Abstract
Purpose
Treatment of patients with localized neuroblastoma with unfavorable biologic features is controversial. To evaluate the outcome of children with low-stage MYCN-amplified neuroblastoma and develop a rational treatment strategy, data from the International Neuroblastoma Risk Group (INRG) database were analyzed.
Patients and Methods
The database is comprised of 8,800 patients. Of these, 2,660 patients (30%) had low-stage (International Neuroblastoma Staging System stages 1 and 2) neuroblastoma, known MYCN status, and available follow-up data. Eighty-seven of these patients (3%) had MYCN amplified tumors.
Results
Patients with MYCN-amplified, low-stage tumors had less favorable event-free survival (EFS) and overall survival (OS) than did patients with nonamplified tumors (53% ± 8% and 72% ± 7% v 90% ± 1% and 98% ± 1%, respectively). EFS and OS were statistically significantly higher for patients whose tumors were hyperdiploid rather than diploid (EFS, 82% ± 20% v 37% ± 21%; P = .0069; OS, 94% ± 11% v 54% ± 15%; P = .0056, respectively). No other variable had prognostic significance. Initial treatment consisted of surgery alone for 29 (33%) of 87 patients. Details of additional therapy were unknown for 14 patients. Twenty-two patients (25%) underwent surgery and moderate-intensity chemotherapy; another 22 underwent surgery, intensive chemotherapy, and radiation therapy. Nine of the latter 22 underwent stem cell transplantation. Survival in patients who received transplantation did not differ from survival in those who did not receive transplantation.
Conclusion
Among patients with low-stage, MYCN-amplified neuroblastoma, outcomes of patients with hyperdiploid tumors were statistically, significantly better than those with diploid tumors. The data suggest that tumor cell ploidy could potentially be used to identify candidates for reductions in therapy. Further study of MYCN-amplified, low-stage neuroblastoma is warranted.
INTRODUCTION
Outcomes for the vast majority of patients with low-stage (International Neuroblastoma Staging System [INSS] stages 1 and 2) neuroblastoma are excellent. Of the 900 patients for whom follow-up data were available from the recently completed Children's Oncology Group study P9641, 811 had stage 1 or stage 2 disease, and 3-year event free survival (EFS) and overall survival (OS) rates were 85.2% ± 1.7% and 97.4% ± 0.8%, respectively.1 Previously published reports also indicate that most patients with low-stage neuroblastoma can be successfully treated with surgery alone.2-5 Local recurrences can typically be managed with surgery and/or radiation therapy. Metastatic recurrences are rare and often treated successfully with chemotherapy.
However, some patients still fare poorly despite low disease stage at diagnosis, and treatment of patients with localized tumors with unfavorable biologic features, particularly MYCN amplification, remains controversial. Numbers of patients with stage 1 or 2 MYCN-amplified neuroblastoma enrolled on previous cooperative group treatment studies have been small,6-9 thus it has been difficult to develop an evidence-based therapeutic strategy for this group. To establish the basis for a rational therapeutic approach to low-stage neuroblastoma with unfavorable biologic features, information regarding outcomes for these patients was obtained from the International Neuroblastoma Risk Group (INRG) database. The INRG is a collaborative effort among groups from Europe, North America, Australia, New Zealand, and Japan. The database contains information regarding more than 8,000 children with neuroblastoma. This resource was used to analyze age, stage, histologic category, MYCN status, tumor cell ploidy (defined as DNA index ≤ 1.0 v > 1.0), chromosome 1p and 11q aberration, serum lactate dehydrogenase (LDH) level, initial treatment, and outcome of patients with INSS stage 1 and 2 neuroblastoma.
PATIENTS AND METHODS
INRG Database
A total of 8,800 unique patients form the database.10 Patients younger than 21 years of age with pathologically confirmed neuroblastoma diagnosed between January 1, 1990, and December 31, 2002, are included. Patients were enrolled on neuroblastoma studies in Germany, Japan, Italy, Spain, or the United Kingdom, or were enrolled on a Children's Oncology Group study or the International Society of Pediatric Oncology Europe Neuroblastoma Group LNESG1 study. Members of INRG are listed in Appendix Table A1 (online only). In addition to date of diagnosis and follow-up data, information on 35 potential risk factors are included in the database. Of the 8,800 patients, 2,978 had low-stage (INSS stage 1 and 2) neuroblastoma and follow-up data. From these 2,978, the analytic cohort for this report is comprised of the subset of 2,660 patients with known MYCN status (30% of patients in the database).
