Abstract
Dynamic nuclear polarization is combined with temperature jump methods to develop a new 2D 13C- 13C NMR experiment that yields a factor or 100-170 increase insensitivity. The polaization step is performed at ∼100 K and the sample is subsequently melted with a 10.6 mm laser pulse to yield a sample with highly polarized 13C spins. 13C detected 2D 13C- 13C spectroscopy is performed in the usual manner.
Over the last decade there has been a resurgence of interest in the application of dynamic nuclear polarization (DNP) techniques directed at increasing the sensitivity in nuclear magnetic resonance (NMR) experiments 1-7. The interest is driven by the possibility of enhancing signal intensities in NMR spectra by factors of 102 - 103 which historical precedent suggests will open many new avenues of research. The experiments were initially directed at signal enhancement in magic angle spinning (MAS) spectra of solids4, 8-15, and, since they were performed at magnetic fields ≥ 5T, they required development of gyrotron microwave sources operating in the range ≥140 GHz for irradiation of the EPR spectrum of the exogenous paramagnetic polarizing agent 4, 13, 16, 17. There is also considerable interest in applying these techniques to high field solution-state NMR experiments, and, recently there have been three efforts aimed at achieving this goal. The first relied on scalar relaxation, a mechanism that is operative at high fields, but is, unfortunately, only applicable in special cases 18. The second is the “dissolution experiment” where the solid sample is polarized at low field, dissolved with excess superheated solvent, and then transferred to a second spectrometer for observation of the NMR spectrum 19, 20. Dissolution DNP was recently integrated with the single scan multi-dimensional NMR technique to obtain two-dimensional (2D) 15N-1H correlation spectra 21. In the third approach, we demonstrated the possibility of performing the polarization and spectroscopy in the same spectrometer, the latter being achieved by melting the sample with CO2 laser irradiation 5. Our approach has the advantage of circumventing the polarization loss associated with shuttling the sample to another spectrometer. Thus, we refer to this experiment as in situ temperature jump DNP (TJ-DNP). Further, and in contrast to the dissolution experiment, the TJ-DNP experiment can be recycled and is therefore suitable for the acquisition of multiple scans of comparable intensity. Here we demonstrate that it is possible to incorporate the extensive repertoire of multidimensional solution-state NMR experiments into this scheme.
Figure 1 shows the 13C NMR spectrum of an 800 mM [13C6,2H7,]-glucose solution obtained with and without TJ-DNP. The enhancement, ε† is ∼ 170 for C1, ∼140 for C2-5, and ∼100 for C6. We are exploring approaches to make the intensities more quantitative, for example, by improving the rate of melting. Note also that ε†=ε(Tobs/T∞wave) includes a factor accounting for the increased Boltzmann population due to the lower temperature, where ε is the enhancement at temperature where polarization occurs, T∞wave (in this case 100 K), and Tobs is the temperature where the liquid state spectra are recorded 5. Incorporation of the TJ-DNP experiment into a multidimensional solution experiment is illustrated in Figure 2, and is accomplished by inserting an evolution and mixing period into the sequence following the melting step. In the case depicted here, we employ TJ-DNP together with a TOCSY mixing sequence to record a 13C-13C correlation spectrum 23. The resulting 2D spectrum is shown in Figure 3, and immediately obvious is the fact that the presence of the paramagnetic polarizing agent does not lead to severe broadening of the NMR signals. This is best seen in the expansion of the 13C1 region that clearly shows the one-bond 13C-13C scalar coupling constants present in the α and β forms of glucose.
Figure 1.
