Abstract
The pan-global marine appendicularian, Oikopleura dioica, shows considerable promise as a candidate model organism for cross-disciplinary research ranging from chordate genetics and evolution to molecular ecology research. This urochordate, has a simplified anatomical organization, remains transparent throughout an exceptionally short life cycle of less than 1 week and exhibits high fecundity. At 70 Mb, the compact, sequenced genome ranks among the smallest known metazoan genomes, with both gene regulatory and intronic regions highly reduced in size. The organism occupies an important trophic role in marine ecosystems and is a significant contributor to global vertical carbon flux. Among the short list of bona fide biological model organisms, all share the property that they are amenable to long-term maintenance in laboratory cultures. Here, we tested diet regimes, spawn densities and dilutions and seawater treatment, leading to optimization of a detailed culture protocol that permits sustainable long-term maintenance of O. dioica, allowing continuous, uninterrupted production of source material for experimentation. The culture protocol can be quickly adapted in both coastal and inland laboratories and should promote rapid development of the many original research perspectives the animal offers.
INTRODUCTION
Biomedical research has long benefitted from the availability of model organisms that can be maintained and manipulated in the laboratory under controlled conditions. The application of genome and proteome scale approaches to such organisms has accelerated understanding of biological mechanisms and has aided the treatment and prevention of disease. These approaches are now also being ever more rapidly applied in ecological, environmental, agricultural and aquaculture research. However, a frequent limitation in such research programs is the absence of well adapted model organisms permitting in depth experimentation and testing of ideas generated by catalogues of “omic” and systems biology data. Technical development of defined and durable laboratory culture of appropriate model organisms will, therefore, play an indispensible role in efficiently driving forward research in non-biomedical areas of investigation. One such emerging model organism is the marine zooplanktonic appendicularian, Oikopleura dioica, an animal with considerable promise in cross-disciplinary research ranging from chordate genetics to environmental toxicology to vertical global carbon flux studies.
Appendicularians are among the second or third most abundant component in marine mesozooplankton communities (Gorsky and Fenaux, 1998), and were first described in 1821 by Chamisso and Eysenhart (cited by Flood and Deibel, 1998). There are more than 70 species, distributed in 3 families, the Oikopleuridae; Fritillaridae and Kowalevskiidae, occurring at depths ranging from the surface to more than 2000 m (Fenaux et al., 1998). They occupy an important trophic position in marine food webs, permitting rapid energy transfer from micro-scale phytoplankton primary producers and bacteria to macro-scale zooplanktivorous predators (Gorsky and Fenaux, 1998). They accomplish this by living inside a complex gelatinous house, composed of glycopolysaccharides, mucopolysaccharides and cellulose (Kimura et al., 2001; Spada et al., 2001; Thompson et al., 2001), allowing capture of particles down to 0.2 µm. To maintain high filtering capacity, the house is repetitively synthesized and discarded in concert with the growth of the animal. Discarded houses, including trapped material, are a substantial fraction of marine snow and contribute significantly to vertical carbon flux in the world's oceans (Alldredge, 1976; Silver and Alldredge, 1981, Robison et al., 2005).
Among appendicularians, the only non-hermaphroditic dioecious species, O. dioica, presents original perspectives as a biological model organism. As a member of the class Urochordata, they have a simplified chordate body plan with a notochord, dorsal neural tube, gill slits and endostyle. Recently, it has been shown that the urochordates, and not the cephalochordates, are the closest living relatives to the vertebrates (Delsuc et al., 2006). For a chordate, O. dioica has an exceptionally short life cycle (6 days at 15°C) with high fecundity (>300 eggs per female, Troedsson et al., 2002). The small organism (∼1 mm adult) remains fully transparent throughout its life cycle (Fig. 1), greatly facilitating experimental investigation. Despite its evolutionary position, at about 70 Mb, O. dioica has one of the smallest known metazoan genomes (Seo et al., 2001). Gene density is high (one gene per 4–5 kb) and gene regulatory regions are compact. A number of genes are trans-spliced and polycistronic transcription is frequent (Ganot et al., 2004). The genome has been sequenced at 14-fold coverage in a collaboration involving the Sars Centre, Genoscope and the Max-Planck Institute for Molecular Genetics. Precise cell lineage fate maps have been determined up to embryo hatching (Fujii et al., 2008; Stach et al., 2008) and juvenile and adult morphologies are well characterized (Fenaux, 1998). All of these features underline the opportunities O. dioica offers in ecology, embryology, developmental cell biology, gene regulation and chordate genetics and evolution. Reliable long-term culture of O. dioica, with cryopreservation of established lines will be central to realization of this potential.
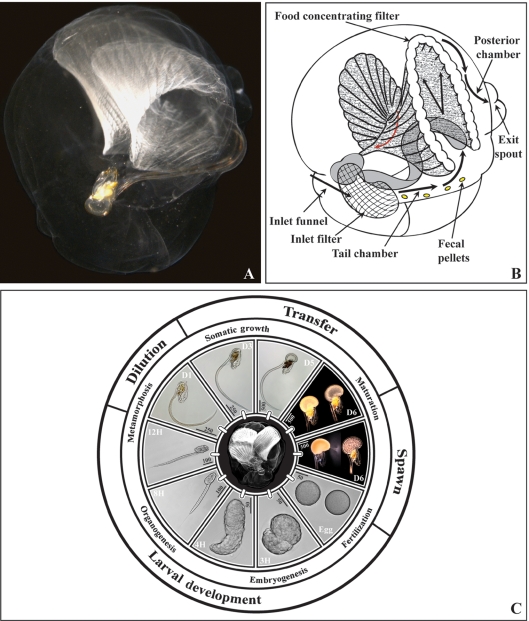
Fig. 1.
Life cycle of the appendicularian, Oikopleura dioica. (A) Oikopleura dioica inside a fully inflated gelatinous filter-feeding house. The ribbed food-concentrating filter is visible at the top. The animal at the centre of the panel is oriented with mouth to the top, gonad to the bottom and tail projecting to the right. (B) Schematic representation of the oikopleurid house modified after Flood and Deibel (1998) and Thompson et al. (2001). Water flow through the house is indicated by black arrows and food particle concentration toward the mouth by a red arrow. (C) The O. dioica life cycle at 15°C. Major transitions in the culture protocol are indicated on the outer ring with corresponding developmental events noted in the ring immediately underneath. Egg: an unfertilized oocyte is shown at right and a fertilized zygote with membrane ruffling shown at left. 3H: tailbud. 4H: just hatched tadpole. 8H: The tail has elongated with respect to the trunk. The tadpole has a visible statocyst, is undergoing mouth and digestive track formation and vacuolation in the notochord. The larval tunic extends in a filament projecting beyond the posterior end of the tail. 12H: The mouth is open, the heart is beating, notochord vacuoles have fused and pre-house secretion is visible on the oikoplastic epithelium covering the trunk. Water flow commences through the digestive tract. Between 12 and 14 h, the metamorphic tail shift occurs, followed by inflation of the first house and initiation of filter-feeding. From D1–D5, the animal grows rapidly through extensive use of endocycling in somatic tissues (Ganot and Thompson, 2002). D6 (upper panel): sexual differentiation is readily apparent with an immature male at left and immature female at right. D6 (lower panel): mature male (left) releasing sperm through the spermiduct located on the anterior dorsal surface of the testes and mature female (right) just prior to rupture of the ovary and release of 100 µm diameter oocytes. Scale bar units are in µm.
