Abstract
MEI-1, the catalytic subunit of the Caenorhabditis elegans “katanin” microtubule-severing complex, is required for meiotic spindle formation. However, MEI-1 must be inactivated after the completion of meiosis to allow formation of the first mitotic spindle. Recent work demonstrated that post-meiotic MEI-1 undergoes ubiquitin-dependent degradation mediated by two independent pathways. Here we describe another level of MEI-1 regulation involving the protein phosphatase 4 (PP4) complex. The PP4 R1 regulatory subunit protein phosphatase four regulatory subunit 1 (ppfr-1) was identified in an RNA interference (RNAi) screen for suppressors of a mei-1(gf) allele that is refractory to post-meiotic degradation. RNAi to the PP4 catalytic subunit PPH-4.1 or to the α4 regulatory PPFR-4 also suppressed lethality of ectopic MEI-1. These results suggest that PP4(+) activates MEI-1, and therefore loss of PP4 decreases ectopic MEI-1(gf) activity. PPH-4.1 and MEI-1 co-immunoprecipitate with one another, indicating that the PP4 complex likely regulates MEI-1 activity directly rather than through an intermediate. The ppfr-1 mutant has subtle meiotic defects indicating that PPFR-1 also regulates MEI-1 during meiosis. MBK-2 is the only kinase known to phosphorylate MEI-1 and triggers post-meiotic MEI-1 degradation. However, genetic interactions between PP4 and mbk-2 were not consistent with an antagonistic relationship between the phosphatase and kinase. Additionally, reducing PP4 in mei-1(gf) did not change the level or localization of post-meiotic MEI-1. Thus, by making use of a genetic background where MEI-1 is ectopically expressed, we have uncovered a third mechanism of MEI-1 regulation, one based on phosphorylation but independent of degradation. The redundant regulatory pathways likely contribute in different ways to the rapid and precise post-meiotic inactivation of MEI-1 microtubule-severing activity.
SEXUAL reproduction utilizes two distinct types of cell division during meiosis and mitosis. In Caenorhabditis elegans, female meiosis is completed upon fertilization, after which the embryo enters the first mitotic cell cycle. Meiosis differs from mitosis not only in the behavior of chromosomes but also in the morphology of the spindle apparatus. Like other animals, the C. elegans meiotic spindle is relatively small, lacks centrosomes, and is located at the cortex (Albertson 1984; Albertson and Thomson 1993; Schatten 1994). In contrast, the first C. elegans mitotic spindle is large, filling the cell, and is nucleated by the centrosomes contributed by the sperm. Clearly, each type of spindle must rely upon gene products unique to each division, and these products must be carefully regulated during the meiosis-to-mitosis transition. This problem is particularly acute in C. elegans where this transition is completed within 15 min (Kemphues et al. 1986; McCarter et al. 1999; Yang et al. 2003). The katanin microtubule-severing complex, encoded by mei-1 and mei-2 (Srayko et al. 2000), is an example of a meiotic-specific function that must be eliminated prior to the first mitotic division. We are interested in determining how the embryo downregulates post-meiotic MEI-1/MEI-2 activity in a rapid yet precise fashion.
MEI-1/MEI-2 colocalize on the meiotic spindle (Clark-Maguire and Mains 1994a; Srayko et al. 2000) where they generate microtubule fragments that nucleate spindle microtubules in the absence of centrosomes (McNally et al. 2006; Srayko et al. 2006). Later, MEI-1/MEI-2 act in spindle shortening and translocation to the cortex (Yang et al. 2003; McNally et al. 2006). Loss-of-function (lf) maternal-effect lethal mutations in either gene result in meiotic failure but the subsequent mitotic divisions are normal (Mains et al. 1990a). After its meiotic function is complete, MEI-1 undergoes ubiquitin-mediated degradation due to the action of two partially redundant pathways (Lu and Mains 2007). One pathway requires the MBK-2 kinase (Quintin et al. 2003; Ming Pang et al. 2004; Stitzel et al. 2006, 2007) while the other includes MEL-26, a substrate-specific adaptor for a CUL-3-based E3 ubiquitin ligase (Dow and Mains 1998; Furukawa et al. 2003; Pintard et al. 2003; Xu et al. 2003). Loss of mel-26 or mbk-2 or the presence of a mei-1 gain-of-function (gf) allele that is refractory to mel-26 inhibition all result in ectopic MEI-1 (and MEI-2) expression during mitosis, resulting in small, misoriented spindles. Here we report a new level of MEI-1/MEI-2 regulation involving the protein phosphatase 4 (PP4) class of protein phosphatases.
