medications. None of these stable angina patients had developed AMI or undergone angioplasty within the 6 months preceding the study.
To evaluate the effect of heparin infusion, we studied 8 additional patients with AMI, for instance, NSTEMI, receiving unfractionated heparin intravenously at the time of the blood collection. This group was similar to non-anticoagulated AMI patients with regard to demographic and clinical features.
The Jagiellonian University Ethical Committee approved the study, and written informed consent was obtained from all patients in accordance with the Declaration of Helsinki.
Laboratory investigations
Blood was drawn into 0.1 volume of 3.2% trisodium citrate from an antecubital vein with minimal stasis (within 15 minutes after hospital admission of AMI patients). Citrated blood samples were centrifuged within 15 minutes of collection and stored at −80°C until used. Lipid profiles, blood cell counts, glucose, creatinine, aminotransferases, activated partial thromboplastin time (aPTT), and cardiac troponin I were assayed by routine laboratory techniques. Fibrinogen was determined using the Clauss method. High-sensitivity CRP was measured by latex nephelometry (Dade Behring, Marburg, Germany). Commercially available immunoenzymatic assays were used to determine plasma interleukin-6 (IL-6; R&D Systems, Abingdon, United Kingdom), TAT (Dade Behring), and soluble CD40 ligand (sCD40L; R&D Systems). All the intra-assay and inter-assay coefficients of variation were below 7%. FII, fVII, fVIII, fIX, fX, and AT were measured by one-stage clotting assays with use of factor-deficient plasmas (Dade Behring). Protein C (PC) was measured using a chromogenic substrate (Dade Behring). Free TFPI (f-TFPI) was determined by enzyme-linked immunosorbent assay (ELISA; Diagnostica Stago, Asnieres, France). Evaluation of coagulation factors and inhibitors yielded values within the reference ranges provided by the manufacturers.
Microvascular injury
Blood coagulation was evaluated at the site of microvascular injury using a Simplate II device (Organon Teknika, Durham, NC) as described previously.24,26 Blood oozing from a standardized bleeding-time wound was collected into heparinized capillary tubes (Kabe Labortechnik, Numbrecht-Elsenroth, Germany) every 60 seconds. Blood samples were then passed into an anticoagulant cocktail (50 mM EDTA, 20 mM benzamidine, 50 μM D-Val-Leu-Lys-chloromethylketone [Sigma-Aldrich, St Louis, MO] in 0.9% NaCl). In the supernatant samples, obtained after centrifugation at 4°C for 20 minutes, we determined: (1) TAT and sCD40L by ELISA; and (2) prothrombin activation, fVa light and heavy chain generation, fVa inactivation and fibrinogen cleavage by quantitative immunoblotting.3,26,31 Given interindividual variability of bleeding time values, the sampling interval was limited to the first 5 minutes.
Gel electrophoresis and Western blotting
Tris-HCl and HEPES were purchased from Sigma-Aldrich and Tween-20 from Baker (Phillipsburg, NJ). A burro monospecific polyclonal antibody that recognizes prothrombin, prethrombin 2, fragment 2, and thrombin B-chain was prepared at Mayo Clinic (Rochester, MN). Monoclonal antibodies against the Aα-chain of fibrinogen (α-Fbg2e), the fVa-HC (α-fVa-HC#17), and fVa-LC (α-fVa-LC#9) were previously described.10,31,32 Horseradish peroxidase–labeled secondary antibodies were purchased from Southern Biotech (Birmingham, AL). Molecular standards were from Invitrogen (Carlsbad, CA). Chemiluminescent substrate was purchased from NEN Life Science Products (Boston, MA).
Sodium dodecyl sulfate–polyacrylamide gel analysis (SDS-PAGE) immunoblotting and quantitation were performed as previously described8,24,26,31,33 with concentrations estimated from serial dilutions of purified standard proteins.
Statistical analysis
Data are expressed as the mean plus or minus SEM or as otherwise stated. The Kolmogorov-Smirnov test was used to determine normal distribution. Between groups, comparisons were done by Student unpaired t test for normally distributed data, otherwise by the Mann-Whitney U test and by the χ2 test for categorical data. Serial data were analyzed within groups by Friedman repeated measures ANOVA with subsequent post-hoc procedures for multiple comparisons. Spearman rank correlation coefficient was calculated to test associations between 2 variables. A P value less than .05 was considered statistically significant.
Results
The groups did not differ with regard to age, sex, coronary risk factors, including lipid profiles, or concomitant medication. Comparison of AMI to CAD showed elevated cardiac troponin I (5.23 ± 1.14 ng/mL) versus undetectable in the CAD patients. Fibrinogen (8.37 ± 3.5 vs 2.49 ± 2.2 mg/L; P = .011), CRP (3.67 ± 0.5 vs 2.72 ± 0.2 g/L; P = .005), and IL-6 (3.29 ± 1.22 vs 2.05 ± 0.72 pg/mL; P < .001) were higher in the AMI patients than stable subjects, respectively. AMI patients had elevated prothrombin (121 ± 3 vs 105 ± 4%; P = .002), fVIII (158 ± 6 vs 115 ± 6%; P < .001), f-TFPI (24.4 ± 2.7 vs 18.2 ± 3.3 ng/mL; P < .001), and lower AT (90 ± 2 vs 112 ± 3%; P = .006) and PC (95 ± 6 vs 111 ± 7%; P = .008) compared with stable CAD patients, respectively. TAT was higher in the AMI patients than in stable CAD subjects (6.93 ± 0.27 vs 3.19 ± 0.13 μg/L; P < .001).
Bleeding times were similar in AMI patients and those with stable CAD (398.8 ± 26.5 vs 409.2 ± 19.6 seconds; P = .3). The total volume of blood collected was similar in both groups (P = .4). Thus, analyses of analyte concentration and total amount, in both patient groups were similar. Therefore, the concentration-based analyses24–28 are presented in the current study.