Analysis of MYCN Status, Ploidy, and Histology
MYCN amplification was determined according to standard methods and definitions used by each cooperative group at the time of enrollment onto a trial. All cooperative groups have used fluorescence in situ hybridization (FISH) to evaluate MYCN status during the past decade. However, in the early 1990s, assays included Southern blot and polymerase chain reaction (PCR), as well as immunohistochemistry with semiquantitative PCR.11-14 DNA index was determined by flow cytometry and was reported as ≤ 1.0 versus > 1.0. It was not possible to distinguish tetraploid tumors from other hyperdiploid tumors based on information in the database. Aberrations in chromosomes 1p and 11q status were detected by FISH or PCR. Histology was classified as favorable or unfavorable according to criteria described by Shimada et al.15
Statistical Methods
Events for the EFS analysis were defined as relapse, progressive disease, secondary malignancy, or death from any cause. Time to event was calculated as time from enrollment to first event, or to time of last patient contact if no event occurred. Time to event for OS analysis was time from enrollment until death, or time of last contact if the patient was alive. The methods of Kaplan-Meier were used to generate survival curves; curves were compared using a log-rank test.16 The sample size of 87 patients provides 80% power (at a .05 significance level) to detect a 26% difference (30% v 56%) or a 22% difference (70% v 92%) in 5-year EFS or OS. EFS and OS are quoted at the 5-year time point as the survival estimate with or without SE, with SEs calculated per the methods of Peto.17 P values lower than .05 were considered statistically significant.
RESULTS
Patient Characteristics
Data regarding 2,660 patients with low stage neuroblastoma and known MYCN status were analyzed. Amplification was detected in tumors from 87 patients (3%). The median age in this cohort was 401 days (range, 0 days to 16.7 years). Only one patient was older than 12 years of age. Additional patient characteristics are presented in Table 1. Fifty-five percent of the children had INSS stage 1 disease; 45% had INSS stage 2 disease. Information regarding histology was available for 47 patients. Histology was classified as favorable in tumors from 56% of these patients and unfavorable in 44%. Data regarding tumor cell ploidy were available for 39 patients. Tumors from 17 (44%) were hyperdiploid while tumors from 22 (56%) were diploid. Lactate dehydrogenase (LDH) level at diagnosis was ≥ 580 U/L in 49% of the 63 patients for whom LDH values were known. Data regarding 1p or 11q aberrations were available for only 25 and 15 patients, respectively.
Table 1.
Characteristics of Patients With INSS Stage 1 or 2 Neuroblastoma and MYCNAmplification (N = 87)
| Characteristic | Total
|
5-Year EFS
|
5-Year OS
|
|||||
|---|---|---|---|---|---|---|---|---|
| No. | % | % | SE | P | % | SE | P | |
| Overall patients | 87 | 53 | 8 | 72 | 7 | |||
| INSS stage | ||||||||
| 1 | 48 | 55 | 50 | 12 | .2760 | 76 | 9 | .3568 |
| 2a/2b | 39 | 45 | 57 | 12 | 67 | 11 | ||
| Histology | ||||||||
| Favorable | 32 | 56 | 58 | 15 | .2029 | 86 | 9 | .1065 |
| Unfavorable | 25 | 44 | 40 | 18 | 62 | 19 | ||
| Unknown | 30 | |||||||
| Ploidy | ||||||||
| Hyperdiploid | 17 | 44 | 82 | 20 | .0069 | 94 | 11 | .0056 |
| Diploid | 22 | 56 | 37 | 21 | 54 | 15 | ||
| Unknown | 48 | |||||||
| 1p | ||||||||
| No loss or aberration | 11 | 44 | 73 | 27 | .4177 | 73 | 27 | .4197 |
| LOH or aberration | 14 | 56 | 56 | 17 | 85 | 12 | ||
| Unknown | 62 | |||||||
| LDH, U/L | ||||||||
| < 580 | 32 | 51 | 63 | 12 | .3522 | 83 | 9 | .0883 |
| ≥ 580 | 31 | 49 | 48 | 14 | 62 | 14 | ||
| Unknown | 24 | |||||||
| Age, days | ||||||||
| < 547 | 54 | 62 | 58 | 10 | .4320 | 77 | 8 | .1798 |
| ≥ 547 | 33 | 38 | 45 | 14 | 63 | 14 | ||
Abbreviations: INSS, International Neuroblastoma Staging System; EFS, event-free survival; OS, overall survival; LOH, loss of heterozygosity; LDH, lactate dehydrogenase.