Over the past 35 years, short- to medium-term culture of O. dioica has been developed in a few laboratories (Paffenhöfer, 1973; Fenaux and Gorsky, 1979; Sato et al., 1999; Bassham and Postelthwait, 2005). Here we present data over a 6-year period on the field collection of this species in relation to the physical parameters of depth, temperature and salinity. We further present testing of feeding regimes, spawn densities and dilutions and seawater treatment, leading to optimization of a detailed culture protocol that permits sustainable long-term maintenance of the animal, allowing continuous, uninterrupted production of source material for experimentation.
METHODS AND RESULTS
Seawater
Seawater used for culture should have stable characteristics (temperature, salinity, pH) and be free of particles that can negatively impact the development of O. dioica. The animals can adapt to some changes in the physical and chemical properties of the seawater provided they are gradually imposed. We use natural seawater obtained at 4 to 8 m depth (salinity, 29 ± 2°/oo; pH, 8.0 ± 0.1). The water is adjusted to 15°C and filtered through three Hytrex II Cartridge Filters (20, 10 and 1 µm, respectively) before passage through an activated charcoal filter (Sigma/Fulka Norit, 96831) with a 1–2 mm gradient and exposure to an Aqua-Care UV-light (254 nm, 10 W). Finally, the water is left to decant over night, with charcoal (VWR charcoal 1–3 mm gradient, 12700-5) in an opaque fiberglass tank (150 L), equipped with an opaque cover to protect the water from direct light and particle contamination. Oikopleura dioica can also be cultured using artificial seawater (Rohto-Marine, Rei-Sea Co. salt, Tokyo, Japan; Fujii et al., 2008), but our access to natural seawater is unlimited and more cost effective.
Algal culture
We have tested various combinations of the following algae in the culture of O. dioica: Chaetoceros calcitrans, Emiliana huxleyi, Isochrysis sp., Rhinomonas reticulata, Synechococcus sp., Tetraselmis suecica and Thalassiosira pseudomonas. Those retained produced satisfactory results in the culture of O. dioica and could themselves be cultured reliably over long time periods. Reference features of the selected algae are summarized in Table I.
Table I:
Algae used as feed in the culture of Oikopleura dioica
| Algal species | Size | Reference |
|---|---|---|
| Chaetoceros calcitrans fo pumilus (Bacillariophyceae) | 4×3 µm (l × w) | CCAP 1010/11a |
| Isochrysis sp. (Prymnesiophyceae) | 6 µm diameter | CCAP 927/14a |
| Rhinomonas reticulata variation reticulata (Crytophyceae) | 14×7 µm (l×w) | CCAP 995/2a |
| Synechoccoccus sp. (Cyanobacteria) | 1 µm diameter | K0408b |
aCulture Collection of Algae and Protozoa, http://www.ccap.ac.uk.
bScandinavianCulture Centre for Algae and Protozoa: http://www.sccap.bot.ku.dk.
Algal strains were maintained in 100 mL Erlenmeyer flasks, under restricted growth conditions (15°C with a photoperiod of 12H), in 0.2 µm filtered (cellulose acetate; Sartorius, 11107.50.N) and autoclaved (120°C, 25 min.) seawater, enriched with half a dose of Conway media (Walne, 1966) and supplemented with silicate (0.07 mM Na2SiO3-9H2O, Sigma 5904) for C. calcitrans. Synechococcus sp. is grown in 1 L Erlenmeyer flasks under the same conditions used in maintenance of the reference strain. For larger scale culture of the other algal species, seawater was successively filtered through 20, 10 and 1 µm cartridge filters, exposed to UV light and supplemented with Conway medium prior to inoculation with algae. For C. calcitrans, silicate (0.14 mM Na2SiO3-9H2O) was added. Pre-production cultures were set up in 500 or 1000 mL glass flasks. Production scale culture was carried out in 1–3 L transparent plastic culture bags (250 mm, 110 µm). These were uni-algal but not axenic. Bag cultures, inoculated with pre-production flasks, were suspended on rods and continuously back-illuminated with 100 µmol photon ms−1 white fluorescent light (OSRAM L 36W/20; cool white). Cultures were incubated at 20±1°C, and cells suspended by continuous filtered (0.2 µm) air bubbling.
Algal growth curves
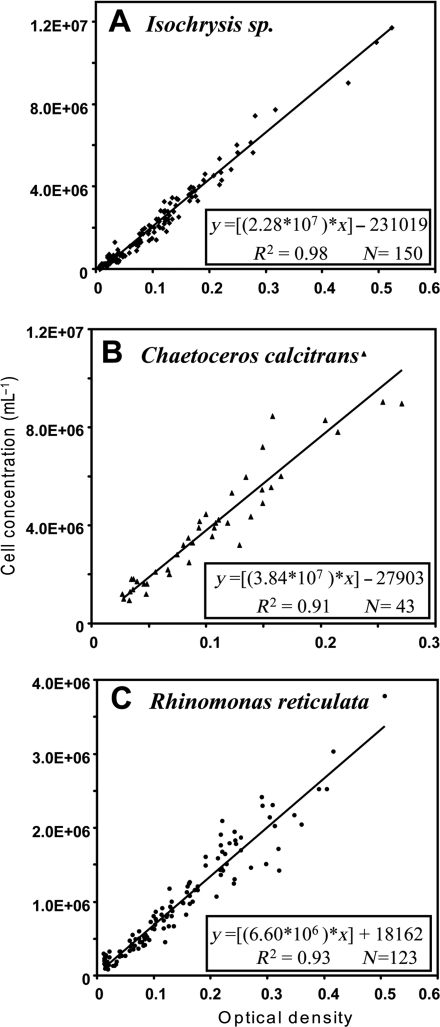
Various methods are commonly used to estimate algal cell density including manual counting with a haemacytometer, automated counting with a Coulter Counter (Multisizer) or spectrophotometric or fluorometric determinations. The latter measurements can be influenced by variations in pigment content of cells as they are cultured over time (Lavens and Sorgeloos, 1996), but nonetheless offer a quick and reasonable estimate of instantaneous cell density. We established standard equations relating optical density at 600 nm (Spectrophotometer Beckman Du-640®, 1 cm cuvette) to algal cell counts using a haemacytometer (Fuchs-Rosenthal). One drop of 5% formalin was added to 10 mL aliquots of algae to fix the cells prior to counting. Relationships of optical densities to cell numbers for the different algal species are shown in Fig. 2. These plots were used to generate equations (Fig. 2A–C) enabling routine calculation of algal cell density from measurements of optical density.