Thrombin generation
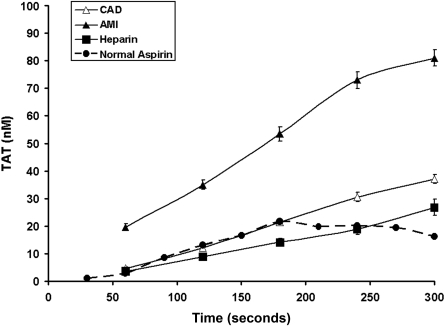
In both the AMI and CAD populations, no detectable initiation phase of TAT formation was seen in bleeding time samples, thus indicating that it is shorter than 60 seconds (the first sample) (Figure 1). TAT formation during the propagation phase proceeded at almost twice the rate (0.31 ± 0.03 nM/second) in AMI patients than that observed for CAD patients (0.16 ± 0.02 nM/second; P = .004). The TAT formation rate in CAD patients was similar to that seen in our historical data for healthy individuals taking aspirin24 (Figure 1, dashed line). The maximum TAT at 300 seconds was similarly increased in the AMI group (81.0 ± 2.5 vs 37.0 ± 1.6 nM; P < .0001). The maximum rate of TAT production correlated with plasma TAT levels only in stable CAD patients (r = 0.44, P = .031), whereas in the AMI patients similar associations were found for its maximum levels after injury (r = 0.43, P = .036). Troponin I levels, a measure of the extent of myocardial necrosis, were correlated with maximum velocity of TAT generation (r = 0.48, P = .03). In the AMI group, but not in stable CAD subjects, the maximum rate of TAT increase at the site of vascular injury was weakly associated with plasma IL-6 levels (r = 0.44, P = .04 and r = 0.41, P = .42, respectively), while the variations in TAT generation did not correlate with plasma CRP levels (P > .1).
Figure 1.
Time course formation of TAT complexes. The formation of TAT for CAD patients (▵, n = 28), AMI patients (▴, n = 28), and heparin-treated AMI (■, n = 8). Values are plotted as means plus or minus SEM for all subjects in each cohort. Historic data for healthy volunteers taking aspirin are also presented ( , ●).
, ●).
Analyses of potential correlations between coagulation factors and inhibitors versus variables of the microvascular injury model showed that in the AMI patients, the only significant negative associations were observed between maximum rate of TAT formation and AT levels (r = −0.45, P = .035) and PC levels (r = −0.49, P = .02). f-TFPI was correlated with TAT only at the first time point in AMI (r = −0.52, P = .008) and in stable CAD patients (r = −0.44, P = .03). Other coagulation factors or inhibitors showed no significant associations with parameters describing TAT generation in stable CAD patients (data not shown).
TAT formation was markedly reduced in heparin-treated patients (aPTT range, 70-220 seconds; Figure 1). The maximum velocity and TAT formation level were significantly lower (0.14 ± 0.02 nM/second; P < .0001, and 23.3 ± 1.4 nM; P < .001, respectively) in the heparin-treated individuals. These variables are similar to those obtained in subjects with stable CAD (maximum velocity: 0.16 ± 0.02 nM/second and TAT level: 37.0 ± 1.6 nM) and the historical experience with the healthy taking aspirin.
Platelet activation
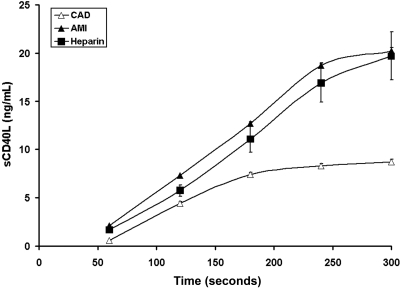
Platelet counts did not differ between the AMI and stable CAD patients (2.43 ± 0.42 vs 2.39 ± 0.31 × 108 platelets/mL; P = .7). Plasma sCD40L concentrations in peripheral blood were higher in the AMI patients (807.1 ± 60.3 vs 226.8 ± 10.5 pg/mL; P < .0001). The profiles of sCD40L release from platelets at the site of vascular injury (Figure 2) consisted of a phase of a rapid increase and a plateau phase seen in all subjects at the last minute, similar to a previous report.28 AMI patients had increased maximum rates of sCD40L release (0.091 ± 0.008 vs 0.063 ± 0.006 ng/mL/second; P < .0001) and maximum levels of sCD40L (20.16 ± 0.36 vs 8.74 ± 0.25 ng/mL; P < .0001) compared with stable CAD, respectively.
Figure 2.
Time course for sCD40L. The formation of sCD40L in stable CAD patients (▵, n = 28), AMI patients (▴, n = 28), and heparin-treated AMI (■, n = 8). Data are plotted as means plus or minus SEM.
In the AMI group, plasma sCD40L and sCD40L release after injury showed no association. Correlations existed between maximum rates and level of sCD40L release and the corresponding parameters for TAT formation in AMI (r = 0.49, P = .02, and r = 0.68, P = .004, respectively). In the stable CAD group, only the maximum velocities for sCD40L release and TAT formation were correlated (r = 0.44, P = .03). No associations between sCD40L release and inflammatory or myocardial necrosis markers, or coagulation factors/inhibitors were observed (all P > .1).
In patients with AMI on unfractionated heparin, sCD40L levels in shed blood and in plasma did not differ from those found in AMI patients (Figure 2), suggesting that the platelet activation seen in AMI is not thrombin-dependent.
Prothrombin activation after peripheral injury
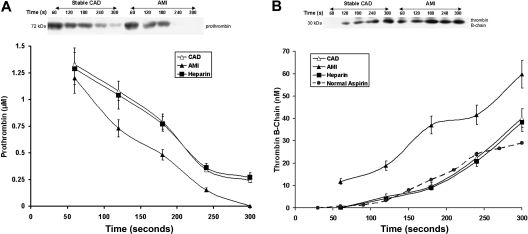
Prothrombin (Mr = 72 kDa, Figure 3A), was consumed after 180 to 240 seconds in the AMI patients, but still detectable by 300 seconds in 18 of 28 CAD subjects (P = .025). In the AMI patients, prothrombin disappeared at a peak rate of 0.0078 ± 0.001 μM/seconds, which was 28% higher than that observed in patients with stable CAD (P = .012). Initial prothrombin concentrations were similar in both groups (1.33 ± 0.07 vs 1.21 ± 0.06 μM; P = .3).
Figure 3.
Prothrombin consumption and thrombin formation. (A) Prothrombin activation in bleeding-time blood. A representative immunoblot of prothrombin on a nonreduced gel (5%-15%) from a patient with stable CAD and a patient with AMI. Prothrombin concentrations, determined by quantitative densitometry of immunoblots, are presented as a function of time (seconds) for all patients in each cohort; stable CAD (▵, n = 28), AMI (▴, n = 28), and heparin-treated AMI (■, n = 8). Data are plotted as means plus or minus SEM. (B) A representative reduced immunoblot (5%-15%) for thrombin B-chain formation in bleeding-time blood. Thrombin B-chain concentration is presented as a function of time (seconds) for patients with stable CAD (▵, n = 28), AMI (▴, n = 28), and heparin-treated AMI (■, n = 8). Data are plotted as means plus or minus SEM. Historic data for healthy individuals on aspirin (●,  ) are presented for comparison.
) are presented for comparison.
