Abstract
We investigated the effect of different light conditions on primary production and growth rates of three closely related freshwater picocyanobacterial strains from three different ribotypes in laboratory cultures. The primary goal was to test whether not only different pigment types (PC-rich versus PE-rich) but also other physiological characteristics suggested by different phylogenetic positions could affect growth and photosynthetic rates of picocyanobacteria. Secondly, we tested whether photacclimation is strain specific. Experiments were conducted over light intensities ranging from 6 to 1500 μmol photons m−2 s−1 with cultures that were acclimated to low (10 μmol photons m−2 s−1) and moderate (100 μmol photons m−2 s−1) irradiance. The PE-rich strain was sensitive to high light conditions and reached highest photosynthesis and growth rates at low light intensities. The relative effect of photoacclimation was different between the two PC-rich strains, with one strain showing only moderate changes in growth rates in response to the light level used during the acclimation period. Overall, growth rates differed widely in response to light intensity and photoacclimation. Photoacclimation significantly affected both primary production and growth rates of all three strains investigated. We conclude that strain-specific photoacclimation adds to the niche partitioning among closely related freshwater picocyanobacteria.
INTRODUCTION
Picocyanobacteria are an integral part of the autotrophic picoplankton in fresh and marine waters, contributing substantially to primary production, in particular in oligotrophic ecosystems (Bell and Kalff, 2001; Callieri, 2008). Picocyanobacteria serve as food for many protists and small invertebrate species (Weisse, 1988; Weisse, 1993; Stockner et al., 2000; Callieri and Stockner, 2002). At the genus level, several freshwater clusters of Synechococcus and Cyanobium gracile are cosmopolitan (Crosbie et al., 2003). The closely related strains, with an average sequence identity of the 16S rRNA gene of 98% (Crosbie et al., 2003; Ernst et al., 2003), differ in several phenotypic features such as their photosynthetic response (Callieri et al., 2005), pigmentation (Ernst et al., 1992; Callieri et al., 1996; Postius and Ernst, 1999) and nutritional value for protist grazers (Moser & Weisse, in prep.). Pigmentation is the main phenotypic difference within the similarly sized, planktonic freshwater picocyanobacteria. Red PE-rich picocyanobacteria use phycoerythrin, green PC-rich ones phycocyanin as major light-harvesting pigments (Stockner and Antia, 1986; Stockner, 1988; Callieri et al., 1996). Although there is a correlation between the pigment type and their phylogenetic position, both pigment types have been found in several of the at least 7 nonmarine picocyanobacterial clusters (Crosbie et al., 2003). A new clade, sister to Cyanobium, was recently reported from oceanic waters, based upon phylogenetic analysis of concatenated 16S rDNA and rpoC1 data sets (Everroad and Wood, 2006). This large clade includes both PE-rich and PC-rich strains. Similarly, the marine cluster B (MC-B) contains PE-rich and PC-rich strains, and this cluster is polyphyletic, consisting of at least two different subclusters (Chen et al., 2006). Similar to the results from Everroad and Wood (Everroad and Wood, 2006), phylogeny derived from the cpcBA operon of the green PC pigment was better able to separate differently pigmented picocyanobacteria than 16S rRNA-ITS phylogeny (Haverkamp et al., 2008). Distinct genotypes have already been linked to different ecotypes for marine picocyanobacteria (Moore et al., 1995; Moore et al., 1998; Rocap et al., 2002; Haverkamp et al., 2008). For freshwater picocyanobacteria, more ecophysiological evidence is needed to decide whether the different genotype clusters should be given species rank (Crosbie et al., 2003).
The aim of our study was to extend the previous work of Callieri et al. (Callieri et al., 2005) on characterizing distinct ecotypes using ecophysiological parameters. Niche partitioning with respect to photophysiology should lead to differential fitness of the respective ecotypes. In asexually reproducing taxa such as cyanobacteria population growth rate can be used as a direct proxy for their fitness (Weisse, 2006). Callieri et al. (Callieri et al., 2005) had reported significant differences in the maximum photosynthetic rate (Pmax), the maximum light utilization coefficient (α), the light saturation coefficient (Ek) and the cellular chlorophyll a content between two PE-rich and two PC-rich freshwater picocyanobacterial strains of the Group B and I clusters (Crosbie et al., 2003). In the previous investigation, the two strains of each pigment type originated from the same ribotype cluster. In the present study, we investigated the photosynthetic and growth response of three strains representing different genotype clusters, Group A (C. gracile cluster), Group B and Group I (Crosbie et al., 2003), and pigment types, which may help in defining species boundaries among the ribotype clusters. The ecophysiology of the large C. gracile cluster, which is present both in freshwater and in the oceans (Everroad and Wood, 2006), has not been studied previously.
The outcome of laboratory experiments may be sensitive to acclimation to specific laboratory conditions such as irradiance and temperature. In the field, calm periods of relatively constant light levels during the day, when photoacclimation is likely, may be followed by periods of weather with rapidly changing light conditions, when photoacclimation appears unlikely. Accordingly, we investigated whether photoacclimation to different light intensities would affect the rates of photosynthesis and growth of the picocyanobacteria. Our null hypotheses were that (i) there was no effect of photoacclimation and (ii) that there was no difference in primary production and growth rates of the closely related, but differently pigmented picocyanobacterial strains.