Fig. 2.
Correlation between optical density at 600 nm and cell number determined using a haemacytometer for algal species used as food sources. Linear regression equations, correlation coefficients (R2) and sample numbers (N) are given for each species.
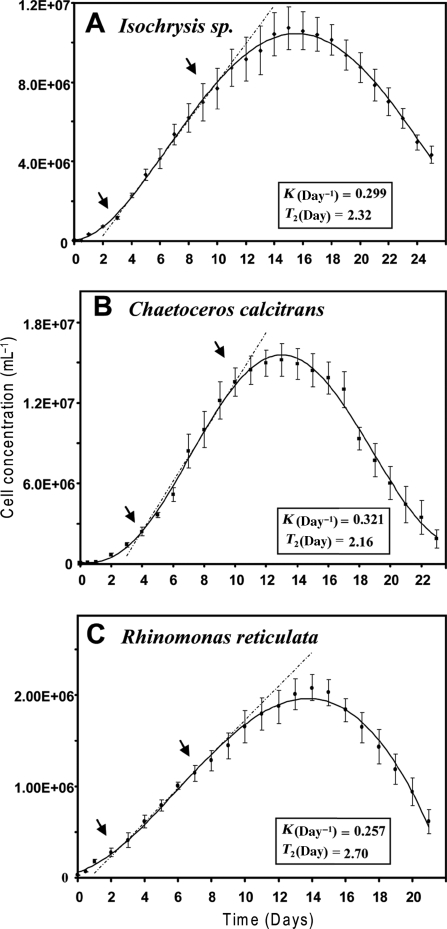
To examine growth rates of the algal strains, cultures were inoculated with an initial cell density of 2.5×104 cells mL−1. Sampling for optical density measurement was carried out twice on the first day and then once per day thereafter (Fig. 3). Growth rates (K) and doubling times (T2) during the linear portion of the exponential growth curves were calculated as indicated in the figure legend. Cell densities of Isochrysis sp. and C. calcitrans increased rapidly with a minor lag phase, whereas R. reticulata grew less rapidly. For routine use in the feeding of O. dioica, minimum cell densities of 1×106 cells mL−1 were reached after 48 h for Isochrysis and 72 h for C. calcitrans. For R. reticulata, a minimum density of 5×105 cells mL−1 was attained after 96 h. Based on these growth curves and to ensure optimal nutritive quality of the algae, cultures used for feeding were discarded after a maximum of 168 h for Isochrysis and C. calcitrans (≈5–6×106 cells mL−1), and 264 h for R. reticulata (≈2×106 cells mL−1).
Fig. 3.
Growth curves at 20°C under continuous illumination for algae used as food sources. Cell concentrations (C, cells mL−1) at times (T, days), were calculated from optical densities using the species specific equations defined in Fig. 2. Growth rates were determined using the equation K = (lnC2−lnC1)/(T2−T1): where K is the growth rate (days−1) and lnCx are the natural logarithms of cell concentrations at the time points Tx. The intersection points (arrows) of the broken lines with the growth curves indicate the lower (T1) and upper (T2) limits of the linear growth phases of the algal cultures that were used for feeding. Doubling times (T2, days) were calculated as T2 = ln2/K. Vertical bars indicate standard errors (N = 6).
Infrastructure for culture of Oikopleura dioica
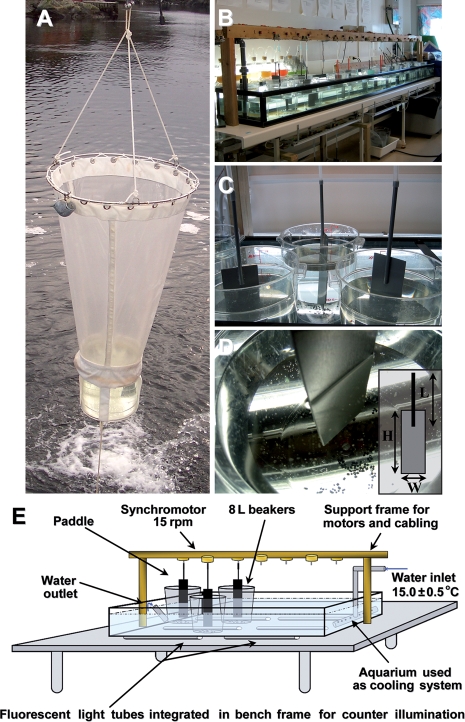
The culture protocol for O. dioica is based on a system originally developed by Fenaux and Gorsky (Fenaux and Gorsky, 1985). The animals are raised at 15.0 ± 0.5°C in 8 L polycarbonate beakers (Cambro camwear® RFSCW8), containing 6 L of seawater (Fig. 4). Animals and algal feed are maintained in suspension by the rotation of a polyvinylcarbonate paddle connected to an electric motor (Synchromotor Crouzet, 15 rpm; 82.334.5 15 rpm AIG; Tufvassons PFS 150S transformer, 6124-0080, 230-24 V, 50–60 Hz, 6.3 A). Motors are mounted in parallel, and are individually protected by a fuse (1/4 A 250 V). The animals in the beakers with their forage organisms are cultivated in 10–12 h light and 14–12 h dark cycles. Under these conditions the life cycle is 6 days. To manipulate the animals, we use polypropylene beakers for dilution at days 1 and 2, and plastic Sterilin pipettes with blunt cut tips to transfer the animals to fresh beakers on days 3–6. The inside diameter of the transfer pipettes varies from 6 to 10 mm as a function of animal house size. To maintain water quality in beakers over a 24 h period, ∼10 g of pre-rinsed activated charcoal pellets (VWR charcoal 1–3 mm gradient, 12 700-5), are added to each beaker, with the exception of beakers used to accomplish the spawn. Charcoal pellets can be washed under running seawater and re-used up to four times. To facilitate routine observation and work with the animals, counter-illumination (OSRAM light tubes cool white 36 W) is installed in the tables supporting the temperature-controlled aquaria. Portable light boxes made of wood covered with an 8 mm plexiglass plate can be used for manipulating animals outside the wet lab or in the field. The inside of the boxes are painted black to accentuate the light/shadow transition, and they are equipped with a circular light bulb (OSRAM Circolux 24W/827, Lumilux warm white 220-240 V-E27).