Treatment Delivered
Initial treatment consisted of tumor excision alone for 29 (40%) of 87 patients with low-stage, MYCN-amplified neuroblastoma (Table 2). Most of these patients (25 of 29) had stage 1 disease. Twenty-two patients (30%) underwent surgery and subsequently received moderate intensity chemotherapy (two to eight cycles), while another 22 patients (30%) were treated with intensive chemotherapy plus radiation therapy. Among patients treated with moderate intensity chemotherapy, 15 had stage 2 disease and seven had stage 1 disease. Of the patients treated with more intensive chemotherapy plus radiation therapy, nine underwent high-dose chemotherapy with stem cell transplantation as part of first-line treatment. All patients who underwent transplantation had stage 2 disease. Details of nonsurgical therapy were unknown for 14 patients. Data regarding second-line treatment were not available.
Table 2.
Outcomes of Patients With Low-Stage, MYCN-Amplified Neuroblastoma Based on Initial Post-Surgical Therapy
| Treatment | Total
|
5-Year EFS
|
5-Year OS
|
|||||
|---|---|---|---|---|---|---|---|---|
| No. | % | % | SE | P | % | SE | P | |
| Surgery alone | 29 | 40 | 50 | 16 | .2938* | 81 | 11 | .5115* |
| Moderate intensity chemotherapy (2-8 cycles) plus surgery | 22 | 30 | 50 | 14 | 64 | 13 | ||
| Intensive chemotherapy + radiotherapy | ||||||||
| Specific type unknown | 6 | 8 | 50 | 25 | 67 | 22 | ||
| No stem cell or bone marrow transplant | 7 | 10 | 71 | 22 | 71 | 22 | ||
| Plus stem cell or bone marrow transplant | 9 | 12 | 50 | 25 | 62 | 27 | ||
| Unknown | 14 | |||||||
Abbreviations: EFS, event-free survival; OS, overall survival.
Comparison of surgery alone or surgery plus moderate intensity chemotherapy v surgery plus intensive chemotherapy plus radiotherapy with or without transplant.
Outcome of Patients With Low-Stage, MYCN-Amplified Neuroblastoma
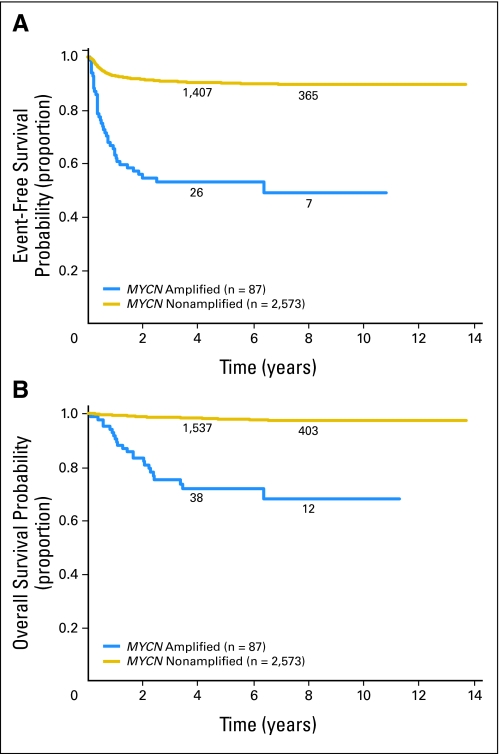
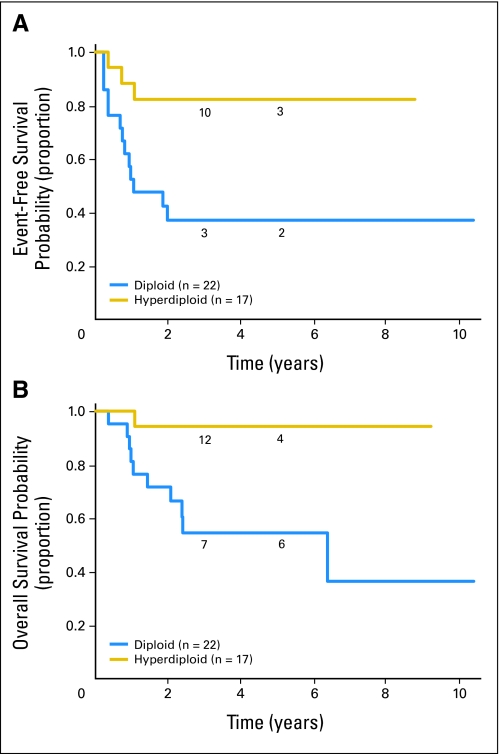
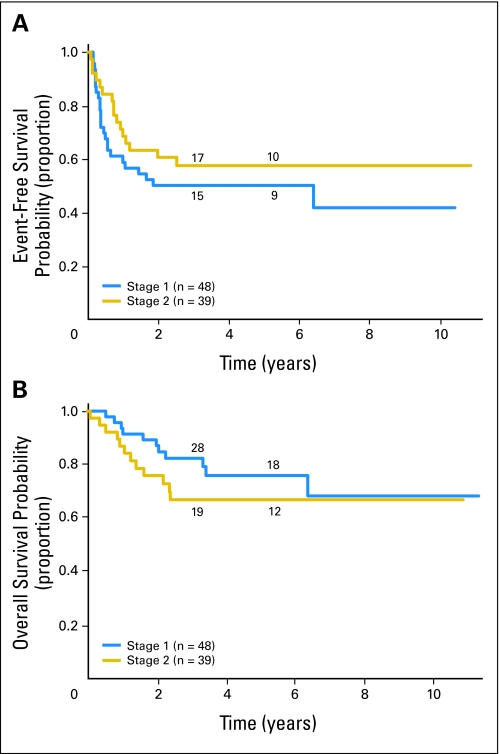
Patients with MYCN-amplified, low-stage tumors had less favorable EFS (P < .0001) and OS (P < .0001) than patients with nonamplified tumors did (53% ± 8% and 72% ± 7% v 90% ± 1% and 98% ± 1%, respectively; Fig 1). Outcome data for patients with MYCN-amplified tumors and information regarding additional biologic and clinical variables are presented in Table 1. Among patients with MYCN amplification, both EFS and OS were statistically significantly higher for patients whose tumors were hyperdiploid rather than diploid (EFS, 82% ± 20% v 37% ± 21%; P = .0069; OS, 94% ± 11% v 54% ± 15%; P = .0056, respectively; Fig 2). No other clinical or biologic variable evaluated had prognostic significance in this cohort. EFS and OS were not statistically significantly different for those with stage 1 versus stage 2 