METHOD
Stock and preliminary cultures
The strains were chosen according to the ribotype clusters from the maximum-likelihood tree published by Crosbie et al. (Crosbie et al., 2003). Two of these strains (MW4C3, Group B, and MW100C3, Group I) were isolated from Lake Mondsee (Crosbie et al., 2003). The strain BO8801 (Group A) had been isolated from Lake Constance in 1988 (Ernst, 1991). The isolate MW4C3 is a red, PE-rich strain, while the isolates BO8801 and MW100C3 are green, PC-rich strains. All stock cultures of ∼40-mL volume were kept non-axenically in 50-mL culture flasks with medium BG11 (Allen, 1968).
One culture (50 mL) of each strain was acclimated to low light levels of 10 µmol photons m−2 s−1 (LL in the following), a second culture of each strain was acclimated to moderate light levels of 100 µmol photons m−2 s−1 (ML in the following). The photoacclimation period, at a temperature of 20°C, lasted for 2 months. New batch cultures (initial abundance, ∼106 cells mL−1) were inoculated when cell numbers in the original cultures had reached concentrations of ∼109 cells mL−1.
Measurement of photosynthesis/irradiance (P/E) curves
We conducted P/E and growth experiments with all three strains in parallel for the LL and ML acclimated cyanobacteria. The experiments were started when cell concentrations in the photoacclimation cultures had reached (1–5) × 108 cells mL−1; preliminary experiments (data not shown) had revealed that the background of heterotrophic bacteria was lowest (∼104 cells mL−1) at this picocyanobacterial level. For the experiments, the cultures were diluted in autoclaved 1-L glass flasks with medium BG11 to yield an initial picocyanobacterial abundance of∼1 × 106 cells mL−1 and a volume of 800–900 mL. This cell density, corresponding to an optical density (OD750) of ∼0.06, was chosen to avoid any self-shading effects (Oberhaus et al., 2007); OD was measured at 750 nm using a Perkin-Elmer Lambda 2 Spectrophotometer (Perkin-Elmer Life and Analytical Sciences, Waltham, MA, USA). Each acclimated culture was then kept in these 1-L glass flasks at their initial light and temperature conditions for 2 h to minimize the risk of a lag-phase during the experiments. Thereafter, the cultures were split and pipetted into a series of 25-mL glass vials. We then added 30 µL of NaH14CO3 with an activity of 20 µCi mL−1 to each vial, yielding an initial activity of 0.03 µCi mL−1 (equivalent to 1.11 kBq mL−1) in the experimental containers. Immediately thereafter, the vials were incubated at nine different light intensities (1495, 1245, 697, 614, 498, 141, 71 14, 5.8 µmol photons m−2 s−1) in an incubator filled with circulating water at a constant temperature of 20 ± 0.2°C (Callieri and Piscia, 2002; Callieri et al., 2004). Light was provided by three tungsten halogen lamps (1000W Osram 64 740 Haloline) with spectral characteristics similar to the Atlas OHS2000 lamps used by Cullen and Lewis (Cullen and Lewis, 1988). The vials rotated slowly (4 rpm) during the incubation period of 4 h. We used three replicates for each light intensity; three additional vials kept at the same temperature in the dark served as controls. Another 100-mL sample of the acclimated cultures was used to estimate the initial chlorophyll a concentration in the experimental containers. These samples were filtered on 0.2-µm polycarbonate filters (Nuclepore); chlorophyll a was measured with an LS2 fluorometer after 1 h of methanol extraction of the pigments (Talling and Driver, 1961, Holm-Hansen and Riemann, 1978).
After the 4 h incubation time, a 1-mL sample was taken from each glass vial for the measurement of the total 14C activity. Radioactivity was measured with a Beckmann LS 6000 TA scintillation counter after the addition of the scintillation cocktail “Instant gel” (Packard). The remaining volume of the 25-mL glass vials was filtered on a 0.22 µm nitrocellulose membrane filter (Millipore), using plastic disposable syringes and a 25-mm plastic filter holder. These filters were acidified with 200 µL 1 N HCl for 1 h to remove the remaining dissolved, surplus 14C. The scintillation cocktail “Filter count” (Packard) was then added, and the assimilated carbon was measured with the scintillation counter. Radioactive uptake measured in the dark bottle at time 0 was subtracted from the experimental results (reviewed by Callieri and Stockner, 2002).
The P/E data were normalized to chlorophyll a. We used the non-linear least-squares regression model by Eilers and Peeters (Eilers and Peeters, 1988) to fit the curves and to estimate the parameters Pmax (maximum photosynthetic rate), α (maximum light utilization coefficient) and Ek (light saturation). This model yielded the best fit to the data and was used earlier for similar experimental work (Callieri and Piscia, 2002; Callieri et al., 2005).
Growth rate experiments
The acclimated cultures (800 mL) for the growth experiment were also pre-incubated in sterile 1-L glass flasks for 2 h to account for a potential lag-phase. Thereafter, 34-mL subsamples were fixed with formalin (2% vol/vol final concentration) to estimate the initial cyanobacterial concentration. Similar to the P/E experiments, sterile 25-mL glass vials were then filled with the acclimated experimental cultures and incubated in the rotating, water circulating incubator at the nine different light intensities reported above, for 24 h. After the incubation, 4-mL subsamples from each vial were fixed with formalin for measurements of final picocyanobacterial abundance. The experiments were conducted with three replicates at each light intensity.