Fig. 4.
Collection and culture of Oikopleura dioica. (A) Animals are collected using a customized plankton net with a transparent, large, plexiglass cod end. (B) Overview of the culture system diagrammed in E. (C) Animals are cultured in 6 L of seawater in 8-L polycarbonate beakers and maintained in suspension by a polyvinylcarbonate (PVC) paddle rotating at 15 rpm. (D) The beakers are supplemented with grains of activated charcoal to regulate water quality over time. The paddles are made with a 3 mm thick PVC plate attached to an 8 mm diameter PVC rod using PVC glue. Dimensions (cm) of the paddle (inset) are adjusted according to developmental stage. H×L×W = 25×30×8 for spawning, days 1 and 2. The dimensions for other developmental stages are 25×30×7. (E) Schematic illustration of physical organization of the culture system. The fluorescent lighting mounted in the bench top greatly facilitates working with the transparent animals without the need to displace beakers to light boxes.
Field collection of Oikopleura dioica and culture initiation
Collection of appendicularians using traditional plankton nets or pumping is too damaging to these fragile animals. Therefore, O. dioica were collected at slow tow speeds (0.5–0.7 knots) using a customized plankton net (50 or 150 µm mesh size as a function of floral and plankton abundance in the water column) with an opening diameter of 0.8 m and a length of 1.5 m (Fig. 4). The net terminated in a large-volume transparent 15 L cod end bucket made of plexiglass. Primary collection sites were in fjords near Bergen, Norway, at 60°34'N 5°2'E (Site 1) and 60°15'N 5°20'E (Site 2). After each tow, the material in the cod end was transferred to a 20 L beaker (Cambro camwear® RFSCW22) by gently submerging a 1 L polypropylene beaker with handle and carefully emptying the contents into the recipient beaker. The beakers were sealed with a lid and returned to the laboratory. On a daily basis for a minimum 1-week period, animals at the day 4 to 5 stage of development were picked out into 8 L beakers containing 6 L of seawater. The animals are left 3–5 h in order to rinse and expand a new house and are then transferred to a second 8 L beaker. During this process, animals with obvious infections are eliminated. Two types of infection are common and easily detected by eye. The first, a bacterial infection, is manifested by a whitish-yellow appearance of the animals (Flood, 1991). The second is the ectoparasite Oodinium pouchetti (Lemmermann, 1899), appearing as pink balls on the tail of the animal (Fenaux and Gorsky, 1979). If necessary, Oodinium sp. can be eliminated by brief incubation in 0.2 mg mL−1 ethyl 3-aminobenzoate methanesulfonate salt (Sigma; A5040), but in routine practice infected animals are not selected. Collection of 20 females and 15 males is usually sufficient to initiate a culture population.
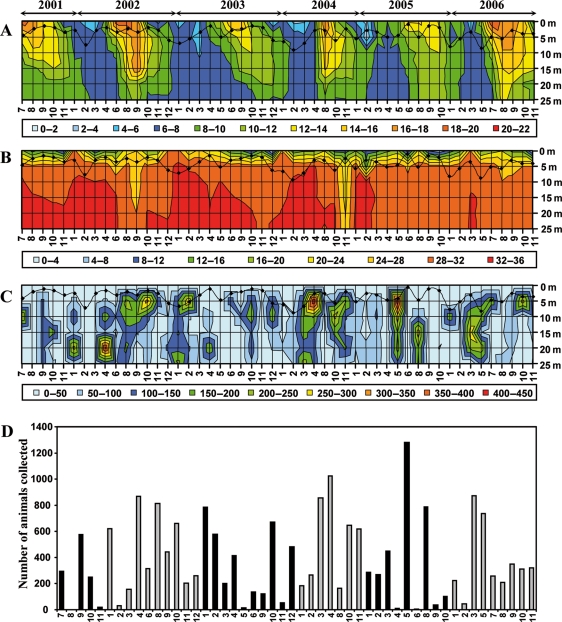
Over a 6-year period, we have carried out near monthly sampling of O. dioica at Site 1, which is fed by a small freshwater stream, generating a gradient of brackish water in the surface layer and it is connected to the fjord via a shallow outlet sill. Temperature and salinity were measured at the beginning of each collection day using a conductivity, temperature and depth (CTD) sensor. The temperature profile (Fig. 5A) alternates between an essentially homogenous 6–8°C during winter and early spring, and a more stratified water column from mid-spring to the end of autumn. Salinity was characterized by a deeper penetration of the brackish layer during periods of temperature stratification in the summer and autumn and the emergence of a zone of higher salinity at depth during the winter months (Fig. 5B). In general, more than 3000 O. dioica were collected per year, with numbers varying substantially from cruise to cruise. Over the sampling period, no obvious correlations were observed between animal abundance (Fig. 5C) and temperature or salinity, although there was a correlation with depth (Table II), indicating the animals were principally found in the upper layers of the water column.
Fig. 5.
Sampling of Oikopleura dioica at Rosslandspollen over a 6-year period. Recordings are given from 0 to 25 m depth for (A) temperature (°C); (B) salinity (°/oo) and (C) number of animals collected. The black curves on each plot represent estimations of turbidity using visibility of the top of the white plankton net as a measure. Months are indicated on the abscissa with 1 to 12 corresponding to January to December. (D) Plot of the number of animals collected per month from 2001 to 2006.
Table II:
Correlations for number of animals collected, versus temperature, salinity and depth
| Mean | Std. Dev. | Temperature | Salinity | Depth | |
|---|---|---|---|---|---|
| Animals | 77 | 84 | −0.017 | −0.039 | −0.158 |
Correlations indicated in bold were significant (P < 0.05, N = 250).