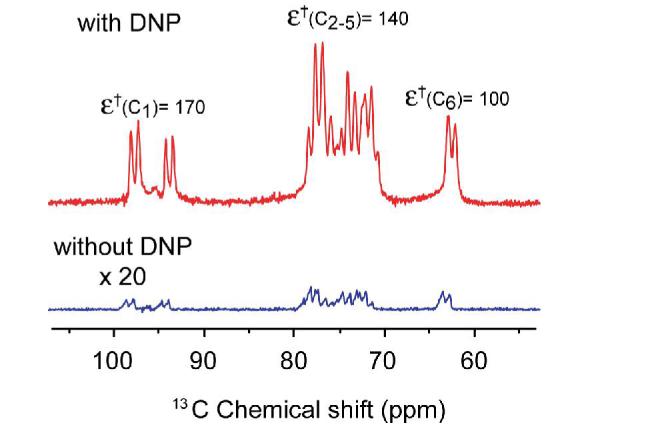
13C NMR spectra of 800 mM 2H7, 13C6 glucose solution prepared with 2H6-DMSO/2H2O/H2O = 5/4/1 and 5 mM TOTAPOL, with (top) and without (bottom) TJ-DNP. For TJ-DNP, 140 GHz microwave irradiation was applied for 30 s at 100 K. A 0.8 s CO2 laser pulse was used to melt the sample. The room temperature spectrum without DNP (bottom) was scaled up by 20 for ease of comparison. The enhancement was ∼ 170 for C1 (both α and β forms), ∼140 for C2-5, and ∼100 for C6. TJDNP spectrum was recorded with a single scan, and the room temperature spectrum without DNP is the result of 2048 scans. The polarizing agent TOTAPOL [1-(2,2,6,6,-Tetramethyl-1-oxyl-4-piperidinyl)oxy-3-(2,2,6,6-tetramethyl-1-oxy-4-oioeridinyl)amino-propan-2-ol] 22] supports the cross effect mechanism 12, 14, 15. The concentration of TOTAPOL was 5 mM. All experiments were conducted on a 5 T (211 MHz for 1H, 53.31 MHz for 13C, and 32 MHz for 2H) magnet with a home built multi-channel DNP NMR probe. An 8 - 10 ∞L sample was placed in a 2.5 mm o.d. quartz rotor. The temperature was maintained below 100 K by circulating cold N2 gas. Samples were spun at 200-800 Hz to rotationally average the unidirectional microwave and laser irradiation. 140 GHz microwaves were produced by a gyrotron. For a sample with 5 mM TOTAPOL, the enhanced signal is typically saturated after 30 s of microwave irradiation. The temperature jump was achieved by irradiation of 10.6 ∞m light from a 10 W CO2 laser through a silver coated silica hollow waveguide.
Figure 2.
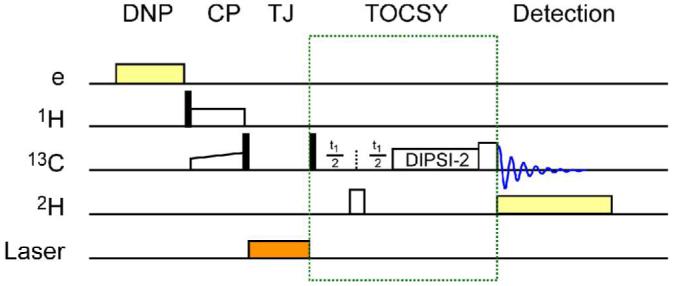
Experimental scheme for 2D TJ-DNP experiment. The sample was irradiated with 140 GHz microwaves at 100 K, followed by 1H-13C cross polarization. The magnetization on 13C was stored along the z-axis to minimize polarization loss by relaxation during melting. Immediately following melting, a 2D NMR pulse sequence was initiated. For TOCSY, a 180° 2H pulse was applied in the middle of t1 evolution for decoupling. DIPSI-2 spin locking was on for 4.5 ms followed by 1 ms trim pulse to reduce anti-phase line shape components. The 13C signal was detected with WALTZ 2H decoupling.
Figure 3.
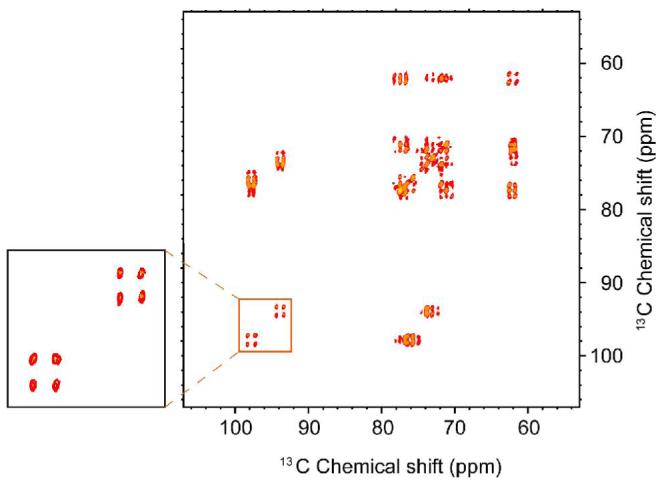
13C-13C TOCSY NMR spectrum of [2H7, 13C6]-glucose solution, with DNP (10 s)-TJ (0.8 s) and 40 s recycle delay. 1 transient of 1024 data points was accumulated for 96 t1 increments. 4.5 ms of DIPSI-2 mixing with ω1/2π = 29 kHz was used for TOCSY. The 2D data matrix was acquired with 2 * 96 * 1024 points, with a single transient per data file and a recycle time of 40 s required by the refreezing. 2H decoupling was applied in both dimensions.