PP4 belongs to the highly conserved protein phosphatase family of serine/threonine phosphatases and is most closely related to PP2A and PP6 phosphatases. This ubiquitous phosphatase complex is implicated in a wide variety of processes, including signal transduction, splicing, organelle assembly, DNA repair, and chromatin function (Cohen et al. 2005). In all organisms investigated so far, including C. elegans, Drosophila, and mammals, PP4 catalytic subunits (PP4c) localize to the centrosomes (Brewis et al. 1993; Helps et al. 1998; Sumiyoshi et al. 2002; Toyo-Oka et al. 2008). Consistent with its location, PP4 is required for proper spindle formation and/or microtubule stability. A hypomorphic Drosophila mutant (cmm) lacks microtubules connecting chromosomes to the spindle poles, disrupting mitotic spindle assembly (Helps et al. 1998). In worms, one of the two catalytic subunits of PP4, pph-4.1, is essential for mitotic centrosome maturation while the other, pph-4.2, is dispensable. Centrosomal recruitment of γ-tubulin and polo kinase is abnormal in pph-4.1(RNAi). pph-4.1 also functions during sperm nuclear division and chiasma formation in the female germline (Sumiyoshi et al. 2002).
PP4 catalytic subunits form complexes with a variety of structural and regulatory subunits that may serve to narrow substrate specificity (Cohen et al. 2005; Gingras et al. 2005). For example, the regulatory subunits R1 and R2 form distinct complexes with PP4c in mammals (Kloeker and Wadzinski 1999; Hastie et al. 2000; Wada et al. 2001; Gingras et al. 2005). The R1 and R2 subunits associate specifically with PP4c, and R2 subunits colocalize with PP4c to centrosomes of cultured cells (Hastie et al. 2000; Toyo-Oka et al. 2008). Functions of R1 and R2 may involve inhibiting activity of the catalytic subunit PP4c or narrowing substrate specificity (Kloeker and Wadzinski 1999; Hastie et al. 2000; Cohen et al. 2005). The R3 regulatory subunit is implicated in recruiting PP4 to chromatin during DNA repair (Gingras et al. 2005; Kim et al. 2007). A fourth regulatory subunit, α4, which may also inhibit activity or narrow substrate specificity, differs from the PP4R1 and PP4R2 subunits in that it can also associate with the PP2A and PP6 (Chen et al. 1998; Nanahoshi et al. 1999).
A single catalytic subunit could participate in a large number of essential biological processes, and so be lethal, but a specific phosphatase pathway could be revealed by screening for genetic interactions with a mutation in a putative substrate. We identified ppfr-1, the C. elegans homolog of the PP4R1 subunit, by performing an RNA interference (RNAi) screen for genes that suppress ectopic mitotic MEI-1 activity of a mei-1(gf) allele that is resistant to post-meiotic degradation. We show that one of the two C. elegans catalytic subunits (PPH-4.1) and one of the other regulatory subunits (α4/PPFR-4) also suppress ectopic MEI-1, but the worm homologs of R2/PPFR-2 and R3/SMK-1 do not. MBK-2 kinase triggers MEI-1 degradation and therefore was an attractive candidate for the kinase that opposes PP4 activity, but ppfr-1 has only mild genetic interactions with mbk-2. Furthermore, ppfr-1 did not change ectopic MEI-1 levels or location, indicating that PPFR-1 does not influence MEI-1 degradation. PPH-4.1 does co-immunoprecipitate with MEI-1, so the action of the PP4 complex may be direct to counteract an inhibiting phosphorylation of MEI-1/MEI-2. Thus, by compromising post-meiotic MEI-1 protein degradation, we were able to detect this MEI-1 phosphorylation system. Because ppfr-1 alone has only subtle meiotic defects, this mode of MEI-1 regulation would have been difficult to detect by other means because of its partial redundancy with the other MEI-1 regulatory systems.
MATERIALS AND METHODS
Nematode culture and strains:
C. elegans were cultured under standard conditions (Brenner 1974) unless otherwise specified. Temperature-sensitive (ts) strains were upshifted at the L4 stage 24 hr before embryos were collected. Hatching rates were scored on the basis of complete broods of >500 embryos from at least four hermaphrodites (Mains et al. 1990b). Mutations used included the following: LG1—mei-2(ct98); mei-1(ct46gf and ct46ct101), mel-26(ct61gf and ct61sb4), unc-13(e1091), daf-8(e1393), unc-29(e1072), ppfr-1(tm2180), lin-11(n566), and tba-2(sb51gf); LG II—zyg-9(b244); LG III—unc-116(rh24gf), tbb-2(sb26), and pph-4.1(tj20); LG IV—mbk-2(dd5ts); LG V—smk-1(mn156). mei-1, mei-2, and mel-26 strains included the cis-lined marker unc-29, which does not alter hatching rates (Mains et al. 1990b). Lethal strains were balanced with the translocation hT2[myo-2∷ GFP] (I;III) or hT2[bli-4(e937) let(h661)] (I;III) (Edgley et al. 2006).