The spawn
The spawn is set up in an 8 L beaker containing 5 L of seawater supplemented with a “conditioner”: 1 mg L−1 ethylenediaminetetraacetic acid (EDTA, Merck 108417) and 20 mg L−1 of sodium metasilicate (Na2SiO3·9H2O, Sigma 5904) (Helm et al., 2004). The principal function of the conditioner is to remove trace metal pollutants from the seawater. EDTA is a common chelator for heavy metal detoxification and reduces water hardness by complexing Ca2+ and Mg2+. Sodium metasilicate is used in water treatment as an agent that binds larger molecule pollutants. We compared the number of healthy day 1 animals produced from 206 standard spawns in the absence of conditioner to 402 standard spawns in the presence of conditioner. After verifying homogeneity of variance between the two treatments [Bartlett's test (Bc = 1.51; χ2(0.05, 1)=3.84; P = 0.218)] an ANOVA (F = 89.73; P < 0.001) revealed that the use of the conditioner significantly improved spawn success. Average spawn density was 16.5 animals per 10 mL (52% healthy animals at day 1) compared with 22 animals per 10 mL (70% healthy animals at day 1) in the absence and presence of conditioner, respectively. The spawn beaker contents are vigorously aerated overnight before use. Prior to transfer of mature animals into the spawn beaker, 2000 cells mL−1 each of Isochrysis sp. and C. calcitrans are added. To set up the spawn, 40–45 mature females and 20 mature males are then transferred into the beaker using a wide bore pipette. For optimum success, it is important to select the animals such that they are as synchronous as possible in their maturation. The beaker is left to incubate for 18–24 h at 15°C, a period corresponding to the time required for the new generation of animals to complete metamorphosis and expand their first house. Spawn density is estimated by counting healthy animals in two 10 mL samples in deep cavity staining blocks (Hecht Assistant, 2020/1), one each from the upper and lower half of the spawn volume, under a binocular microscope. The spawn is then diluted into five fresh 8 L beakers to attain 1500 day 1 animals per beaker. Dilutions are performed by slowly and carefully submerging a polypropylene beaker in the spawn volume and then gently submerging and emptying this in the recipient beaker. Following subsequent dilution protocols described below, this will routinely yield a density of 300–500 animals per beaker at day 3.
The culture cycle and feeding
The five beakers obtained from the dilution of the spawn are cultured for 24 h and then each beaker is diluted 50% with fresh seawater at day 2. The dilution procedure is as above. At day 3, the animals have grown sufficiently to be rapidly distinguished from empty houses and are transferred by pipetting. Here we generate six fresh beakers each containing 150 animals. An effort is made to select animals of homogenous size in order to facilitate subsequent culture and spawning. Then successively, at days 4 and 5, the animals from each beaker are transferred to fresh beakers. The density per beaker is set to 130 animals at day 4 and 110 animals at day 5. At day 6, animals are selected for spawning (see above). In the event that maturation is retarded, animals are also transferred to fresh beakers at day 6 in order to complete maturation.
To optimize growth and fecundity of the animals, we tested a number of dietary regimes (Table III). The results on the growth and fecundity of O. dioica are presented in Table IV. When comparing trunk length at day 5, diet had a significant effect on growth (Table V) ranging from a mean of 366 µm on diet A to 948 µm on diet J. Growth of the animals fed diet J was superior to all other diets tested except I (Table VI). Similar effects of diet on fecundity were observed (Table VII) with oocyte production per female ranging from a mean of 142 on diet A to 388 on diet J, with again diet J significantly superior to all diets except I (Table VIII). Therefore, diet J has been selected as our standard culture diet and permits successful long-term propagation of O. dioica. The main features and rationale of this diet are as follows. Morning and evening feeding is supplied from days 1 to 5. Isochrysis sp. and C. calcitrans are fed throughout the life cycle, the morning feeding being twice the amount of the evening feeding. Feed quantities are doubled after day 3. The small Synechococcus sp. is added only during the first half of the life cycle, while the large R. reticulata is used during the second half. In addition to filtering and feeding on live particles, appendicularians are known to concentrate and feed on dead particles down to about 0.2 µm in diameter (Flood and Deibel, 1998; Fernández et al., 2004). Therefore, feeding is also supplemented with a soluble extract of R. reticulata. Rhinomonas reticulata cells from a culture volume of 3–8 L (1.7–2×106 cells mL−1) were concentrated by centrifugation at 4°C, 6000 rpm for 20 min (Dupont Sorvall® RC5C plus), using 250 mL Sorvall® bottles. Since an individual sample volume capacity of the hydraulic press is 35 mL, the cells were then resuspended in a minimal (35 or 70 mL) volume using a short Paster pipette and a pipette bulb. The samples were kept on ice in falcon tubes until pressing. Using a French Cell press, the cells were crushed at 750–1000 PSI. The resultant soluble extract was aliquoted in Eppendorff tubes and frozen at −80°C. Aliquoted stocks of the frozen extract were thawed and diluted just before use. One culture beaker dose corresponds to an extract from 6 million cells. Live R. reticulata cells are too large to be assimilated by O. dioica up to day 3. The production of extract delivers the beneficial nutritional components of this alga in an absorbable form at earlier developmental stages.
Table III:
Diets used in testing of food regimes for Oikopleura dioica culture
| Developmental stage | |||||
|---|---|---|---|---|---|
| Diet | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 |
| A | 0.333/0.167 | 0.333/0.167 | 0.333/0.167 | 0.666/0.333 | 0.666/0.333 |
| B | 2/1 | 2/1 | 2/1 | 2/1 | 2/1 |
| C | 4/2 | 4/2 | 4/2 | 4/2 | 4/2 |
| D | 6/3 | 6/3 | 6/3 | 6/3 | 6/3 |
| E | 10/5 | 10/5 | 10/5 | 10/5 | 10/5 |
| F | 2/1 | 2/1 | 2/1 | 4/2 | 6/3 |
| G | 2/1 | 2/1 | 2/1 | 4/2 | 4/2 |
| H | 2/1 | 2/1 | 2/1 | 4/2 | 4/2 |
| 0/0 | 0/0 | 0/1 | 1/1 | 1/1 | |
| I | 2/1 | 2/1 | 2/1 | 4/2 | 4/2 |
| 0/0 | 0/0 | 0/1 | 1/1 | 1/1 | |
| ER/ER | ER/ER | ER/ER | ER/ER | ER/ER | |
| J | 2/1 | 2/1 | 2/1 | 4/2 | 4/2 |
| 0/0 | 0/0 | 0/1 | 1/1 | 1/1 | |
| ER/ER | ER/ER | ER/ER | ER/ER | ER/ER | |
| Sa/Sb | Sa/Sb | Sa/– | –/– | –/– | |
Numbers are given in 1000 cells mL−1 as a final concentration in the 6 L culture volume. For diets A to G, only Isochrysis sp. and Chaetoceros calcitrans were used. For example, 2/1 denotes 2000 cells mL−1 of each of the two algal species were added in the morning and 1000 cells mL−1 of each of the two algal species were added in the evening. In the more complex diets H to J, the first row is as above. The second row refers to morning and evening feeding of an additional, larger alga, Rhinomonas reticulata. ER/ER indicates morning and evening feeding of a soluble extract of R. reticulata obtained from the equivalent of a final concentration of 1000 cell mL−1 of this alga. Sa/Sb denotes morning and evening feeding of Synechococcus sp.: “a” indicates a dose of 80 000 cells mL−1 final concentration and “b” indicates 40 000 cells mL−1 final concentration.