The direct detection of 13C in solution offers a valuable alternative to indirect detection via 1H, provided that the sensitivity of 13C can be enhanced, as demonstrated here. 13C detected experiments dramatically shorten the duration of the pulse sequence when compared to their corresponding 1H detected counterparts, thereby reducing losses due to transverse relaxation. 1H solvent suppression is also unnecessary. 13C detected experiments are particularly beneficial for the study of paramagnetic samples because the paramagnetic contributions to relaxation are reduced by a factor of 10 compared to 1H. 13C detected experiments also facilitate the investigation of spectral regions where 1H detection may be difficult such as those undergoing chemical or conformational exchange. Finally, as illustrated here, 13C detected experiments also enable measurements in completely deuterated samples. In total, these advantages of direct 13C detection should enable new experiments for studies of larger bio-molecular systems. Other groups have appreciated these features in recent years and as a consequence there has been considerable effort devoted to developing experiments that utilize direct 13C detection 24-26. The sensitivity gain available from TJ-DNP could rapidly accelerate or improve the realization of many of these advantages.
Despite the dramatic increase in sensitivity reported here we anticipate that the experimental protocol could be improved in several respects. First, the temperature at which the polarization is performed could be lowered from 100 K to 10 K, yielding another factor of ≥10 in signal intensity as the Boltzmann factor and the polarization enhancements from DNP would both be larger. Second, we are currently using quartz rotors and most of the heat from the laser pulse is expended in bringing the rotor from 100 K to 300 K. Alternative rotor materials may allow faster and more uniform melting, reducing losses due to relaxation, and improving the line shape. Finally, it would be beneficial to move the experiments to a higher operating frequency where contemporary solution NMR experiments are currently performed. We anticipate that 2D TJDNP will be widely applicable to NMR experiments involving small molecules, for example in metabolomics, and perhaps to some proteins.
ACKNOWLEDGMENTS
We thank J. Sirigiri and R.J.Temkin for their assistance with the 140 GHz gyrotron source used in these experiments and Jeff Bryant for his superb technical assistance. This research was supported by grants from the National Institutes of Health EB002804 and EB002026.
References
- 1.Wind RA, Duijvestijn MJ, Vanderlugt C, Manenschijn A, Vriend J. Progress in Nuclear Magnetic Resonance Spectroscopy. 1985;17:33–67. [Google Scholar]
- 2.Afeworki M, Mckay RA, Schaefer J. Macromolecules. 1992;25(16):4084–4091. [Google Scholar]
- 3.Afeworki M, Schaefer J. Macromolecules. 1992;25:4092–4096. [Google Scholar]
- 4.Becerra LR, Gerfen GJ, Temkin RJ, Singel DJ, Griffin RG. Physical Review Letters. 1993;71(21):3561–3564. doi: 10.1103/PhysRevLett.71.3561. [DOI] [PubMed] [Google Scholar]
- 5.Joo C-G, Hu K-N, Bryant JA, Griffin RG. J. Am Chem. Soc. 2006;128:9428–9432. doi: 10.1021/ja0611947. [DOI] [PubMed] [Google Scholar]
- 6.Maly T, Debelouchina GT, Bajaj VS, Hu K-N, Joo C-G, Mak-Jurkauskas ML, Sirigiri JR, Wel P. C. A. v. d., Herzfeld J, Temkin RJ, Griffin RG. J. Chem Physics. 2008;128:052211. doi: 10.1063/1.2833582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Wel PCA, Hu K-N, Lewandoswki J, Griffin RG. J. Am Chem. Soc. 2006;128:10840–10846. doi: 10.1021/ja0626685. [DOI] [PubMed] [Google Scholar]