Thrombin B-chain formation (Figure 3B) was markedly accelerated in the AMI patients (P = .007) and approximately 10 nM thrombin was visible on immunoblots within the first 60 seconds after injury. This observation was unique to the AMI patients. The maximum rate of thrombin generation in AMI patients (0.33 ±0.04 nM/second) was higher by 57% compared with stable CAD patients (0.21 ± 0.03 nM/second, P < .001). Thrombin generation in CAD patients was similar to that observed previously for healthy individuals taking aspirin (dashed line).34 The maximum level of thrombin B-chain reported in bleeding-time blood was also higher in AMI (59.8 ± 6.2 vs 40.2 ± 1.6 nM; P < .001). These levels were associated with the maximum TAT levels in both groups (r = 0.64, P < .0001, and r = 0.59, P < .0001, respectively). There were weak associations between the rates of thrombin B-chain generation and of sCD40L release in both patient groups (r = 0.36, P = .044 and r = 0.41, P = .033, respectively). Positive correlations were observed between IL-6 levels and both the maximum velocities and levels of thrombin B-chain (r = 0.44, P = .02, and r = 0.53, P = .004, respectively) in the AMI group.
Heparin treatment in AMI suppressed prothrombin activation and thrombin formation to rates similar to those for stable CAD (Figure 3A,B). Thrombin B-chain generation in both the CAD before and after heparin treatment is not affected. Thrombin B-chain development in these individuals is similar to that observed for thrombin B-chain generation in previous studies of healthy individuals on aspirin (dashed line) with this model.24
Factor V activation and inactivation
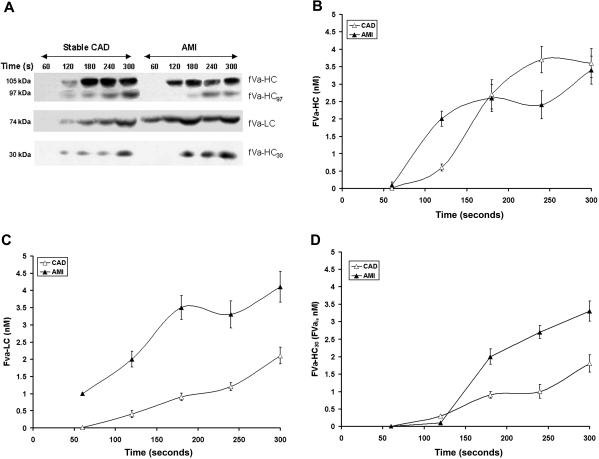
Factor V activation proceeds with the initial presentation of the heavy chain (cleavage at R709).7,35 FVa-HC (fVa-HC, Mr = 105 kDa, Figures 4A,B) appears in considerable amounts on average at 120 seconds in the AMI patients and slightly later in stable CAD patients, by approximately 60 seconds (P = .029). The fVa-HC97 fragment cleaved as a result of thrombin cleavage at Arg643 (fVai 643)33,36 was visualized in both groups approximately 60 seconds after fVa-HC (P < .001) in low amounts (∼ 0.6 nM) with levels increasing slowly over time (to ∼ 1.3 nM). The maximum concentration of fVai643 was 50% higher (P < .001) in stable CAD patients. Despite significantly higher thrombin formation in the AMI group, no association was observed between fVai643 and the rate of thrombin B-chain or TAT formation after vascular injury. The maximum rates of fVa-HC generation (0.020 ± 0.004 vs 0.017 ± 0.002 nM/second; P = .3, respectively) and the maximum concentrations of fVa-HC in bleeding-time blood (3.43 ± 0.36 nM vs 3.62 ± 0.42 nM; P = .7) were similar in both groups. In the AMI population, the maximum levels of fVa-HC showed associations with the maximum level of sCD40L (r = 0.5, P = .009), TAT (r = 0.44, P = .032), and thrombin (r = 0.4, P = .042).
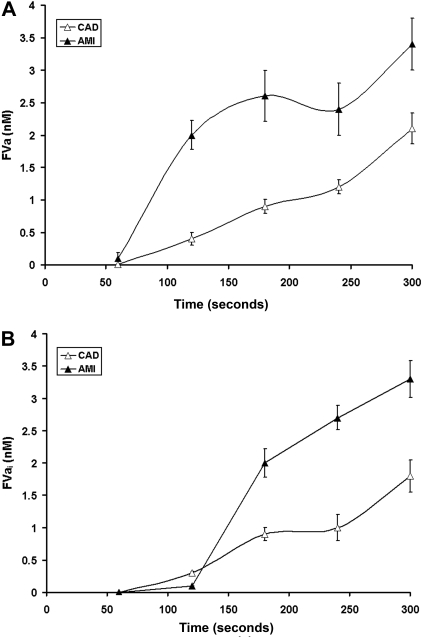
Figure 4.
Factor V activation and inactivation. (A) Representative immunoblots for fV cleavage products identified in consecutive 60-second bleeding time blood samples separated on a 5% to 15% linear gradient SDS-PAGE gel under reducing conditions from a stable CAD patient and a patient with AMI. The products identified include fVa-HC, fV-HC cleaved by thrombin (fVa-HC97) fVa-LC, and fVa-HC 30-kDa fragment produced by activated protein C. (B) FVa-HC production is presented as a function of time for the stable CAD patient population (▵) and the AMI patient population (▴). Data are plotted as the means plus or minus SEM. (C) FVa-LC production for the 2 patient populations are presented as a function of time. Data are plotted as mean plus or minus SEM for the AMI population (n = 28, ▴) and the CAD population (n = 28, ▵). (D) Time course of presentation of the 30-kDa fragment of fVa-HC is presented for the AMI cohort (▴) and the CAD cohort (▵). Each data point represents the entire cohort (n = 28) in each case and are plotted as the mean plus or minus SEM.
The formation of fVa-LC (fVa-LC, Mr = 74 kDa) and thus fVa activity could be detected on immunoblots within the first 60 seconds after injury in the AMI patients (Figure 4A,C). Detection of fVa-LC in the stable CAD population was not seen until 120 to 180 seconds (P = .013). The maximum rates of fVa-LC generation were increased in the AMI patients compared with stable subjects (0.025 ± 0.002 vs 0.014 ± 0.002 nM/second; P = .003; Figure 4C). Maximum concentrations of fVa-LC were also higher in the AMI patients (4.13 ± 0.41 vs 2.17 ± 0.24 nM; P < .001). As anticipated, correlations between the rate of fVa-LC generation and that of thrombin B-chain production were observed in both patient groups (r = 0.48, P = .014 and r = 0.51, P = .009, respectively).