Table IV:
Growth and fecundity of Oikopleura dioica as a function of diet
| Trunk length (µm) |
||||||
|---|---|---|---|---|---|---|
| Diet | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Fecundity (no. of oocytes) |
| A | 137 ± 17 | 192 ± 36 | 311 ± 83 | 358 ± 60 | 366 ± 118 | 142 ± 64 |
| B | nd | nd | 314 ± 30 | 478 ± 50 | 668 ± 80 | nd |
| C | nd | nd | 275 ± 35 | 423 ± 56 | 709 ± 107 | nd |
| D | 168 ± 9 | 193 ± 12 | 318 ± 40 | 463 ± 66 | 720 ± 120 | 182 ± 64 |
| E | 168 ± 9 | 190 ± 20 | 318 ± 42 | 445 ± 87 | 693 ± 133 | 162 ± 56 |
| F | nd | nd | 353 ± 54 | 513 ± 62 | 797 ± 99 | nd |
| G | 153 ± 14 | 195 ± 21 | 328 ± 55 | 490 ± 122 | 766 ± 165 | 256 ± 96 |
| H | 168 ± 9 | 208 ± 21 | 305 ± 41 | 487 ± 82 | 819 ± 131 | 272 ± 77 |
| I | 194 ± 29 | 291 ± 29 | 357 ± 44 | 601 ± 76 | 879 ± 124 | 368 ± 179 |
| J | 168 ± 9 | 213 ± 21 | 345 ± 49 | 533 ± 85 | 948 ± 116 | 388 ± 104 |
Means and standard deviations are given. A minimum of 10 measurements per replicate of two to five replicates were made for each stage of each diet. A minimum of 14 measurements were made for fecundity values. nd, not determined. Diets A to J are defined in Table III.
Table V:
Oikopleura dioica day 5 trunk length versus diet: ANOVA
| Variation | SS | DF | MS | F | P |
|---|---|---|---|---|---|
| Total | 72 9270 | 39 | 18 699 | ||
| Group | 63 6643 | 9 | 70 738 | 22.91 | <0.001 |
| Error | 92 627 | 30 | 3088 |
The analysis based on the 10 diets A to J was performed using Statistica 8 Statsoft software, after verification of homogeneity of variances with the Bartlett's test: [Bc = 16.21; χ2(0.05, 9)=16.92; P = 0.063].
Table VI:
Multiple pairwise comparisons of the effect of diets on mean trunk length at day 5
| Diet | A | B | C | D | E | F | G | H | I | J |
|---|---|---|---|---|---|---|---|---|---|---|
| A | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| B | 0.987 | 0.943 | 1.000 | 0.069 | 0.317 | 0.018 | <0.001 | <0.001 | ||
| C | 1.000 | 1.000 | 0.458 | 0.903 | 0.179 | 0.005 | <0.001 | |||
| D | 0.999 | 0.632 | 0.972 | 0.292 | 0.010 | <0.001 | ||||
| E | 0.243 | 0.699 | 0.078 | 0.002 | <0.001 | |||||
| F | 0.998 | 1.000 | 0.537 | 0.017 | ||||||
| G | 0.929 | 0.151 | 0.002 | |||||||
| H | 0.869 | 0.027 | ||||||||
| I | 0.759 | |||||||||
| J |
Post hoc Tukey test with unequal sample size was applied. Significant pairwise differences in the effects of diets (Table IV) on growth are indicated in bold.
Table VII:
Oikopleura dioica fecundity versus diet: ANOVA
| Variation | SS | DF | MS | F | P |
|---|---|---|---|---|---|
| Total | 4.803 | 135 | 0.036 | ||
| Group | 2.371 | 6 | 0.395 | 20.95 | <0.001 |
| Error | 2.432 | 129 | 0.019 |
The analysis based on the 10 diets A to J was performed using Statistica 8 Statsoft software, after logarithmic transformation and verification of homogeneity of variances with the Bartlett's test: [Bc = 12.58; χ2(0.05,6)=16.59; P = 0.05].
Table VIII:
Multiple pairwise comparison of the effect of diets on fecundity
| Diet | A | D | E | G | H | I | J |
|---|---|---|---|---|---|---|---|
| A | 0.911 | 0.753 | <0.001 | <0.001 | <0.001 | <0.001 | |
| D | 0.999 | 0.034 | 0.006 | <0.001 | <0.001 | ||
| E | 0.205 | 0.108 | <0.001 | <0.001 | |||
| G | 1.000 | 0.244 | 0.021 | ||||
| H | 0.338 | 0.030 | |||||
| I | 0.943 | ||||||
| J |
Post hoc Tukey test with unequal sample size was applied. Significant pairwise differences in the effects of diets (Table IV) on fecundity are indicated in bold.
The current capacity of our culture system is to produce up to 6000 mature animals per week. Stages up to day 3 are available at considerably higher numbers. We routinely maintain four to five populations permitting use of most developmental stages on any given day. At this scale, seawater requirements are 1800 L per week.
DISCUSSION
A routine, durable and reliable culture protocol is essential to successful implantation of O. dioica as a modern model organism in an increasing number of laboratories. During initial phases of elaboration of the detailed protocol presented here, loss of the culture was not an uncommon occurrence. Symptoms of impending failure often included decreasing animal size and reduced fecundity in successive generations and an inability of many animals to inflate their first house. These difficulties were largely overcome through control of water quality, implementation of a sufficiently diverse diet, attention to detail in the cultivation of forage algae, careful selection against parasite contamination and improvement in the spawning medium resulting in higher and more consistent reproductive success. Using the current protocol, culture crash events are now very rare and we currently have an individual population that, at the time of writing, has been maintained for 89 successive generations in the absence of any out crossing.
In practice, and certainly during initial set up of a culture facility, it remains useful to have access to an external source of O. dioica. One option is a local field collection site. Of our two field sites, site 2 allows collection from mid-spring to mid-autumn, a time when O. dioica is usually easily found in Norwegian coastal waters. Site 1, Rosslandspollen, permits year round collection and seems to serve as an example of a refuge area for this species during the winter months. An alternative is to obtain live seed animals from an existing culture facility such as ours. We have successfully shipped live animals to destinations in Europe and North America. The optimum method is to set up several mini-spawns in 250 mL flasks filled with spawning media and containing 3–4 males and 12 females. Flasks are packed in a polystyrene box and sent via express carrier. Delivery within 24 to 48 h generally allows recovery of sufficient numbers of viable seed animals.