disease (EFS, 50% ± 12% v 57% ± 12%; P = .2760; OS, 76% ± 9% v 67% ± 11%; P = .3568, respectively; Fig 3). In addition, no differences in outcome were observed based on age at diagnosis. EFS and OS for those ≥ 18 months old at diagnosis were 45% ± 14% and 63% ± 14% compared with 58% ± 10% and 77% ± 8% for patients younger than 18 months old. No statistically significant difference in outcome was found based on histology, although a trend toward improved OS in patients with favorable histology was observed. EFS and OS were 58% ± 15% and 86% ± 9% for patients with favorable histology compared with 40% ± 18% and 62% ± 19% for patients with unfavorable histology. Similarly, no significant difference in outcome was observed based on level of LDH at diagnosis, but a trend toward a more favorable outcome for patients with LDH lower than 580 U/L was detected. EFS and OS for patients with LDH lower than 580 were 63% ± 12% and 83% ± 9% compared with 48% ± 14% and 62% ± 14% for patients with LDH ≥ 580. 1p and 11q aberrations did not have prognostic significance in this cohort, but the number of patients for whom this information was available was small (25 and 15 patients, respectively).
Fig 1.
International Neuroblastoma Staging System stage 1 and 2 patients. (A) Event free and (B) overall survival curves for MYCN nonamplified (n = 2,573) versus amplified (n = 87) patients. The numbers of patients at risk for an event are shown along the curves at years 4 and 8.
Fig 2.
MYCN amplified International Neuroblastoma Staging System stage 1 and 2 patients (n = 87). (A) Event free and (B) overall survival curves for hyperdiploid (n = 17) versus diploid (n = 22) patients. The numbers of patients at risk for an event are shown along the curves at years 3 and 5.
Fig 3.
MYCN amplified International Neuroblastoma Staging System stage 1 and 2 patients (n = 87). (A) Event free and (B) overall survival curves for stage 1 (n = 48) versus stage 2 (n = 39) patients. The numbers of patients at risk for an event are shown along the curves at years 3 and 5.
Initial therapy did not appear to have a significant impact on survival among patients with MYCN amplified tumors. EFS and OS among patients initially treated with surgery alone were 50% ± 16% and 81% ± 11%, respectively, while EFS and OS for the entire cohort of patients with MYCN-amplified, low-stage tumors were 53% ± 8% and 72% ± 7%. Nine patients underwent high-dose chemotherapy with stem cell transplantation as part of initial therapy. EFS and OS for these patients were 50% ± 25% and 62% ± 27%. In comparison, EFS and OS for all patients with stage 2 MYCN amplified tumors were 57% ± 11.9% and 67% ± 11.1%. Hematopoietic stem cell transplantation in the upfront setting was not associated with an improvement in either EFS or OS in this group of patients.