Total inactivation of fVa after cleavage at Arg506 and Arg306 by APC (fVai306,506) is associated with a 30 kDa fVa-HC fragment (fVa-HC30, residues 307 to 506).7 The generation of this product of APC cleavage is greatly enhanced in AMI, an indication increased expression of the thrombin-thrombomodulin system either because of the enhanced thrombin availability, the enhanced expression of thrombomodulin and/or the endothelial protein C receptor (EPCR) or all of the above.
Interestingly, the maximum rate of the accumulation of the fVa 30-kDa fragment in the AMI patients was 76% greater than in CAD (0.018 ± 0.006 vs 0.075 ± 0.008 nM/second; P < .001; Figure 4D). The quantity of this fragment at the last sample was greater in the AMI patients (3.34 ± 0.29 vs 1.87 ± 0.25 nM; P < .001), indicating enhanced PC activation. The maximum level of fVai was correlated with the maximum amount of thrombin formation, in both AMI (r = 0.49, P = .011) and CAD (r = 0.47, P = .016). Similar correlations were observed for the maximum TAT levels in our model (r = 0.51, P = .006 and r = 0.53, P = .003, respectively). In heparin-treated AMI patients, the APC inactivation of fVa discerned by the appearance of the 30-kDa fragment was similar to that observed for stable CAD patients (data not shown).
The interplay between fVa heavy and light chain formation and fVa-HC inactivation by APC cleavage dictates the concentration of functional fVa. The fVa concentration based upon the limiting components (Figures 4B,C) is illustrated in Figure 5A and the concentrations of the inactive fVai (Figure 4D) in Figure 5B. Under normal circumstances, fVa activity is limited by formation of the light chain. As seen in Figure 4C in CAD, light chain generation is the limiting component in the expression of fVa (Figure 5A). In contrast, in AMI because of destruction of the heavy chain by APC, the fVa concentrations are limited by the heavy chain (Figure 5A). This is clearly a consequence of fVa inactivation (Figure 4D) illustrated by the increased formation of fVai (Figure 5B) as evidenced by the activation fragment of the heavy chain in AMI. The total fVa-HC generated corresponds to the sum of fVa-HC and 30-kDa fragment. Therefore, the total fVa-HC approaches 7 nM in AMI, similar to that produced in CAD (5 nM).
Figure 5.
Time course for fVa availability. Inferred levels of active fVa (A) and FVai (B) in AMI (▴) and CAD (▵) based upon the limiting (heavy or light) chain concentrations (Figure 4).
The patterns of fVa-HC and fVa-LC generation did not differ between heparin-treated AMI patients, those with stable CAD, and healthy taking aspirin24 (data not shown).
Fibrinogen consumption
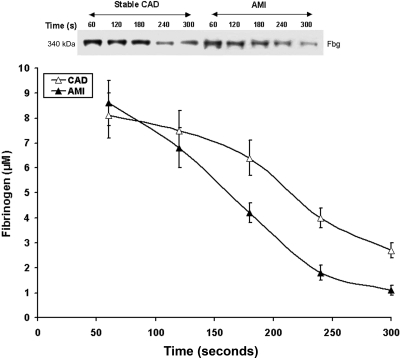
Fibrinogen consumption in bleeding time samples for both AMI and CAD patients is seen in Figure 6. Initial fibrinogen concentrations in the AMI and stable CAD groups were similar (8.6 ± 0.9 vs 8.1 ± 0.9 μM, respectively; P = .5); levels at 300 seconds were elevated in the stable CAD group (2.7 ± 0.3 vs 1.1 ± 0.2 μM, respectively; P < .001). The velocity of fibrinogen removal from shed blood was higher in the AMI patients than the stable CAD subjects (0.041 ± 0.004 vs 0.030 ± 0.004 μM/second, P = .02, respectively; Figure 6). In both CAD and AMI patients there was a correlation between the rate of fibrinogen consumption and thrombin production (r = 0.49, P = .03 and r = 0.53; P = .004, respectively) and IL-6 (r = 0.38, P = .04 and r = 0.39, P = .04, respectively).
Figure 6.
Fibrinogen consumption. Representative nonreduced immunoblot (5%-15%) of fibrinogen from consecutive 60-second bleeding-time blood samples for a stable CAD patient and a patient with AMI. Fibrinogen concentrations were determined by densitometry of immunoblots as described in “Fibrinogen consumption.” Data for all CAD patients (n = 28, ▵) and AMI patients (n = 28, ▴) are plotted as the means plus or minus SEM.
In heparin-treated AMI patients, the pattern of fibrinogen consumption was similar to that observed for stable CAD patients (data not shown).
Discussion
This study shows enhanced coagulant reactions induced by vascular injury in the peripheral circulation in patients with AMI as evidenced by accelerated systemic thrombin generation, enhanced fibrinogen proteolysis, and fV activation/inactivation, as well as markedly increased release of sCD40L from platelets and higher IL-6 levels compared with well-matched patients with stable CAD. Qualitative and quantitative alterations in the kinetics of coagulant reactions observed after an occlusive thrombotic event in the coronary circulation, for instance, AMI suggests that systemic coagulation pathways are hyperreactive or stimulated and additional injury is a potent trigger resulting in markedly increased thrombin formation and platelet activation. Previous studies on blood coagulation at sites of hemostatic plug formation have been limited to the evaluation of healthy subjects,24 patients at high risk of CAD,26 and patients with stable angina.29 Importantly, all the findings refer to subjects on aspirin treatment, suggesting that thrombin formation and thrombin-mediated reactions may be even faster in CAD patients not receiving aspirin.37 Given the fact that patients with a recent MI have an increased risk of recurrent coronary events,12 detailed information on the kinetics of systemic coagulant reactions and platelet activation in peripheral circulation, along with their determinants, in patients with activated coagulation associated with ACS might help explain the pathophysiology of this clinical observation and shed some light on the relationship between a local thrombotic event and systemic inflammatory activation.