Though careful maintenance of multiple algal strains requires an additional effort, it is clearly beneficial in the culture of O. dioica. A combination of two or three species of high nutritional value, including a suitable-sized diatom and flagellate invariably provide improved growth rates in marine culture (Helm et al., 2004). Unicellular microalgae are commonly used in aquaculture, and increasing numbers of strains are commercially available. These species vary in nutritional value, related to their shape, size, toxicity, digestibility and biochemical composition (Brown and Farmer, 1994). Biochemical composition can also vary as a function of culture conditions, including media (Wikfors et al., 1984); temperature (James et al., 1989; Thompson et al., 1992); light intensity (Thompson et al., 1990) and aeration. Isochrysis, C. calcitrans, R. reticulata and Synechococcus are commonly used in marine culture. In addition to their suitable characteristics as food for O. dioica, we also found these species to be relatively stable in culture, unlike other species we tested such as Thalassiosira pseudonana, Tetraselmis suecica and Emiliana huxleyi. Troedsson et al. (Troedsson et al., 2005) observed that the use of a mixture of Isochrysis and C. calcitrans, as the core diet for O. dioica provided a balanced regime of complementary fatty acid composition. In addition to nutritional features of algae, it is essential to consider the prey size and type in the case of appendicularia, because only a fraction of particles cleared from the water column will be ingested, the rest remaining trapped in the house (Troedsson et al., 2007). It is important not to over feed appendicularians in the culture. Over feeding leads to clogging of filters, which has been shown to make animals escape their house prematurely during short-term incubations (Tiselius et al., 2003). Recent studies have also indicated that high concentrations of Chaetoceros calcitrans may hinder the effective clearance of other algae (Troedsson et al., 2007). Thus, over feeding can have detrimental effects on animal production.
With sufficient vigilance, the detailed culture protocol presented here permits uninterrupted long-term culture of O. dioica. Perspectives for further improvement and increased accessibility of the culture can now be oriented in several directions. Nishida and colleagues (Fujii et al., 2008) have adopted the culture protocol to the use of artificial seawater, permitting inland culture of O. dioica and allowing significant expansion of use of the model beyond coastal laboratories equipped with seawater. In routine practice, we out cross populations after 10–20 generations of culture, either to other culture populations or with animals collected in the field. This avoids genetic bottlenecks that can impair routine production of animals, but for genetic experimentation it would be useful to establish inbred strains. It would also be useful for this purpose to have larger scale production of animals in smaller lots, particularly when envisaging a genetic screen. We have carried out sib-matings for 18 successive generations. The main difficulty lies in repetitive selection of synchronously maturing males and females to maximize reproductive success at each generation. For this we used 1 L glass beakers. Each cross “1 male × 1 female” was set up in 600–700 mL of seawater+algae [paddle (H×L×W): 18×30×4]. To initiate the protocol, at day 1 following the mating of one pair, offspring were diluted in 6 L seawater in an 8 L beaker, and fed only the standard morning feeding. Animals were not diluted at day 2, and again fed once. From day 3 onwards sib-cultures were continued using the standard culture protocol. For subsequent generations of sib-matings, it was important to set up several candidate sib-mating pairs in order to select the most successful crossing to carry on with.
A current limitation in the O. dioica culture protocol is the labour required for manual transfer of animals during days 3–5. This is required to maintain a healthy culture because at 15°C, animals excrete a fecal pellet every 2–3 min (López-Urrutia and Acuña, 1999), and discard houses every 4 h (Fenaux, 1985, Acuña and Kiefer, 2000; Sato et al., 2001). Over a 24 h period, the metabolic waste and particles that accumulate degrade water quality and negatively affect the animals. We are currently assessing approaches to automating water cleansing/replacement to eliminate animal transfer during this portion of the life cycle. We are experiencing success in doing this with manual transfers limited to reducing animal density at day 3 and selection of animals for spawning at day 6, considerably reducing man hours required for culture. It is also possible to set up shorter term cultures of the animals for those not wishing to undertake a year round enterprise. Cryopreservation of lines will also be essential to development of this animal model. Currently, we are able to cryopreserve sperm (unpublished data) but have not yet had success with embryos.
It is our hope that the data and protocols provided in this manuscript will facilitate other investigators in adopting this important, fascinating, emergent model organism, O. dioica, in their own research.
FUNDING
This work was supported by grants 133335/V40 and 145326/432 from the Norwegian Research Council (E.M.T.).
ACKNOWLEDGEMENTS
We acknowledge G.-A. Paffenhöfer and R. Fenaux for their pioneering work in the culturing and study of Appendicularia. We thank G. Gorsky for expert advice in initiating our O. dioica culture and D. Eversen for her help with algal culture. We thank the crews of R/V Hans Brattstrøm and R/V Aurelia, for help in collecting field animals. Finally, we thank the technical staff of the Sars Centre Appendicularian culture facility and the ILAB personnel for their continued efforts.
REFERENCES
- Acuña J. L., Kiefer M. Functional response of the appendicularian Oikopleura dioica. Limnol. Oceanogr. 2000;45:608–618. [Google Scholar]
- Alldredge A. Discarded Appendicularian houses as sources of food, surface habitats, and particulate organic matter in planktonic environments. Limnol. Oceanogr. 1976;21:14–23. [Google Scholar]
- Bassham S., Postlethwait J. H. The evolutionary history of placodes: a molecular genetic investigation of the larvacean urochordate Oikopleura dioica. Development. 2005;132:4259–4272. doi: 10.1242/dev.01973. [DOI] [PubMed] [Google Scholar]
- Brown M. R., Farmer C. A. Riboflavin content of six species of microalgae used in marineculture. J. Appl. Phycol. 1994;6:61–65. [Google Scholar]
- Delsuc F., Brinkmann H., Chourrout D., et al. Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature. 2006;439:965–968. doi: 10.1038/nature04336. [DOI] [PubMed] [Google Scholar]