DISCUSSION
Risk assessment in neuroblastoma depends on integration of data regarding both clinical and biologic features. In most cases, there is concordance among prognostic variables. For example, MYCN amplification is strongly associated with advanced disease stage,11 and both MYCN amplification and advanced stage disease are associated with an unfavorable prognosis. Similarly, loss of heterozygosity at chromosome 1p is associated with age older than 1 year, advanced clinical stage, and MYCN amplification.18 Again, all features portend a less favorable prognosis. The challenge for clinicians, however, is to appropriately assign a risk designation in the relatively rare circumstances in which there is a lack of concordance among prognostic variables, such as low stage and MYCN amplification. While the number of patients with low-stage neuroblastoma and MYCN amplification is relatively small, because clinical and biologic features must be integrated for risk designation, the conundrum presented by these patients is of considerable interest.
Efforts have been made to address the issue of conflicting prognostic information in neuroblastoma, but small numbers make outcome analyses challenging. In a Pediatric Oncology Group (POG) study of 850 children with localized neuroblastoma, only six had MYCN-amplified tumors.6 Three patients remained disease free after therapy while three experienced recurrences. In a subsequent POG study, only 11 of 329 children with POG stage A disease had MYCN-amplified tumors.7 Outcomes for these patients varied: four children never experienced recurrent disease, four relapsed but survived with additional therapy, and three died of disease.7 Numbers from a contemporaneous Children's Cancer Group study were similar. Of 374 children with Evans stages I and II disease enrolled on CCG3881, only seven had MYCN-amplified tumors.8 Although a statistically significant difference in outcome based on MYCN status was found for the overall cohort studied,8 the small number of patients with MYCN-amplified tumors precluded further analysis of outcome within this subgroup. A recent publication described the Children's Oncology Group experience with patients with low stage neuroblastoma and MYCN amplification. Schneiderman and colleagues19 assessed outcomes of 32 patients with MYCN-amplified neuroblastoma, but included patients with stages A, B, and Ds disease. EFS for this population was approximately 50%, however the number of patients with stages 1 and 2 disease for whom complete data were available limited the extent to which subgroup analyses could be performed.
Numbers have also limited the ability of European groups to address this issue of lack of concordance of prognostic variables in neuroblastoma. In the recent LNESG1 study, only 16 of the 427 patients with low-stage neuroblastoma had MYCN-amplified tumors (seven stage 1; nine stage 2; M. Beck-Popovic, personal communication, May 2008). Even in a cohort that included a somewhat larger number of patients with low-stage, MYCN-amplified neuroblastoma, it was not possible to establish the importance of other prognostic data. George and colleagues9 evaluated the relationship of histology and MYCN amplification in patients with neuroblastic tumors, 42 of whom had stage 1 or stage 2 neuroblastoma. Only 11% of tumors from patients with localized disease were MYCN amplified, and when histopathology and disease stage were considered in relation to MYCN status and outcome, the numbers of patients in each subgroup prohibited further study. For example, only one patient with stage 2 disease had a tumor with favorable histology and MYCN amplification.9
The INRG database presents a unique opportunity to study rare subgroups of patients, including those with unusual combinations of clinical and biologic features. The international database makes it possible to assess outcomes for more than three times as many patients as had been analyzed previously (n = 87 in our patient group). In addition, the large database permits examination of the contribution of variables that were not collected via single cooperative group efforts. For example, Schneiderman and colleagues19 were not able to evaluate the prognostic importance of Shimada histology in the COG patients with low-stage, MYCN-amplified neuroblastoma due to missing data. In contrast, information regarding histology was available for 57 patients in the INRG data set. These results suggest that there is a trend toward improved outcomes in patients with favorable histology, although the level of statistical significance was not attained. This work thus highlights the importance of international collaboration for assessment of multiple prognostic variables, particularly when a rare disease entity is being studied. Efforts are being made to standardize the information to be collected for patients entered into the INRG database in the future. Data regarding a more uniform set of variables (including ploidy and histology) will be collected, and analyses of subsets of patients with low-stage, MYCN-amplified neuroblastoma may have greater power to detect differences in outcome in the near future.