Major differences between patients during the acute phase of MI and those with stable CAD patients are as follows: (1) TAT formation triggered by vascular injury is almost 2-fold faster in ACS patients than in stable CAD subjects. This acceleration appears to be related to platelet activation and the extent of myocardial necrosis. Importantly, the velocity of TAT or thrombin B-chain formation at the site of injury cannot be predicted by TAT levels in plasma of peripheral venous blood in the AMI patients; (2) FVa generation is faster (by ∼ 30%) in the AMI patients as evidenced by fVa-LC formation, ordinarily the limiting component in fVa generation,7 as also shown in the Simplate model.24 Generation of fVa in our model suddenly accelerates at 2 minutes in the AMI patients. This is probably a consequence of massive thrombin formation and release of platelet-derived fVa locally. Twenty percent of fV is stored in platelet α-granules and is partially activated.38 This pool of fV39 is 2- to 3-fold more resistant to APC and contributes to sustained activation of coagulation with platelets deposited at the site of injury.40,41 The inferred contribution of platelet-derived fVa to the accumulation of fVa-HC visualized on immunoblots is supported by the correlation between its estimated concentration and that found for the platelet activation marker, sCD40L. (3) The lack of intergroup differences in fVa-HC generation at the site of vascular injury is explained by the nearly simultaneous increase in the rate of degradation of fVa-HC associated with enhanced PC activation in AMI patients. Moreover, AMI results in markedly accelerated (by 76%) APC release of the 30-kDa fragment (R307-506) of fVa-HC with concentrations at 6 minutes approaching that of intact fVa-HC. One might suspect that increased thrombin generation in AMI will lead to earlier appearance of the 30-kDa fragment of APC-mediated fVa degradation, as thrombin in complex with thrombomodulin is abundant in the capillary bed, to efficiently activate protein C.42 In addition, platelet factor 4 released from aggregated platelets has been shown to increase APC activity.43 Also, peripheral vascular endothelial cells have been reported to provide the surface on which APC cleaves fVa more efficiently compared with the reaction on the platelet surface.44 These findings might at least in part account for increased amounts of the 30-kDa fragment of fVa degraded by APC upon vascular injury in ACS patients compared with results in stable subjects. (4) The detection of the 97-kDa fragment of fVa-HC, a product of fVa proteolysis by thrombin in the presence of endothelial cells and/or platelets,33,45 is delayed in AMI relative to CAD. The presence of the 97-kDa fragment was previously detected in asymptomatic individuals24,26 and CAD patients.29 Unexpectedly, despite increased thrombin, formation of the 97-kDa fragment is reduced in AMI. It might be hypothesized that in a state of increased thrombin generation, for example, AMI, thrombin binds preferentially to thrombomodulin, accelerating APC-catalyzed inactivation of fVa as shown in this study and thus alternative pathway of fVa degradation through the 97-kDa fragment formation is less pronounced compared with stable CAD because of depletion of the fVa-HC substrate. (5) The fibrinogen consumption observed in the ACS patients was increased by approximately 30% compared with stable patients, though the magnitude of this intergroup difference was smaller that than found for thrombin generation. Mechanisms underlying this observation may involve enhanced fibrinogen binding to platelet-derived thrombospondin46 and endothelial cells via thrombin-mediated process in the AMI patients,47 leading to smaller amounts of this protein in the fluid phase. (6) A small difference in the maximum rates of prothrombin consumption at the site of injury observed in ACS patients compared with stable CAD subjects could also be related to increased binding to the exposed endothelium and activated platelets.48
The model of peripheral microvascular injury is a valuable tool to assess the magnitude of platelet activation both by measuring the expression of activation markers by flow cytometry49 and release of various proteins mostly derived from platelet α-granules, such as β-thromboglobulin,30 vascular endothelial growth factor (VEGF),28,50 platelet-derived growth factor (PDGF),28 and sCD40L.28,51 We used sCD40L as a platelet activation marker because more than 95% of circulating sCD40L is platelet derived.52 As expected, the peak rate of sCD40L release and maximum sCD40L levels in bleeding-time blood were markedly higher in ACS than in stable subjects. However, the kinetics of sCD40L release at sites of vascular injury cannot be predicted by sCD40L levels in venous blood in either group, suggesting the major contribution is by local mechanisms probably identical to those enhancing thrombin formation and platelet activation upon injury.
This study is the first to assess the impact of coagulation factors (except fXI and fXII) and coagulation inhibitors such as f-TFPI, AT, and PC on the kinetics of coagulant reactions in the Simplate model. We found that only AT and PC correlated with the rate of bleeding time blood thrombin production. These close associations might help explain clinical observations showing that low normal levels of these 2 inhibitors increase the risk of recurrent cardiovascular events in patients with ACS.53 Previously, we have shown in this patient population that simulated Tf-initiated thrombin generation based upon factor composition can discriminate between acute and stable CAD.22 We have also demonstrated that these ACS and CAD MI patients have circulating fXIa that correlates with markers of coagulation and inflammation.54
It is known that the half-life of thrombin in plasma is 10 to 15 se-conds largely through the inhibitory properties of AT enhanced by glycosaminoglycan cofactors.55 Butenas et al56 showed that prothrombin and AT in the normal ranges (50%-150%) were major determinants in thrombin formation in a synthetic plasma model. Using a cell-based model of coagulation, Allen et al57 showed that almost all coagulation factors (range, 0%-200% of pooled plasma levels) affect the rate of thrombin production. A correlation of thrombin formation with APC, which is in keeping with studies using other models,10 further corroborates the concept that at least in small vessels anticoagulant proteins are crucial determinants of hemostatic balance controlling potent triggers such as Tf and collagen.55 The negative correlation between f-TFPI and the 60-second TAT level, which in CAD patients at the site of injury, may be considered as a substitute of the time of a lacking lag phase that is determined by the activity of the Tf-fVIIa complex,58 confirms that TFPI plays a crucial role in initiating and shutting down the Tf-mediated coagulant reactions.11 As expected, f-TFPI had no effect on the subsequent rate of thrombin formation.59,60 Importantly, our findings suggest that in contrast to several in vitro coagulation models,56,57 the microvascular injury model, with vascular injury in vivo, is, to a large extent, determined by cellular factors exposed and/or produced in loco, such as Tf expression.25 This supports the concept of a major contribution of local mechanisms in the maintenance of hemostatic balance upon vascular damage. Pathak et al61 reported that thrombin generation occurs in the absence of platelets on the surface of vascular tissue of the arteries. Upon arterial injury, regulation of thrombin generation is most likely controlled by Tf expression on cell surfaces, however significant correlations are observed between the kinetics of thrombin formation and platelet activation in ACS patients. This confirms the importance of platelets in thrombin generation at sites of microvascular injury.