Cell numbers of picocyanobacteria were measured with a flow cytometer (FacsCalibur, Becton Dickinson), using the software programmes “Cellquest” and “Attractors” (Becton Dickinson). Cyanobacterial growth rates (µ, day−1) were calculated from their initial (N0) and final (N24) cell numbers according to µ = ln(Nt/N0)/t.
Statistical analyses were performed with Sigma Stat 2.03 (SPSS Inc.) using t-test and one-way ANOVA to evaluate the differences in photosynthetic and growth parameters reported in Table I. In particular, differences between cultures adapted to LL and ML were compared pairwise. The results were considered significant if P was <0.05.
Table I:
Photosynthetic and growth rate parameters of the three picocyanobacterial strains measured after photoacclimation to low light (LL, 10 µmol photons m−2 s−1) or moderate light (ML, 100 µmol photons m−2 s−1) conditions, respectively
| LL |
ML |
|||||
|---|---|---|---|---|---|---|
| Parameter | MW4C3 | MW100C3 | BO8801 | MW4C3 | MW100C3 | BO8801 |
| Pmax | 3.06 | 0.16 | 0.72 | 1.73 | 2.09 | 2.04 |
| Ek | 50.3 | 15.3 | 3.0 | 73.6 | 48.2 | 31.4 |
| α | 0.061 | 0.010 | 0.239 | 0.023 | 0.031 | 0.067 |
| µmax | 0.31 ± 0.03 | 0.23 ± 0.03 | 0.40 ± 0.05 | 0.09 ± 0.01 | 0.90 ± 0.15 | 0.42 ± 0.02 |
| Chlcell | 2.76 ± 0.10 | 19.05 ± 0.25 | 7.29 ± 0.47 | 1.63 ± 0.07 | 5.05 ± 0.07 | 4.25 ± 0.09 |
Photosynthetic parameters reported in the first three rows were derived from the curve fits shown in Fig. 2. Values reported are means ± standard deviation. Pmax= maximum photosynthetic rate (mg C mg Chl a−1 h−1); Ek = light saturation coefficient (µmol photons m−2 s−1); α = initial linear slope of the P/E curve or maximum light utilization coefficient (mg C (mg Chl a)−1 h−1 (µmol photons m−2 s−1)−1); µmax = maximum growth rate (day−1); Chlcell = cell-specific Chl a at time zero (fg Chl a cell−1).
RESULTS
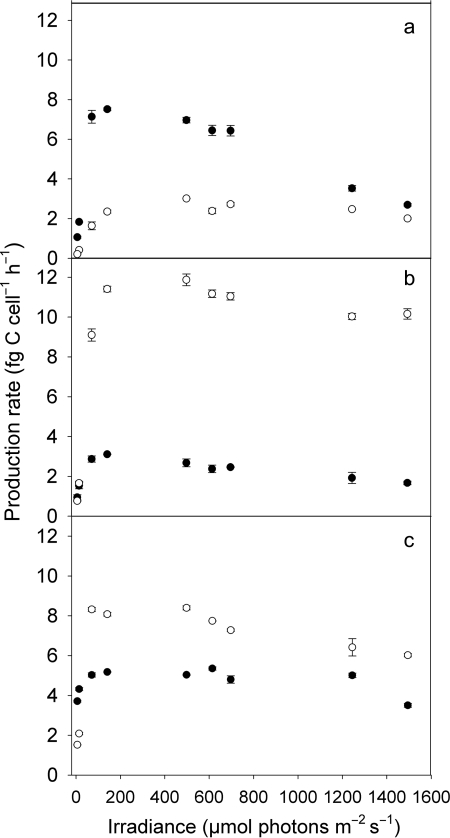
The three strains showed maximum cell-specific production rates ranging from 3.1 to 11.9 fg C cell−1 h−1 (Fig. 1). The PE-rich, LL adapted strain MW4C3 showed high production rates at ML levels (∼70–500 µmol photons m−2 s−1) and photoinhibition at irradiance >600 µmol photons m−2 s−1 (Fig. 1a), whereas the ML adapted culture of this strain and the two PC-rich strains (Fig. 1b and c) were poorly photoinhibited or appeared to be inhibited only at the highest light intensity, irrespective of their adaptation to LL or ML. Strain BO8801, in particular its LL adapted culture, reached high production rates at the lowest irradiance tested (5.8 µmol photons m−2 s−1, Fig. 1c), while the other PC-rich strain did not (Fig. 1b).
Fig. 1.
Cell-specific production rate (fg C cell−1 h−1) of PE-rich picocyanobacterial strain MW4C3 (a) and of two PC-rich strains, MW100C3 (b) and BO8801 (c), after photoacclimation to low light (LL, 10 µmol photons m−2 s−1; filled symbols) and moderate light (ML, 100 µmol photons m−2 s−1; open symbols) intensity. Symbols denote mean values and standard error.