Although the present database provides the patient numbers needed to begin to study outcomes for rare subsets of children with neuroblastoma, there are caveats that must be considered as data are interpreted. Cooperative groups from around the world have approached laboratory analysis of MYCN status and tumor cell ploidy differently over time. During the years 1990 to 2002, cooperative groups in Japan, Europe, and North America used Southern blotting, PCR, immunohistochemistry with PCR, and/or FISH to assess MYCN copy number. Threshold values used to define MYCN amplification have differed among the groups as well. Although DNA index was determined by flow cytometry in all participating groups, the definition of hyperdiploidy varied slightly from group to group. Therefore, differences in assay methods and differences in the definitions of MYCN amplification and hyperdiploidy could confound this analysis. However, when Ambros and colleagues20 compared results of Southern blotting, PCR, and FISH assays for MYCN status in 160 neuroblastoma tumors, they found an error rate of lower than 4%. Ambros’ group carried out laboratory analyses according to consensus standard operating procedures, which likely minimized interlaboratory variability. Nonetheless, the low rate of conflicting results in the MYCN analyses suggests that differences in techniques are unlikely to substantially change the findings now reported. Uniform international approaches to laboratory analysis of biologic markers and definitions of relevant terms remain highly desirable, however. Efforts to standardize neuroblastoma staging have greatly facilitated comparisons of results of clinical trials worldwide,21,22 and efforts to standardize interpretation of morphologic features of neuroblastic tumors has led to near global use the International Neuroblastoma Pathology Classification (INPC) system.23 One of the goals of the INRG is to develop international consensus with regard to assessment of other key prognostic variables in neuroblastoma, including standard operating procedures for the analysis of MYCN status and tumor cell ploidy as well as uniform nomenclature to describe results of these analyses.
Despite its present limitations, the INRG database provides an unprecedented opportunity to use the international experience to critically evaluate long-held assumptions regarding subsets of patients with neuroblastoma. Interestingly, this analysis demonstrates that there is no statistically significant difference in outcome between patients with stage 1 versus stage 2 disease in the presence of MYCN amplification. Patients with stage 1 disease were more likely to have been treated with surgery alone while patients with stage 2 disease were more likely to have received aggressive therapy. However, this does not mitigate the fact that patients with stage 2 MYCN-amplified neuroblastoma fared as well as patients with stage 1 MYCN-amplified tumors. A limitation of analyses using this database is the lack of information regarding precise chemotherapy doses delivered. Nonetheless, it is striking that survival for patients who received moderate dose therapy was similar to that of patients who received intensive therapy including transplant.
In contrast, the data suggest that patient outcomes differ based on tumor cell ploidy, as patients whose tumors were hyperdiploid had an OS rate of 94% ± 11.4% while OS among patients whose tumors were diploid was 54% ± 15%. In light of these data, it appears that intensive chemoradiotherapy may not be necessary for patients with low-stage, MYCN-amplified neuroblastoma whose tumors are hyperdiploid. In risk stratification systems currently used in North America and Europe, patients with stage 2 MYCN-amplified neuroblastoma are designated as having high-risk disease, regardless of age, histology, or tumor cell ploidy. These patients are treated with aggressive therapy, including stem cell transplantation. Our data do not support substratification of patients with stage 2 MYCN-amplified neuroblastoma based on age or histology, but the data do suggest that hyperdiploidy may be used to identify stage 2 patients who could be candidates for reductions in currently designated intensive therapy. Given trends toward higher OS in patients with low levels of LDH at diagnosis and in patients with favorable Shimada histology, these variables may also be useful in defining a group of patients with low-stage, MYCN-amplified neuroblastoma who could receive less toxic first-line therapy.
An international prospective trial of reduced therapy for patients with hyperdiploid MYCN-amplified, low-stage neuroblastoma would provide an ideal way to further study this question. However, the international database generated over a 12-year period included only 17 patients with hyperdiploid, MYCN-amplified, low-stage tumors (approximately one patient/year). Therefore, logistics preclude such a trial. Instead, a more cost-effective approach is to use the INRG framework to obtain more complete data regarding 1p, 11q, and other chromosomal aberrations as well as more detailed data regarding first-line and second-line therapy for patients with low-stage, MYCN-amplified neuroblastoma. These expanded datasets could be used to validate the results of the current analysis. In addition, ongoing studies that take a genome-wide approach to analysis of differential regulation of signaling pathways in diploid and hyperdiploid MYCN-amplified tumors from patients with low-stage neuroblastoma could provide greater insights into the biology underlying clinical outcomes.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Rochelle Bagatell, Susan L. Cohn, Maja Beck-Popovic
Collection and assembly of data: Rochelle Bagatell, Wendy B. London, Yang Zhang
Data analysis and interpretation: Rochelle Bagatell, Maja Beck-Popovic, Wendy B. London, Yang Zhang, Susan L. Cohn
Manuscript writing: Rochelle Bagatell, Wendy B. London, Susan L. Cohn
Final approval of manuscript: Rochelle Bagatell, Maja Beck-Popovic, Wendy B. London, Yang Zhang, Andrew D.J. Pearson, Katherine K. Matthay, Tom Monclair, Peter F. Ambros, Susan L. Cohn
Appendix
Table A1.