An interesting finding is the observation that IL-6, a major proinflammatory cytokine, is positively correlated with kinetic parameters of thrombin formation in the ACS patients in our model. No such associations were found for CRP, which is in contrast to previous observations in stable CAD patients treated with the cholesterol-lowering agent, simvastatin, which also reduces CRP.29 Our findings provide additional evidence for bidirectional links between inflammation and coagulation at several stages in CAD patients, including those with ACS, as previously suggested.62
Unsatisfactory dampening of thrombin formation in ACS patients treated with intravenous heparin was demonstrated based on plasma levels of thrombin markers, indicating indirectly the potency of the damaged coronary plaque as a trigger of blood coagulation. Our current findings (Figures 1,3B) suggest that heparin administration in doses that prolonged aPTT to desirable levels decreases thrombin generation only to the values close to those observed in stable CAD patients in terms of both the velocity of the process and the maximum level measured at the site of microvascular injury. These findings are in line with the previous study on healthy volunteers.63 It is worth noting that low-molecular-weight heparin suppresses thrombin generation similarly to unfractionated heparin,63 suggesting that our observations may be extrapolated to ACS patients receiving the former.
The number of the patients enrolled in this study was limited; however, patient selection resulted in a reasonably homogenous population, which together with well-matched controls, mitigate against significant recruitment bias. Further, patients with unstable angina, who represent a large percentage of ACS patients, were not participants in this study.
The description of complex prothrombotic processes at the platelet-vessel interface preceded by the vascular injury model provides insights into the effect of arterial thrombosis on the systemic hemostatic balance. It might be speculated that a systemic inflammatory state associated with activated clotting pathways may activate local mechanism leading to atherosclerotic plaque instability and rupture and thus to ACS.
Acknowledgments
This work was supported by a grant of the Polish Ministry of Science no. 2 P05B 094 29 (A.U.) and by Program Project Grant no. HL46703 (Project 1 and Core B) and R01 no. HL34575 from the National Institutes of Health (K.G.M.).
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.U. designed the research protocol, conducted patient experiments, performed research, analyzed the data, and wrote the paper; K.S. performed the human research protocols; K.E.B.-Z. contributed analytical tools, performed laboratory research, and contributed to writing the paper; W.T. performed the human research protocols; K.Z. performed the human research protocols; and K.G.M. analyzed data and contributed to writing the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kenneth G. Mann, Department of Biochemistry, 208 S Park Drive, Suite 2, University of Vermont, Burlington, VT 05446; e-mail: kenneth.mann@uvm.edu.
References
- 1.Mann KG, Butenas S, Brummel KE. The dynamics of thrombin formation. Arterioscler Thromb Vasc Biol. 2003;23:17–25. doi: 10.1161/01.atv.0000046238.23903.fc. [DOI] [PubMed] [Google Scholar]
- 2.Orfeo T, Brufatto N, Nesheim ME, et al. The factor V activation paradox. J Biol Chem. 2004;279:19580–19591. doi: 10.1074/jbc.M400727200. [DOI] [PubMed] [Google Scholar]
- 3.Brummel KE, Paradis SG, Butenas S, Mann KG. Thrombin functions during tissue factor-induced blood coagulation. Blood. 2002;100:148–152. doi: 10.1182/blood.v100.1.148. [DOI] [PubMed] [Google Scholar]
- 4.Orfeo T, Butenas S, Brummel-Ziedins KE, Mann KG. The tissue factor requirement in blood coagulation. J Biol Chem. 2005;280:42887–42896. doi: 10.1074/jbc.M505506200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mann KG. Thrombin can't live without it; probably die from it. Chest. 2003;124:1S–3S. doi: 10.1378/chest.124.3_suppl.1s. [DOI] [PubMed] [Google Scholar]
- 6.Esmon CT. The protein C pathway. Chest. 2003;124:26S–32S. doi: 10.1378/chest.124.3_suppl.26s. [DOI] [PubMed] [Google Scholar]
- 7.Mann KG, Kalafatis M. Factor V: a combination of Dr Jekyll and Mr Hyde. Blood. 2003;101:20–30. doi: 10.1182/blood-2002-01-0290. [DOI] [PubMed] [Google Scholar]
- 8.Kalafatis M, Rand MD, Mann KG. The mechanism of inactivation of human factor V and human factor Va by activated protein C. J Biol Chem. 1994;269:31869–31880. [PubMed] [Google Scholar]
- 9.Mann KG, Hockin MF, Begin KJ, Kalafatis M. Activated protein C cleavage of factor Va leads to dissociation of the A2 domain. J Biol Chem. 1997;272:20678–20683. doi: 10.1074/jbc.272.33.20678. [DOI] [PubMed] [Google Scholar]
- 10.van 't Veer C, Golden NJ, Kalafatis M, Mann KG. Inhibitory mechanism of the protein C pathway on tissue factor-induced thrombin generation: synergistic effect in combination with tissue factor pathway inhibitor. J Biol Chem. 1997;272:7983–7994. doi: 10.1074/jbc.272.12.7983. [DOI] [PubMed] [Google Scholar]
- 11.Mackman N. Role of tissue factor in hemostasis, thrombosis, and vascular development. Arterioscler Thromb Vasc Biol. 2004;24:1015–1022. doi: 10.1161/01.ATV.0000130465.23430.74. [DOI] [PubMed] [Google Scholar]
- 12.Fuster V, Moreno PR, Fayad ZA, Corti R, Badimon JJ. Atherothrombosis and high-risk plaque: part I: evolving concepts. J Am Coll Cardiol. 2005;46:937–954. doi: 10.1016/j.jacc.2005.03.074. [DOI] [PubMed] [Google Scholar]
- 13.Merlini PA, Ardissino D, Oltrona L, et al. Heightened thrombin formation but normal plasma levels of activated factor VII in patients with acute coronary syndromes. Arterioscler Thromb Vasc Biol. 1995;15:1675–1679. doi: 10.1161/01.atv.15.10.1675. [DOI] [PubMed] [Google Scholar]