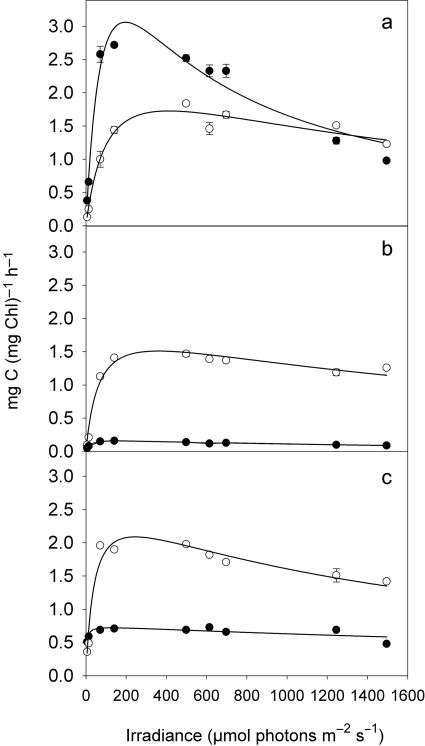
Similar to cellular production rates, chlorophyll a-specific photosynthesis of the LL acclimated strain MW4C3 increased rapidly between 14 and 71 µmol photons m−2 s−1 and peaked at irradiances between 150 and 200 µmol photons m−2 s−1 (Fig. 2a). Photoinhibition was obvious at irradiances of >600 µmol photons m−2 s−1. Specific photosynthesis of the two PC-rich, LL adapted strains was constantly low over a wide range of light intensity (Fig. 2b and c). The P/E curves of the ML cultures of all three strains were more similar, showing only moderate photoinhibition at high irradiance (Fig. 2a–c).
Fig. 2.
P/E curves of the PE-rich picocyanobacterial strain MW4C3 (a) and the two PC-rich strains MW100C3 (b) and BO8801 (c), after photoacclimation to low light (LL, 10 µmol photons m−2 s−1; filled circles) and moderate light (ML, 100 µmol photons m−2 s−1; open circles) intensity. Symbols denote mean values and standard errors. The curves were fit by the Eilers and Peters (Eilers and Peters, 1988) model.
The photosynthetic parameters varied widely, both among the light acclimation treatments of each strain and between the strains; Pmax of the LL culture MW4C3 was close to 3 mg C (mg Chl a)−1 h−1, i.e. from 4 to 19 times higher than Pmax of the PC-rich cultures acclimated to LL (Table I). The initial slope of the P/E curve, α, was highest and the saturation light intensity, Ek, lowest in the LL culture of BO8801. All cultures had significantly higher cell-specific chlorophyll content in the LL treatments than in the ML treatments (Table I). The chlorophyll per cell was highest in strain MW100C3 at both light treatments.
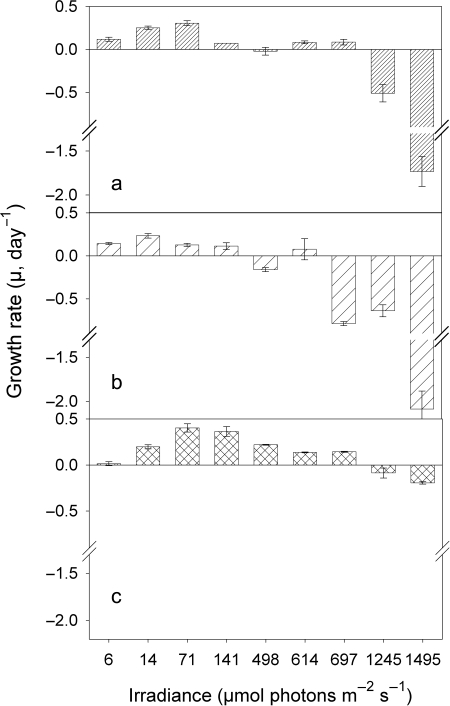
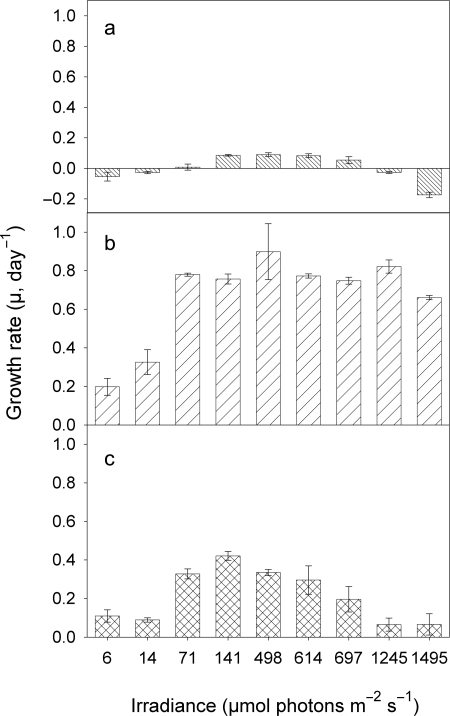
The growth response to light of the LL cultures of all three strains differed widely (Fig. 3). The highest growth rates (µmax), 0.40 day−1, were measured for the green strain BO8801 at 70 µmol photons m−2 s−1 (Fig. 3c and Table I). This isolate showed the clearest, unimodal pattern in its µ versus light response (Fig. 3c); µ of all three strains was negative at the highest two light intensities (Fig. 3a–c). Strain-specific differences in the growth response to light were more pronounced in the ML cultures (Fig. 4). Strain MW100C3 showed high µ, ranging from 0.66–0.90 day−1, over all light intensities except at the two lowest ones (Fig. 4b). Growth rates of the red strain MW4C3 were generally low (<0.1 day−1) and negative at the two highest and the two lowest light intensities (Fig. 4a). The green strain BO8801 (Fig. 4c) showed similar growth rates in the ML and LL cultures (0.40 ± 0.05 day−1, Table I). A shift in positive and maximum µ towards higher light intensities was observed in the other two ML cultures, relative to the respective LL cultures (Figs. 3a and b, 4a and b, and Table I).