- Fenaux R. Rhythm of secretion of oikopleurid‘s houses. Bull. Mar. Sci. 1985;37:498–503. [Google Scholar]
- Fenaux R. Anatomy and functional morphology of the Appendicularia. In: Bone Q., editor. The Biology of Pelagic Tunicates. New York: Oxford University Press; 1998. pp. 25–34. [Google Scholar]
- Fenaux R., Gorsky G. Techniques d'élevages des Appendiculaires. Ann. Inst. Océanogr. Paris. 1979;55:195–200. [Google Scholar]
- Fenaux R., Gorsky G. Nouvelle technique d'élevage des appendiculaires. Rapports et Procès-Verbaux des Réunions (Commission Internationale pour l'Exploration Scientifique de la Mer Méditerranée) 1985;29:291–292. [Google Scholar]
- Fenaux R., Bone Q., Deibel D. Appendicularia distribution and zoogeography. In: Bone Q., editor. The Biology of Pelagic Tunicates. New York: Oxford University Press; 1998. pp. 251–264. [Google Scholar]
- Fernández D., Lopez-Urrutia A., Fernández A., et al. Retention efficiency of 0.2 to 6 µm particles by the appendicularians Oikopleura dioica and Fritillaria borealis. Mar. Ecol. Prog. Ser. 2004;266:89–101. [Google Scholar]
- Flood P. R. Yellow-stained Oikopleurid appendicularians are caused by bacterial parasitism. Mar. Ecol. Prog. Ser. 1991;71:291–295. [Google Scholar]
- Flood P. R., Deibel D. The appendicularian house. In: Bone Q., editor. The Biology of Pelagic Tunicates. New York: Oxford University Press; 1998. pp. 105–124. [Google Scholar]
- Fujii S., Nishio T., Nishida H. Cleavage pattern, gastrulation, and neurulation in the appendicularian, Oikopleura dioica. Dev. Genes. Evol. 2008;218:69–79. doi: 10.1007/s00427-008-0205-4. [DOI] [PubMed] [Google Scholar]
- Ganot P., Thompson E. M. Patterning through differential endoreduplication in epithelial organogenesis of the chordate, Oikopleura dioica. Dev. Biol. 2002;252:59–71. doi: 10.1006/dbio.2002.0834. [DOI] [PubMed] [Google Scholar]
- Ganot P., Kallesøe T., Reinhardt R., et al. Spliced-leader RNA trans splicing in a chordate, Oikopleura dioica, with a compact genome. Mol. Cell. Biol. 2004;24:7795–7805. doi: 10.1128/MCB.24.17.7795-7805.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorsky G., Fenaux R. The role of Appendicularia in marine food webs. In: Bone Q., editor. The Biology of Pelagic Tunicates. New York: Oxford University Press; 1998. pp. 161–169. [Google Scholar]
- Helm M. M., Bourne N., Lovatelli A. Hatchery culture of bivalves, a practical manual. Part 5—Hatchery operation: culture of larvae basic methodology, feeding and nutrition, factors influencing growth and survival, and settlement and metamorphosis. FOA Fisheries Technical Paper. 2004:84–129. [Google Scholar]
- James C. M., Al-Hinty S., Salman A. E. Growth and ω3 fatty acid and amino acid composition of microalgae under different temperature regimes. Aquaculture. 1989;77:337–357. [Google Scholar]
- Kimura S., Ohshima C., Hirose E., et al. Cellulose in the house of the appendicularian Oikopleura rufescens. Protoplasma. 2001;216:71–74. doi: 10.1007/BF02680133. [DOI] [PubMed] [Google Scholar]
- Lavens P., Sorgeloos P. Manual on the production and use of live food for aquaculture. FAO, Fisheries Technical Paper. 1996;vol. 361:295. [Google Scholar]
- López-Urrutia A., Acuña J. L. Gut throughput dynamics in the appendicularian Oikopleura dioica. Mar. Ecol. Prog. Ser. 1999;191:195–205. [Google Scholar]
- Paffenhöfer G. A. The cultivation of an Appendicularian through numerous generations. Mar. Biol. 1973;22:183–185. [Google Scholar]
- Robison B. H., Reisenbichler K. R., Sherlock R. E. Giant larvacean houses: rapid carbon transport to the deep sea floor. Science. 2005;308:1609–1611. doi: 10.1126/science.1109104. [DOI] [PubMed] [Google Scholar]
- Sato R., Jingshan Y., Yuji T., et al. New apparatuses for cultivation of appendicularians. Plankton Biol. Ecol. 1999;46:162–164. [Google Scholar]
- Sato R., Tanaka Y., Ishimaru T. House production by Oikopleura dioica (Tunicata, Appendicularia) under laboratory conditions. J. Plankton Res. 2001;23:415–423. [Google Scholar]
- Seo H. C., Kube M., Edvardsen R. B., et al. Miniature genome in the marine chordate Oikopleura dioica. Science. 2001;294:2506. doi: 10.1126/science.294.5551.2506. [DOI] [PubMed] [Google Scholar]
- Silver M. W., Aldredge A. L. Bathypelagic marine snow: deep-sea algal and detrital community. J. Mar. Res. 1981;39:501–530. [Google Scholar]
- Spada F., Steen H., Troedsson C., et al. Molecular patterning of the oikoplastic epithelium of the larvacean tunicate Oikopleura dioica. J. Biol. Chem. 2001;276:20624–20632. doi: 10.1074/jbc.M100438200. [DOI] [PubMed] [Google Scholar]
- Stach T., Winter J., Bouquet J.-M., et al. Evolution of life-cycles: the embryology of a planktonic tunicate reveals traces of sessility. Proc. Natl Acad. Sci. USA. 2008;105:7229–7234. doi: 10.1073/pnas.0710196105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiselius P., Petersen J. K., Nielsen T. G., et al. Functional response of Oikopleura dioica to house clogging due to exposure to algae of different sizes. Mar. Biol. 2003;142:253–261. [Google Scholar]
- Thompson P. A., Harrison P. J., Whyte J. N. C. Influence of irradiance on the fatty acid composition of phytoplankton. J. Phycol. 1990;26:278–288. [Google Scholar]
- Thompson P. A., Guo M.-X., Harrison P. J. Effects of variation in temperature on the biochemical composition of eight species of marine phytoplankton. J. Phycol. 1992;28:481–488. [Google Scholar]
- Thompson E. M., Kallesøe T., Spada F. Diverse genes expressed in distinct regions of the trunk epithelium define a monolayer cellular template for construction of the oikopleurid house. Dev. Biol. 2001;238:260–273. doi: 10.1006/dbio.2001.0414. [DOI] [PubMed] [Google Scholar]
- Troedsson C., Bouquet J. M., Aksnes D. L., et al. Resource allocation between somatic growth and reproductive output in the pelagic chordate Oikopleura dioica allows opportunistic response to nutritional variation. Mar. Ecol. Prog. Ser. 2002;243:83–91. [Google Scholar]
- Troedsson C., Grahl-Nielsen O., Thompson E. M. Variable fatty acid composition of the pelagic appendicularian Oikopleura dioica in response to dietary quality and quantity. Mar. Ecol. Prog. Ser. 2005;289:165–176. [Google Scholar]
- Troedsson C., Frischer M. E., Nejstgaard J. C., et al. Molecular quantification of differential ingestion and particle trapping rates by the appendicularian Oikopleura dioica as a function of prey size and shape. Limnol. Oceanogr. 2007;52:416–427. [Google Scholar]
- Walne P. R. Experiments in large scale culture of the larvae of Ostrea edulis L. Fish Invest. Ser. II. 1966;25:1–53. [Google Scholar]
- Wikfors G. H., Twarog J. W., Ukeles R. Influence of chemical composition of algae food sources on growth of juvenile oysters, Crassostrea virginica. Biol. Bull. 1984;167:251–263. [Google Scholar]