- 14.Hoffmeister HM, Ehlers R, Buttcher E, et al. Relationship between minor myocardial damage and inflammatory acute-phase reaction in acute coronary syndromes. J Thromb Thrombolysis. 2003;15:33–39. doi: 10.1023/a:1026140317777. [DOI] [PubMed] [Google Scholar]
- 15.Merlini PA, Bauer KA, Oltrona L, et al. Persistent activation of coagulation mechanism in unstable angina and myocardial infarction. Circulation. 1994;90:61–68. doi: 10.1161/01.cir.90.1.61. [DOI] [PubMed] [Google Scholar]
- 16.Hoffmeister HM, Jur M, Wendel HP, Heller W, Seipel L. Alterations of coagulation and fibrinolytic and kallikrein-kinin systems in the acute and postacute phases in patients with unstable angina pectoris. Circulation. 1995;91:2520–2527. doi: 10.1161/01.cir.91.10.2520. [DOI] [PubMed] [Google Scholar]
- 17.Golino P, Ravera A, Ragni M, et al. Involvement of tissue factor pathway inhibitor in the coronary circulation of patients with acute coronary syndromes. Circulation. 2003;108:2864–2869. doi: 10.1161/01.CIR.0000105900.21445.3D. [DOI] [PubMed] [Google Scholar]
- 18.Li YH, Teng JK, Tsai WC, et al. Prognostic significance of elevated hemostatic markers in patients with acute myocardial infarction. J Am Coll Cardiol. 1999;33:1543–1548. doi: 10.1016/s0735-1097(99)00081-9. [DOI] [PubMed] [Google Scholar]
- 19.Merlini PA, Ardissino D, Bauer KA, et al. Persistent thrombin generation during heparin therapy in patients with acute coronary syndromes. Arterioscler Thromb Vasc Biol. 1997;17:1325–1330. doi: 10.1161/01.atv.17.7.1325. [DOI] [PubMed] [Google Scholar]
- 20.Vaziri ND, Kennedy SC, Kennedy D, Gonzales E. Coagulation, fibrinolytic, and inhibitory proteins in acute myocardial infarction and angina pectoris. Am J Med. 1992;93:651–657. doi: 10.1016/0002-9343(92)90198-k. [DOI] [PubMed] [Google Scholar]
- 21.Cucuianu MP, Cristea A, Roman S, et al. Comparative behaviour of the components of the factor VIII complex in acute myocardial infarction. Thromb Res. 1983;30:487–497. doi: 10.1016/0049-3848(83)90183-4. [DOI] [PubMed] [Google Scholar]
- 22.Brummel-Ziedins K, Undas A, Orfeo T, et al. Thrombin generation in acute coronary syndrome and stable coronary artery disease: dependence on plasma factor composition. J Thromb Haemost. 2008;6:104–110. doi: 10.1111/j.1538-7836.2007.02799.x. [DOI] [PubMed] [Google Scholar]
- 23.Morange PE, Blankenberg S, Alessi MC, et al. Prognostic value of plasma tissue factor and tissue factor pathway inhibitor for cardiovascular death in patients with coronary artery disease: the AtheroGene study. J Thromb Haemost. 2007;5:475–482. doi: 10.1111/j.1538-7836.2007.02372.x. [DOI] [PubMed] [Google Scholar]
- 24.Undas A, Brummel K, Musial J, Mann KG, Szczeklik A. Blood coagulation at the site of microvascular injury: effects of low-dose aspirin. Blood. 2001;98:2423–2431. doi: 10.1182/blood.v98.8.2423. [DOI] [PubMed] [Google Scholar]
- 25.Weiss HJ, Lages B. Evidence for tissue factor-dependent activation of the classic extrinsic coagulation mechanism in blood obtained from bleeding time wounds. Blood. 1988;71:629–635. [PubMed] [Google Scholar]
- 26.Undas A, Brummel KE, Musial J, Mann KG, Szczeklik A. Simvastatin depresses blood clotting by inhibiting activation of prothrombin, factor V, and factor XIII and by enhancing factor Va inactivation. Circulation. 2001;103:2248–2253. doi: 10.1161/01.cir.103.18.2248. [DOI] [PubMed] [Google Scholar]
- 27.Undas A, Brummel-Ziedins KE, Potaczek D, et al. Atorvastatin and quinapril inhibit blood coagulation in patients with coronary artery disease following a 28-day therapy. J Thromb Haemost. 2006;4:2397–2404. doi: 10.1111/j.1538-7836.2006.02165.x. [DOI] [PubMed] [Google Scholar]
- 28.Undas A, Celinska-Lowenhoff M, Stepien E, et al. Effects of simvastatin on angiogenic growth factors released at the site of microvascular injury. Thromb Haemost. 2006;95:1045–1047. doi: 10.1160/TH06-01-0022. [DOI] [PubMed] [Google Scholar]
- 29.Undas A, Celinska-Lowenhoff M, Brummel-Ziedins KE, et al. Simvastatin given for 3 days can inhibit thrombin generation and activation of factor V and enhance factor Va inactivation in hypercholesterolemic patients. Arterioscler Thromb Vasc Biol. 2005;25:1524–1525. doi: 10.1161/01.ATV.0000168913.25278.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Undas A, Celinska-Lowenhoff M, Domagala TB, et al. Early antithrombotic and anti-inflammatory effects of simvastatin versus fenofibrate in patients with hypercholesterolemia. Thromb Haemost. 2005;94:193–199. doi: 10.1160/TH05-01-0067. [DOI] [PubMed] [Google Scholar]
- 31.Rand MD, Lock JB, van 't Veer C, Gaffney DP, Mann KG. Blood clotting in minimally altered whole blood. Blood. 1996;88:3432–3445. [PubMed] [Google Scholar]
- 32.Foster WB, Tucker MM, Katzmann JA, et al. Monoclonal antibodies to human coagulation factor V and factor Va. Blood. 1983;61:1060–1067. [PubMed] [Google Scholar]
- 33.Hockin MF, Kalafatis M, Shatos M, Mann KG. Protein C activation and factor Va inactivation on human umbilical vein endothelial cells. Arterioscler Thromb Vasc Biol. 1997;17:2765–2775. doi: 10.1161/01.atv.17.11.2765. [DOI] [PubMed] [Google Scholar]
- 34.Undas A, Brummel K, Musial J, Mann KG, Szczeklik A. Pl(A2) polymorphism of beta(3) integrins is associated with enhanced thrombin generation and impaired antithrombotic action of aspirin at the site of microvascular injury. Circulation. 2001;104:2666–2672. doi: 10.1161/hc4701.099787. [DOI] [PubMed] [Google Scholar]
- 35.Nesheim ME, Mann KG. Thrombin-catalyzed activation of single chain bovine factor V. J Biol Chem. 1979;254:1326–1334. [PubMed] [Google Scholar]
- 36.Erdogan E, Bukys MA, Orfeo T, Mann KG, Kalafatis M. Identification of an inactivating cleavage site for thrombin on the heavy chain of factor Va. Thromb Haemost. 2007;98:998–1006. [PubMed] [Google Scholar]