Fig. 3.
Growth rates versus irradiance of picocyanobacterial strains MW4C3 (a), MW100C3 (b) and BO8801 (c), after photoacclimation to low light conditions (LL, 10 µmol photons m−2 s−1).
Fig. 4.
Growth rates versus irradiance of picocyanobacterial strains MW4C3 (a), MW100C3 (b) and BO8801 (c), after photoacclimation to moderate light conditions (ML, 100 µmol photons m−2 s−1).
DISCUSSION
P/E curves of freshwater picocyanobacteria: evidence from field and laboratory studies
The results of the P/E curves presented in this study were obtained under similar experimental conditions as those used in an earlier study with two PE-rich and two PC-rich freshwater picocyanobacterial isolates (Callieri et al., 2005). Our experimental abundances (∼1 × 106 cells mL−1) and chlorophyll a concentrations (1.8–18.5 µg L−1) were in the range of natural picocyanobacterial levels (reviewed by Weisse, 1993) and our experimental cultures were optically thin, i.e. it is unlikely that shelf-shading affected the experimental results reported (Oberhaus et al., 2007). The shape of the curves and the effect of photoacclimation were similar in both investigations. However, the most surprising and counterintuitive result of our study is the decline of photosynthetic rates in the ML culture of the red strain MW4C3, relative to the corresponding LL culture (Fig. 1a). We can rule out the possibility that the results from these two experimental series were accidentally exchanged. The stronger performance of the LL culture was also reflected by its growth rates, which were higher than those of the corresponding ML culture (Figs 3a and 4a). More importantly, our results are supported by earlier findings from the field. Callieri et al. (Callieri et al., 2005) complimented their laboratory investigations with cultured strains by conducting P/E with natural picocyanobacterial assemblages from Italian subapline Lake Maggiore, which were dominated by the PE pigment type. Samples were taken in July and October at depths of 3 and 15 m. The irradiance and temperature conditions at these depths were close to our ML and LL acclimation, particularly during the July sampling, when irradiance ranged from 40 to 250 µmol photons m−2 s−1 and temperature was 20°C in the epilimnion (0–6 m). The light intensities at 15 m on both occasions corresponded closely to our LL acclimation. The P/E curves obtained by Callieri et al. (Callieri et al., 2005) under identical incubation conditions with the natural, PE-rich dominated picocyanobacteria closely match our results measured with the PE-rich strain MW4C3 from Austrian subapline Lake Mondsee. Similar to the present study, Callieri et al. (Callieri et al., 2005) reported higher photosynthetic rates for the population taken from 15 m depth than for the near-surface population on both occasions. The photosynthetic parameters measured for the natural populations, Pmax, α and Ek, were all close to our values reported in Table I. Photoinhibition became apparent in the natural, deeper water populations at irradiances >600 µmol photons m−2 s−1 but was less obvious in the near-surface populations. Again, these results are very similar to ours reported in Fig. 2a. In Lake Constance, the absolute and relative abundance of the PE-rich genotype BO 8807 (Group B, subalpine cluster I) was maximal at 20 m during the summer stratification, while PC-rich cells (strain BO 8805, subalpine cluster II) occurred preferentially in the littoral habitat (Becker et al., 2007). Evidence for LL adaptation of PE-rich picocyanobacteria was also provided by a recent field study from the Baltic Sea (Haverkamp et al., 2008). These authors observed at four stations in the Gulf of Finland a peak of the red picocyanobacteria in 5–15 m water depth, while cell numbers of the green picocyanobacteria peaked at the surface and gradually declined with depth.
In contrast to the present study, Callieri et al. (Callieri et al., 2005) did not observe a remarkable difference in the photosynthetic response of LL and ML cultures in two other PE-rich strains (MW73D5 and MW10#1) of the subalpine cluster I (Group B). A likely explanation for this discrepancy is that the duration of the acclimation period was different in these two studies. Callieri et al. (Callieri et al., 2005) had acclimated their cultures for three generations only. In the present study, we extended the duration of the photoacclimation period and gradually acclimated the cultures to LL and ML conditions over 2 months, to ensure balanced growth (MacIntyre et al., 2002). This was particularly important for the acclimation to ML levels, which may induce photostress for PE-rich strains. Photoacclimation is a physiological process (Falkowski and LaRoche, 1991; MacIntyre et al., 2002), referring to phenotypic adjustments that arise in response to variations in environmental factors. Accordingly, photoacclimation, contrary to genetically fixed adaptation, is reversible and does not involve a change in the genetic structure of the cells.