The INRG Task Force: Participants of the INRG Meeting (Whistler, Canada, September 17-19, 2005)
| Name | Country and Cooperative Group | Role |
|---|---|---|
| Susan L. Cohn | United States, COG | Chair |
| Andrew D.J. Pearson | United Kingdom, SIOPEN | Chair |
| Statistics committee | ||
| Wendy B. London | United States, COG | Chair |
| Emanuele S.G. d'Amore | Italy, SIOPEN | |
| Andreas Faldum | Germany, GPOH | |
| Barbara Hero | Germany, GPOH | |
| Tomoko Iehara | Japan, JANB/JINCS | |
| David Machin | United Kingdom, SIOPEN | |
| Veronique Mosseri* | France, SIOPEN | |
| Michel Peuchmaur | France, SIOPEN | |
| Hiroyuki Shimada | United States, COG | |
| Biology committee | ||
| Peter F. Ambros | Austria, SIOPEN | Chair |
| Inge M. Ambros* | Austria, SIOPEN | |
| Garrett M. Brodeur | United States, COG | |
| Jerome Couturier | France, SIOPEN | |
| Michelle Haber | Australia | |
| Javed Khan | United States, COG | |
| John M. Maris | United States, COG | |
| Akira Nakagawara | Japan, JANB/JINCS | |
| Gudrun Schleiermacher | France, SIOPEN | |
| Frank Speleman* | Belgium, SIOPEN | |
| Ruediger Spitz | Germany, GPOH | |
| Nadine Van Roy | Belgium, SIOPEN | |
| Metastatic disease committee | ||
| Katherine K. Matthay | United States, COG | Chair |
| Klaus Beiske | Norway, SIOPEN | |
| Sue Burchill | United Kingdom, SIOPEN | |
| Irene Cheung | United States, COG | |
| Francesco Giammarile | France, SIOPEN | |
| Eiso Hiyama | Japan | |
| Jean Michon | France, SIOPEN | |
| Robert C. Seeger | United States, COG | |
| Barry Shulkin | United States, COG | |
| Surgery committee | ||
| Tom Monclair | Norway, SIOPEN | Chair |
| Hervé Brisse | France, SIOPEN | |
| Giovanni Cecchetto | Italy, SIOPEN | |
| Keith S.J. Holmes | United Kingdom, SIOPEN | |
| Michio Kaneko | Japan, JANB/JINCS | |
| Jed G. Nuchtern | United States, COG | |
| Dietrich von Schweinitz | Germany, GPOH | |
| Senior advisors | ||
| Frank Berthold | Germany, GPOH | |
| Victoria Castel | Spain, SIOPEN | |
| Robert P. Castleberry* | United States, COG | |
| Nai-Kong Cheung | United States, COG | |
| Bruno De Bernardi | Italy, SIOPEN | |
| Helen Irving | Australia, COG | |
| Ruth Ladenstein | Austria, SIOPEN | |
| C. Patrick Reynolds | United States, COG | |
| Jinhua Zhang | China | |
| Young investigators | ||
| Julie R. Park | United States, COG | |
| Roswitha Schumacher-Kuckelkorn | Germany, GPOH | |
| Thorsten Simon | Germany, GPOH | |
| Hidetaka Niizuma | Japan, JANB/JINCS | |
| Toby Trahair | Australia | |
| William Guy Forbeck Research Foundation | ||
| Jennifer Forbeck | United States | |
| John T. Kemshead | United Kingdom |
Abbreviations: COG, Children's Oncology Group; SIOPEN, International Society of Pediatric Oncology Europe Neuroblastoma Group; GPOH, German Pediatric Oncology and Hematology Group; JANB/JINCS, Japanese Advanced Neuroblastoma Study Group/Japanese Infantile Neuroblastoma Co-operative Study Group.
International Neuroblastoma Risk Group Task Force members not present at the Whistler meeting.
published online ahead of print at www.jco.org on December 1, 2008.
Supported in part by the William Guy Forbeck Research Foundation, the Little Heroes Cancer Research Fund, and the Caitlin Robb Foundation.