- 8.Hall DA, Maus DC, Gerfen GJ, Inati SJ, Becerra LR, Dahlquist FW, Griffin RG. Science. 1997;276:930–932. doi: 10.1126/science.276.5314.930. [DOI] [PubMed] [Google Scholar]
- 9.Rosay M, Lansing JC, Haddad KC, Bachovchin WW, Herzfeld J, Temkin RJ, Griffin RG. J. Am. Chem. Soc. 2003;125:13626–27. doi: 10.1021/ja036898k. [DOI] [PubMed] [Google Scholar]
- 10.Rosay M, Weis V, Kreischer KE, Temkin RJ, Griffin RG. Journal of the American Chemical Society. 2002;124(13):3214–3215. doi: 10.1021/ja0176752. [DOI] [PubMed] [Google Scholar]
- 11.Bajaj VS, Farrar CT, Hornstein MK, Mastovsky I, Vieregg J, Bryant J, Elena B, Kreischer KE, Temkin RJ, Griffin RG. J. Mag. Res. 2003;160:85–90. doi: 10.1016/s1090-7807(02)00192-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu KN, Yu HH, Swager TM, Griffin RG. Journal of the American Chemical Society. 2004;126(35):10844–10845. doi: 10.1021/ja039749a. [DOI] [PubMed] [Google Scholar]
- 13.Bajaj VS, Hornstein MK, Kreischer KE, Sirigiri JR, Woskov PP, Mak-Jurkauskas ML, Herzfeld J, Temkin RJ, Griffin RG. Journal of Magnetic Resonance. 2007;189(2):251–279. doi: 10.1016/j.jmr.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu K-N, Bajaj VS, Rosay MM, Griffin RG. J. Chem. Phys. 2007;126:044512. doi: 10.1063/1.2429658. [DOI] [PubMed] [Google Scholar]
- 15.Hu K-N, Song C, Yu H.-h., Swager TM, Griffin RG. J. Chem. Phys. 2008;128:052321. doi: 10.1063/1.2816783. [DOI] [PubMed] [Google Scholar]
- 16.Becerra LR, Gerfen GJ, Bellew BF, Bryant JA, Hall DA, Inati SJ, Weber RT, Un S, Prisner TF, McDermott AE, Fishbein KW, Kreischer KE, Temkin RJ, Singel DJ, Griffin RG. Journal of Magnetic Resonance Series A. 1995;117(1):28–40. [Google Scholar]
- 17.Joye CD, Griffin RG, Hornstein MK, Hu K-N, Kreischer KE, Rosay M, Shapiro MA, Sirigiri JR, Temkin RJ, Woskov PP. IEEE Transactions on Plasma Science. 2006;34:518–523. doi: 10.1109/TPS.2006.875776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loening NM, Rosay M, Weis V, Griffin RG. Journal of the American Chemical Society. 2002;124(30):8808–8809. doi: 10.1021/ja026660g. [DOI] [PubMed] [Google Scholar]
- 19.Ardenkjaer-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M, Golman K. Proc. of the Natl Acad. of Sci. of. 2003;100(18):10158–10163. doi: 10.1073/pnas.1733835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolber J, Ellner F, Fridlund B, Gram A, Johannesson H, Hansson G, Hansson LH, Lerche MH, Mansson S, Servin R, Thaning M, Golman K, Ardenkjaer-Larsen JH. Nuclear Instruments & Methods in Physics Research Section a-Accelerators Spectrometers Detectors and Associated Equipment. 2004;526(12):173–181. [Google Scholar]
- 21.Frydman L, Blazina D. Nature Physics. 2007;3(6):415–419. [Google Scholar]
- 22.Song C, Hu K-N, Swager TM, Griffin RG. J. Am Chem. Soc. 2006;128:11385–90. doi: 10.1021/ja061284b. [DOI] [PubMed] [Google Scholar]
- 23.Eletsky A, Moreira O, Kovacs H, Pervushin K. Journal of Biomolecular Nmr. 2003;26(2):167–179. doi: 10.1023/a:1023572320699. [DOI] [PubMed] [Google Scholar]
- 24.Lee D, Vogeli B, Pervushin K. Journal of Biomolecular Nmr. 2005;31(4):273–278. doi: 10.1007/s10858-005-2361-4. [DOI] [PubMed] [Google Scholar]
- 25.Bermel W, Bertini I, Felli IC, Matzapetakis M, Pierattelli R, Theli EC, Turano P. Journal of Magnetic Resonance. 2007;188(2):301–310. doi: 10.1016/j.jmr.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 26.Bermel W, Bertini I, Felli IC, Piccioli M, Pierattelli R. Progress in Nuclear Magnetic Resonance Spectroscopy. 2006;48(1):25–45. doi: 10.1016/j.pnmrs.2014.10.001. [DOI] [PubMed] [Google Scholar]