Growth rates and photosynthetic response are strain-specifically different and sensitive to photoacclimation
The P/E curves obtained from fitting our data to the Eilers and Peeters model (Eilers and Peeters, 1988) showed strain-specifically different photosynthetic behaviour (Fig. 2). We used the model by Eilers and Peeters (Eilers and Peeters, 1988) because it (i) yielded the best fit to our data and (ii) to render our results comparable with similar previous work with cultured and natural picocyanobacteria (Callieri and Piscia, 2002; Callieri et al., 2005). The LL photoacclimation amplified the difference between the PE- and PC-rich picocyanobacterial responses to irradiance. The P/E curve of the PE-rich, red MW4C3, is typical of a culture sensitive to photoinhibition, reaching high Pmax at LL to ML intensities. The two PC-rich, green strains were, if at all, only photoinhibited at the highest irradiance, in accordance with the results from previous experiments (Callieri et al., 2005). Both in LL and in ML acclimated treatments, the green strain BO8801 reached the highest photosynthetic efficiency (highest α and lowest Ek, Table I), i.e. its photosynthesis was saturated at LL.
The range of initial linear slope of the P/E curve, α, that we measured was larger than that reported by Veldhuis et al. (Veldhuis et al., 2005) for marine Synechococcus. Alpha is, however, highly variable and may even overlap between prokaryotic and eukaryotic picophytoplankton (Morel and Bricaud, 1981; Kirk, 1994; Veldhuis et al., 2005). Note that the model used for fitting P/E curves has a profound effect on the estimate of the light-limited rate of photosynthesis, α, but less effect on the light-saturated photosynthesis rate, Pmax (MacIntyre et al., 2002). Of the three photosynthetic parameters reported in Table I, only the light saturation coefficient, Ek, showed an unequivocal response to the light treatments. Although it was strain-specifically different, it was shifted up in the ML acclimated cultures of all three strains (Table I). The light saturation coefficient characterizes the photosynthetic response of an ecotype independent from the actual growth conditions. The maximum photosynthetic rate, Pmax, is related to the growth conditions and pigment type, and increases with increasing cell size (Malinsky-Rushansky et al., 2002). As Ek is defined as Pmax/α, it is also affected by changes in Pmax.
We complemented the P/E experiments with growth experiments over the same irradiance gradient. In contrast to the two PC-rich isolates, negative µ at high irradiance (>1200 µmol photons m−2 s−1) was recorded for the red strain MW4C3 not only for the LL culture, but also for the ML culture, corroborating the sensitivity of this isolate to high light intensities. Photoacclimation to 10-fold higher irradiance did even negatively affect µ of this red strain. These results are similar to those reported by Waterbury et al. (Waterbury et al., 1986) for marine and Callieri and Stockner (Callieri and Stockner, 2002) for freshwater picocyanobacteria, who also measured optimal growth rates at low irradiances (25–35 µmol photons m−2 s−1). Uysal (Uysal, 2001) observed that PE-rich picocyanobacterial clones isolated from 0 and 10 m of the Black Sea grew slower at irradiances >20 µmol photons m−2 s−1 than clones isolated from deeper water. The growth rates that he measured at irradiances ranging from 10 to 100 µmol photons m−2 s−1 varied between 0.1 and 0.5 day−1, i.e. they were similar to those of our freshwater strains. However, high µ value of a marine Synechococcus sp., with no indication of photoinhibition, was measured at high light intensities of up to 2000 µmol photons m−2 s−1 (Kana and Glibert, 1987a, b). Similar to these findings, our two PC-rich strains were tolerant to the highest light intensities tested, ∼1500 µmol photons m−2 s−1, after long-term photoacclimation to ML conditions.
The physiological effects of light quality and quantity on growth rate of PE- and PC-rich strains differ (Callieri et al., 1996). The maximum growth rate was reached by PE cells exposed to green light and by PC cells exposed to red light, reflecting specific light harvesting due to their respective pigment composition. Red PE-rich picocyanobacteria have an absorption peak at 560–570 nm, i.e. in green light, whereas PC-rich strains have an absorption peak at 620–630 nm, i.e. they absorb orange–red light effectively (Ernst et al., 1992; Haverkamp et al., 2008). The influence of light intensity is related to complex, structural changes in Photosystem II (PSII), with a rapid transition from a light-sensitive form of the D1 protein of PSII centres (Form I) to a form less susceptible to photoinhibition (Form II, Clarke et al., 1995). During adaptation to high light intensity, a gene encoding the Form II of the D1 protein is activated (Kulkarni and Golden, 1995; reviewed by Golden, 1995), which alleviates negative effects of photoinhibition.
In conclusion, our results support the earlier conjecture (Callieri et al., 2005) that PE-rich picocyanobacterial freshwater strains from Group B are more sensitive to photoinhibtion than PC-rich strains from Groups I and A. The results obtained with six freshwater picocyanobacterial strains (i.e. three PE-rich and three PC-rich ones) in the previous and the present investigation revealed that ecophysiological differences were primarily related to the pigment type and appeared less affected by the phylogenetic position. Similar to their marine relatives, freshwater picocyanobacteria possess the capability of photoadaptation, but the extent of photoadaptation depends on the duration of photoacclimation, and is strain-specifically different. After gradual and long-term photoacclimation, even PE-rich strains from Group B can withstand high irradiances without a visible photoinhibition effect. In temperate lakes, this may result in a fitness gain in summer, during extended periods of warm, calm weather. The results reported in this study suggest that species-like ecotypes exist among genetically (i.e. at the 16S rRNA level) closely related freshwater picocyanobacteria.