Presented in part at the 13th Advances in Neuroblastoma Research Conference, May 21–24, 2008, Chiba, Japan.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Strother D, London WB, Yap J, et al: Surgery and restricted use of chemotherapy as treatment of low-risk neuroblastoma: Preliminary results of Children's Oncology Group protocol 9641. International Society of Paediatric Oncology (SIOP) XXXVIII Congress Meeting, Geneva, Switzerland, September 17–21, 2006
- 2.Matthay KK, Sather HN, Seeger RC, et al: Excellent outcome of stage II neuroblastoma is independent of residual disease and radiation therapy. J Clin Oncol 7:236-244, 1989 [DOI] [PubMed] [Google Scholar]
- 3.Kushner BH, Cheung NK, LaQuaglia MP, et al: International neuroblastoma staging system stage 1 neuroblastoma: A prospective study and literature review. J Clin Oncol 14:2174-2180, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Evans AE, Silber JH, Shpilsky A, et al: Successful management of low-stage neuroblastoma without adjuvant therapies: A comparison of two decades, 1972 through 1981 and 1982 through 1992, in a single institution. J Clin Oncol 14:2504-2510, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Berthold F, Treuner J, Brandeis WE, et al: Neuroblastoma study NBL 79 of the German Society for Pediatric Oncology: Report after 2 years [in German]. Klinische Padiatrie 194:262-269, 1982 [DOI] [PubMed] [Google Scholar]
- 6.Cohn SL, Look AT, Joshi VV, et al: Lack of correlation of N-myc gene amplification with prognosis in localized neuroblastoma: A Pediatric Oncology Group study. Cancer Res 55:721-726, 1995 [PubMed] [Google Scholar]
- 7.Alvarado CS, London WB, Look AT, et al: Natural history and biology of stage A neuroblastoma: A Pediatric Oncology Group study. J Pediatr Hematol Oncol 22:197-205, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Perez CA, Matthay KK, Atkinson JB, et al: Biologic variables in the outcome of stages I and II neuroblastoma treated with surgery as primary therapy: A children's cancer group study. J Clin Oncol 18:18-26, 2000 [DOI] [PubMed] [Google Scholar]
- 9.George RE, Variend S, Cullinane C, et al: Relationship between histopathological features, MYCN amplification, and prognosis: A UKCCSG study United Kingdom Children Cancer Study Group. Med Pediat Oncol 36:169-176, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Cohn SL, Pearson ADJ, London WB, et al: International Neuroblastoma Risk Group (INRG) Classification System: An INRG Task Force report. J Clin Oncol doi: 10.1200/JCO.2008.16.6785 [epub ahead of print on December 1, 2008] [DOI] [PMC free article] [PubMed]
- 11.Brodeur GM, Seeger RC, Schwab M, et al: Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science 224:1121-1124, 1984 [DOI] [PubMed] [Google Scholar]
- 12.Christiansen H, Delattre O, Fuchs S, et al: Loss of the putative tumor suppressor-gene locus 1p36 as investigated by a PCR-assay and N-myc amplification in 48 neuroblastomas: Results of the German Neuroblastoma Study Group. Prog Clin Biol Res 385:19-25, 1994 [PubMed] [Google Scholar]
- 13.Wada RK, Seeger RC, Brodeur GM, et al: Characterization of human neuroblastoma cell lines that lack N-myc gene amplification. Prog Clin Biol Res 271:57-69, 1988 [PubMed] [Google Scholar]
- 14.Shapiro DN, Valentine MB, Rowe ST, et al: Detection of N-myc gene amplification by fluorescence in situ hybridization: Diagnostic utility for neuroblastoma. American J Pathol 142:1339-1346, 1993 [PMC free article] [PubMed] [Google Scholar]
- 15.Shimada H, Chatten J, Newton WA Jr, et al: Histopathologic prognostic factors in neuroblastic tumors: Definition of subtypes of ganglioneuroblastoma and an age-linked classification of neuroblastomas. J Natl Cancer Inst 73:405-416, 1984 [DOI] [PubMed] [Google Scholar]
- 16.Kaplan EL, Meier P: Nonparametric estimation from incomplete observations. J American Statistical Association 53:457-481, 1958 [Google Scholar]
- 17.Peto R, Peto J: Asymptotically efficient rank invariant test procedures. JRSSA 135:185-198, 1972 [Google Scholar]
- 18.Attiyeh EF, London WB, Mosse YP, et al: Chromosome 1p and 11q deletions and outcome in neuroblastoma. N Engl J Med 353:2243-2253, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Schneiderman J, London WB, Brodeur GM, et al: Clinical significance of MYCN amplification and ploidy in favorable-stage neuroblastoma: A report from the Childrens Oncology Group. J Clin Oncol 26:913-918, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Ambros IM, Benard J, Boavida M, et al: Quality assessment of genetic markers used for therapy stratification. J Clin Oncol 21:2077-2084, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Brodeur GM, Seeger RC, Barrett A, et al: International criteria for diagnosis, staging, and response to treatment in patients with neuroblastoma. J Clin Oncol 6:1874-1881, 1988 [DOI] [PubMed] [Google Scholar]
- 22.Castleberry RP, Pritchard J, Ambros P, et al: The International Neuroblastoma Risk Groups (INRG): A preliminary report. Eur J Cancer 33:2113-2116, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Shimada H, Ambros IM, Dehner LP, et al: The International Neuroblastoma Pathology Classification (the Shimada system). Cancer 86:364-372, 1999 [PubMed] [Google Scholar]