- 37.Undas A, Brummel-Ziedins KE, Mann KG. Antithrombotic properties of aspirin and resistance to aspirin: beyond strictly antiplatelet actions. Blood. 2007;109:2285–2292. doi: 10.1182/blood-2006-01-010645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Janeway CM, Rivard GE, Tracy PB, Mann KG. Factor V Quebec revisited. Blood. 1996;87:3571–3578. [PubMed] [Google Scholar]
- 39.Gould WR, Silveira JR, Tracy PB. Unique in vivo modifications of coagulation factor V produce a physically and functionally distinct platelet-derived cofactor: characterization of purified platelet-derived factor V/Va. J Biol Chem. 2004;279:2383–2393. doi: 10.1074/jbc.M308600200. [DOI] [PubMed] [Google Scholar]
- 40.Camire RM, Kalafatis M, Simioni P, Girolami A, Tracy PB. Platelet-derived factor Va/Va Leiden cofactor activities are sustained on the surface of activated platelets despite the presence of activated protein C. Blood. 1998;91:2818–2829. [PubMed] [Google Scholar]
- 41.Camire RM, Kalafatis M, Cushman M, et al. The mechanism of inactivation of human platelet factor Va from normal and activated protein C-resistant individuals. J Biol Chem. 1995;270:20794–20800. doi: 10.1074/jbc.270.35.20794. [DOI] [PubMed] [Google Scholar]
- 42.Esmon CT. Molecular events that control the protein C anticoagulant pathway. Thromb Haemost. 1993;70:29–35. [PubMed] [Google Scholar]
- 43.Slungaard A, Fernandez JA, Griffin JH, et al. Platelet factor 4 enhances generation of activated protein C in vitro and in vivo. Blood. 2003;102:146–151. doi: 10.1182/blood-2002-11-3529. [DOI] [PubMed] [Google Scholar]
- 44.Oliver JA, Monroe DM, Church FC, Roberts HR, Hoffman M. Activated protein C cleaves factor Va more efficiently on endothelium than on platelet surfaces. Blood. 2002;100:539–546. doi: 10.1182/blood.v100.2.539. [DOI] [PubMed] [Google Scholar]
- 45.Tracy PB, Nesheim ME, Mann KG. Proteolytic alterations of factor Va bound to platelets. J Biol Chem. 1983;258:662–669. [PubMed] [Google Scholar]
- 46.Leung LL, Nachman RL. Complex formation of platelet thrombospondin with fibrinogen. J Clin Invest. 1982;70:542–549. doi: 10.1172/JCI110646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hatton MW, Moar SL, Richardson M. Enhanced binding of fibrinogen by the subendothelium after treatment of the rabbit aorta with thrombin. J Lab Clin Med. 1990;115:356–364. [PubMed] [Google Scholar]
- 48.Byzova TV, Plow EF. Networking in the hemostatic system: integrin alphaiibbeta3 binds prothrombin and influences its activation. J Biol Chem. 1997;272:27183–27188. doi: 10.1074/jbc.272.43.27183. [DOI] [PubMed] [Google Scholar]
- 49.Abrams CS, Ellison N, Budzynski AZ, Shattil SJ. Direct detection of activated platelets and platelet-derived microparticles in humans. Blood. 1990;75:128–138. [PubMed] [Google Scholar]
- 50.Weltermann A, Wolzt M, Petersmann K, et al. Large amounts of vascular endothelial growth factor at the site of hemostatic plug formation in vivo. Arterioscler Thromb Vasc Biol. 1999;19:1757–1760. doi: 10.1161/01.atv.19.7.1757. [DOI] [PubMed] [Google Scholar]
- 51.Stepien E, Szuldrzynski K, Branicka A, et al. The thrombin generation is associated with the PIA1/A2 beta3, integrin polymorphism in aspirin-treated patients with coronary artery disease: a role of statins. Pol Arch Med Wewn. 2007;117:33–40. [PubMed] [Google Scholar]
- 52.Anand SX, Viles-Gonzalez JF, Badimon JJ, Cavusoglu E, Marmur JD. Membrane-associated CD40L and sCD40L in atherothrombotic disease. Thromb Haemost. 2003;90:377–384. doi: 10.1160/TH03-05-0268. [DOI] [PubMed] [Google Scholar]
- 53.Pelkonen KM, Wartiovaara-Kautto U, Nieminen MS, Ahonen K, Sinisalo J. Low normal level of protein C or of antithrombin increases risk for recurrent cardiovascular events. Blood Coagul Fibrinolysis. 2005;16:275–280. doi: 10.1097/01.mbc.0000169220.00679.13. [DOI] [PubMed] [Google Scholar]
- 54.Butenas S, Undas A, Gissel MT, et al. Factor XIa and tissue factor activity in patients with coronary artery disease. Thromb Haemost. 2008;99:142–149. doi: 10.1160/TH07-08-0499. [DOI] [PubMed] [Google Scholar]
- 55.Gomez K, McVey JH, Tuddenham E. Inhibition of coagulation by macromolecular complexes. Haematologica. 2005;90:1570–1576. [PubMed] [Google Scholar]
- 56.Butenas S, van't Veer C, Mann KG. “Normal” thrombin generation. Blood. 1999;94:2169–2178. [PubMed] [Google Scholar]
- 57.Allen GA, Wolberg AS, Oliver JA, et al. Impact of procoagulant concentration on rate, peak and total thrombin generation in a model system. J Thromb Haemost. 2004;2:402–413. doi: 10.1111/j.1538-7933.2003.00617.x. [DOI] [PubMed] [Google Scholar]
- 58.Butenas S, van 't Veer C, Mann KG. Evaluation of the initiation phase of blood coagulation using ultrasensitive assays for serine proteases. J Biol Chem. 1997;272:21527–21533. doi: 10.1074/jbc.272.34.21527. [DOI] [PubMed] [Google Scholar]
- 59.van 't Veer C, Mann KG. Regulation of tissue factor initiated thrombin generation by the stoichiometric inhibitors tissue factor pathway inhibitor, antithrombin-III, and heparin cofactor-II. J Biol Chem. 1997;272:4367–4377. doi: 10.1074/jbc.272.7.4367. [DOI] [PubMed] [Google Scholar]
- 60.Hockin MF, Jones KC, Everse SJ, Mann KG. A model for the stoichiometric regulation of blood coagulation. J Biol Chem. 2002;277:18322–18333. doi: 10.1074/jbc.M201173200. [DOI] [PubMed] [Google Scholar]
- 61.Pathak A, Zhao R, Monroe DM, et al. Thrombin generation in vascular tissue. J Thromb Haemost. 2006;4:60–67. doi: 10.1111/j.1538-7836.2005.01630.x. [DOI] [PubMed] [Google Scholar]
- 62.Esmon CT. The interactions between inflammation and coagulation. Br J Haematol. 2005;131:417–430. doi: 10.1111/j.1365-2141.2005.05753.x. [DOI] [PubMed] [Google Scholar]
- 63.Eichinger S, Wolz M, Nieszpaur-Los M, et al. Effects of a low molecular weight heparin (Fragmin) and of unfractionated heparin on coagulation activation at the site of plug formation in vivo. Thromb Haemost. 1994;72:831–835. [PubMed] [Google Scholar]