FUNDING
This study was supported by the Austrian Academy of Sciences and the Italian National Research Council (Institute of Ecosystem Study).
ACKNOWLEDGEMENTS
We thank Annette Ernst, Nicholas Crosbie and Peter Stadler for isolating and maintaining the cultures used in this study. Constructive comments by two anonymous reviewers are gratefully acknowledged.
REFERENCES
- Allen M. M. Simple conditions for growth of unicellular blue-green algae on plates. J. Phycol. 1968;4:1–4. doi: 10.1111/j.1529-8817.1968.tb04667.x. [DOI] [PubMed] [Google Scholar]
- Becker S., Richl P., Ernst A. Seasonal and habitat-related distribution pattern of Synechococcus genotypes in Lake Constance. FEMS Microb. Ecol. 2007;62:64–77. doi: 10.1111/j.1574-6941.2007.00366.x. [DOI] [PubMed] [Google Scholar]
- Bell T., Kalff J. The contribution of picophytoplankton in marine and freshwater systems of different trophic status and depth. Limnol. Oceanogr. 2001;46:1243–1248. [Google Scholar]
- Callieri C. Picophytoplankton in freshwater ecosystems: the importance of small-sized phototrophs. Freshwater Rev. 2008;1:1–28. [Google Scholar]
- Callieri C., Piscia R. Photosynthetic efficiency and seasonality of autotrophic picoplankton in Lago Maggiore after its recovery. Freshwater Biol. 2002;47:941–956. [Google Scholar]
- Callieri C., Stockner J. G. Freshwater autotrophic picoplankton: a review. J. Limnol. 2002;61:1–14. [Google Scholar]
- Callieri C., Amicucci E., Bertoni R., et al. Fluorometric characterization of two picocyanobacteria strains from different underwater light quality. Int. Rev. Gesamt. Hydrobiol. 1996;81:13–23. [Google Scholar]
- Callieri C., Balseiro E., Bertoni R., et al. Picocyanobacterial photosynthetic efficiency under Daphnia grazing pressure. J. Plankton Res. 2004;26:1471–1477. [Google Scholar]
- Callieri C., Moro S., Caravati E., et al. Strain-specific photosynthetic response of freshwater picocyanobacteria. Verh. Internat. Verein. Limnol. 2005;29:777–782. [Google Scholar]
- Chen F., Wang K., Kan J., et al. Diverse and unique picocyanobacteria in Chesapeake Bay, revealed by 16S–23S rRNA internal transcribed spacer sequences. Appl. Environ. Microbiol. 2006;72:2239–2243. doi: 10.1128/AEM.72.3.2239-2243.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke A. K., Campbell D., Gustafsson P., et al. Dynamic responses of photosystem II and phycobilisomes to changing light in the cyanobacterium Synechococcus pcc 7942. Planta. 1995;197:553–562. [Google Scholar]
- Crosbie N. D., Pöckl M., Weisse T. Dispersal and phylogenetic diversity of nonmarine picocyanobacteria, inferred from 16S rRNA gene and cpcBA-intergenic spacer sequence analyses. Appl. Environ. Microb. 2003;69:5716–5721. doi: 10.1128/AEM.69.9.5716-5721.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen J. J., Lewis M. R. The kinetics of algal photoadaptation in the context of vertical mixing. J. Plankton Res. 1988;10:1039–1063. [Google Scholar]
- Eilers P. H. C., Peeters J. C. H. A model for the relationship between light intensity and the rate of photosynthesis in phytoplankton. Ecol. Model. 1988;42:199–215. [Google Scholar]
- Ernst A. Cyanobacterial picoplankton from Lake Constance: I. Isolation by fluorescence characteristics. J. Plankton Res. 1991;13:1307–1312. [Google Scholar]
- Ernst A., Sandmann G., Postius C., et al. Cyanobacterial picoplankton from Lake Constance. II. Classification of isolates by cell morphology and pigment composition. Bot. Acta. 1992;105:161–167. [Google Scholar]
- Ernst A., Becker S., Wollenzien U. I. A., et al. Ecosystem-dependent adaptive radiations of picocyanobacteria inferred from 16S rRNA and ITS-1 sequence analysis. Microbiology. 2003;149:217–228. doi: 10.1099/mic.0.25475-0. [DOI] [PubMed] [Google Scholar]
- Everroad R. C., Wood A. M. Comparative molecular evolution of newly discovered picocyanobacterial strains reveals a phylogenetically informative variable region of β phycoerythrin. J. Phycol. 2006;42:1300–1311. [Google Scholar]
- Falkowski P. G., La Roche J. Acclimation to spectral irradiance in algae. J.Phycol. 1991;27:8–14. [Google Scholar]
- Golden S. S. Light-Responsive Gene Expression in Cyanobacteria. J. Bacteriol. 1995;177:1651–1654. doi: 10.1128/jb.177.7.1651-1654.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkamp T., Acinas S. G., Doeleman M., et al. Diversity and phylogeny of Baltic Sea picocyanobacteria inferred from their ITS and phycobiliprotein operons. Environ. Microbiol. 2008;10:174–188. doi: 10.1111/j.1462-2920.2007.01442.x. [DOI] [PubMed] [Google Scholar]
- Holm-Hansen O., Riemann B. Chlorophyll a determination: improvements in methodology. Oikos. 1978;30:438–447. [Google Scholar]
- Kana T. M., Glibert P. M. Effect of irradiances up to 2000 µE m−2 s−1 on marine Synechococcus WH7803 – I. Growth, pigmentation, and cell composition. Deep-Sea Res. 1987;a 34:479–495. [Google Scholar]
- Kana T. M., Glibert P. M. Effect of irradiances up to 2000 µE m−2 s−1 on marine Synechococcus WH7803 – II. Photosynthetic responses and mechanisms. Deep-Sea Res. 1987:b 497–516. [Google Scholar]
- Kirk J. T. O. Light and Photosynthesis in Aquatic Ecosystems. 2nd edn. Cambridge: Cambridge University Press; 1994. [Google Scholar]
- Kulkarni R. D., Golden S. S. Form II of D1 is important during transition from standard to high light intensity in Synechococcus sp. strain PCC7942. Photosynthesis Res. 1995;46:435–443. doi: 10.1007/BF00032298. [DOI] [PubMed] [Google Scholar]
- MacIntyre H. L., Kana T. M., Anning T., et al. Photoacclimation of photosynthesis irradiance response curves and photosynthetic pigments in microalgae and cyanobacteria. J. Phycol. 2002;38:17–38. [Google Scholar]
- Malinsky-Rushansky N., Berman T., Berner T., et al. Physiological characteristics of picophytoplankton, isolated from Lake Kinneret: responses to light and temperature. J. Plankton Res. 2002;24:1173–1183. [Google Scholar]
- Moore L. R., Goericke R., Chisholm S. W. Comparative physiology of Synechococcus and Prochlorococcus: influence of light and temperature on growth, pigments, fluorescence and absorptive properties. Mar. Ecol. Prog. Ser. 1995;116:259–275. [Google Scholar]
- Moore L. R., Rocap G., Chisholm S. W. Physiology and molecular phylogeny of coexisting Prochlorococcus ecotypes. Nature. 1998;393:464–467. doi: 10.1038/30965. [DOI] [PubMed] [Google Scholar]
- Morel A., Bricaud A. Theoretical results concerning light absorption in a discrete medium, and application to specific absorption of phytoplankton. Deep-Sea Res. I. 1981;28A:1375–1393. [Google Scholar]
- Oberhaus L., Briand J. F., Leboulanger C., et al. Comparative effects of the quality and quantity of light and temperature on the growth of Planktothrix agardhii and P.rubescens. J. Phycol. 2007;43:1191–1199. [Google Scholar]
- Postius C., Ernst A. Mechanisms of dominance: coexistence of picocyanobacterial genotypes in a freshwater ecosystem. Arch. Microbiol. 1999;172:69–75. doi: 10.1007/s002030050742. [DOI] [PubMed] [Google Scholar]
- Rocap G., Distel D. L., Waterbury J. B., et al. Resolution of Prochlorococcus and Synechococcus ecotypes using 16S-23S rRNA internal transcribed spacer (ITS) region sequences. Appl. Environ. Microbiol. 2002;68:1180–1191. doi: 10.1128/AEM.68.3.1180-1191.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockner J. G. Phototrophic picoplankton: an overview from marine and freshwater ecosystems. Limnol. Oceanogr. 1988;33:765–775. [Google Scholar]
- Stockner J. G., Antia N. J. Algal picoplankton from marine and freshwater ecosytems: a multidisciplinary perspective. Can. J. Fish. Aquat. Sci. 1986;43:2472–2503. [Google Scholar]
- Stockner J., Callieri C., Cronberg G. Picoplankton and other non-bloom forming cyanobacteria in lakes. In: Whitton B. A., Potts M., editors. The Ecology of Cyanobacteria. Dordrecht: Kluwer Academic Publishers; 2000. pp. 195–231. [Google Scholar]
- Talling J. F., Driver D. Some problems in the estimation of chlorophyll a in phytoplankton. 10th Pac. Sci. Cong.; Honolulu. 1961. pp. 142–146. [Google Scholar]
- Uysal Z. Chroococcoid cyanobacteria Synechococcus spp. in the Black Sea: pigments, size, distribution, growth and diurnal variability. J. Plankton Res. 2001;23:175–189. [Google Scholar]
- Veldhuis M. J. W., Timmermans K. R., Croot P., et al. Picophytoplankton; a comparative study of their biochemical composition and photosynthetic properties. J. Sea Res. 2005;53:7–24. [Google Scholar]
- Waterbury J. B., Watson S. W., Valois F. W., et al. Biological and ecological characterization of the marine unicellular cyanobacterium Synechococcus. Can. Bull. Fish. Aquat. Sci. 1986;214:71–120. [Google Scholar]
- Weisse T. Dynamics of autotrophic picoplankton in Lake Constance. J. Plankton Res. 1988;10:1179–1188. [Google Scholar]
- Weisse T. Dynamics of autotrophic picoplankton in marine and freshwater ecosystems. Adv. Microb. Ecol. 1993;13:327–370. [Google Scholar]
- Weisse T. Freshwater ciliates as ecophysiological model organisms – lessons from Daphnia, major achievements, and future perspectives. Arch. Hydrobiol. 2006;167:371–402. [Google Scholar]