ppfr-1(tm2180), a gift of S. Mitani (National Bioresource Project, Japan; Gengyo-Ando and Mitani 2000), was outcrossed five times and then a closely linked sterile was removed by selecting a recombinant between unc-29 and lin-11. The resulting fertile ppfr-1(tm2180) lin-11 strain was crossed to mei-1(ct46gf) unc-29 males, and Unc Lin mei-1(ct46gf) unc-29 ppfr-1(tm2180) lin-11 F2 segregants were chosen. These were then crossed to wild-type males, and F2 Unc non-Lin mei-1(ct46gf) unc-29 ppfr-1(tm2180) animals were isolated. A similar strategy was used to create ppfr-1(tm2180) unc-29 from ppfr-1(tm2180) lin-11. To link ppfr-1(tm2180) to mei-2(ct98), mei-2(ct98) unc-13 daf-8 was crossed to ppfr-1(tm2180) unc-29 males, and mei-2(ct98) unc-13 unc-29 ppfr-1(tm2180) progeny were identified among the F2 Unc-13 non-Daf progeny. The unc-13 allele used does not alter hatching rates. The presence of tm2180 was confirmed by PCR, and complementation tests confirmed other mutations.
RNAi:
The chromosome I RNAi library (Fraser et al. 2000) was screened for suppression of mei-1(ct46gf) lethality at 20°. Subsequent RNAi to PP4 subunits was performed using both feeding (Timmons et al. 2001) and injection (Fire et al. 1998). For injection, RNA was in vitro transcribed using the Megascript system (Ambion, Austin, TX) and purified and annealed as previously described (Fire et al. 1998). Double-stranded RNA (dsRNA) was microinjected at a concentration of ∼2 mg/ml into the gonads or the intestines of young adult hermaphrodites. For RNAi feeding, L4 larvae were placed on plates seeded with dsRNA-producing bacteria (Fraser et al. 2000). In both cases, animals were transferred to fresh plates every 24 hr until egg laying ceased. The first broods were not scored for hatching. The RNAi bacterial lawns are thicker than those of C. elegans' normal laboratory food source OP50. Thicker lawns alone did not result in suppression because we report a number of RNAi bacterial strains that did not alter mei-1(ct46gf) hatching levels compared to growth on OP50.
pph-4.1, pph-4.2, and ppfr-4 RNAi clones:
The feeding clone used for ppfr-1 was obtained from the chromosome I library (Fraser et al. 2000). The targeted sequences were checked using BLAST against the C. elegans genome to ensure RNAi specificity. Since pph-4.1 and pph-4.2 are similar to each other (74% base-pair identity, with a maximum length of 15 identical nucleotides), the 5′-end sequences that lacked significant similarity were chosen for gene-specific RNAi. The fragments of pph-4.1, pph-4.2, and ppfr-4 were amplified from embryonic cDNA generated by RT–PCR (Invitrogen Superscript III) and cloned into the L4440 vector individually. Constructs were confirmed by sequencing and transformed into both JM109 and HT115 (DE3) bacteria. The following primers were used: pph-4.1 forward, TGGCTCTGGCGTGCACCGAC; pph-4.1 reverse, TCGATAACCTGGACGTTGC; pph-4.2 forward, GATCA ATTAGGCCCGAACG; pph-4.2 reverse, CACAGATTGTGACC GGTGT; ppfr-4 forward, TGAAGACGTTCCAACAAACTCG CTG; ppfr-4 reverse, CTCCTCGTAACATCTTTCACTCCAG.
Sumiyoshi et al. (2002) reported higher lethality from pph-4.1(RNAi) in the second generation. However, the genetic interactions that we report here did not increase in the second generation, and so first generation hatching rates are reported.
Indirect immunofluorescence microscopy:
Embryos were freeze-cracked and fixed with methanol or methanol–acetone. MEI-1 and α-tubulin localization was determined as described (Kemphues et al. 1986; Pintard et al. 2003; Lu and Mains 2005). Photographs were taken on a Zeiss Axioplan II microscope equipped with a Hamamatsu ORCA-ER digital camera. The same exposure settings were used for all anti-MEI-1 immunofluorescence images. Embryos were scored as positive when MEI-1 fluorescence intensity was higher at the spindle poles than in the surrounding cytoplasm, a metric that correlates closely with hatching (Lu and Mains 2007). Photographs of embryos were scored independently by two investigators.
Microscopy of live embryos:
L4 larvae were raised at 15° and shifted to the experimental temperatures 24 hr before dissection. One-cell embryos were mounted as in previous descriptions (Sulston et al. 1983). The first mitotic division was recorded by time-lapse Nomarski microscopy using a Zeiss Axioplan 2 microscope in a room adjusted to the experimental temperatures. The fractional spindle length and orientation were scored as described by Gomes et al. (2001). The fractional spindle length was the distance between the spindle poles divided by the length of the embryo's A–P axis. The spindle orientation was the angle between the line connecting the spindle poles and the A–P axis.
ppfr-1(tm2180) embryos were recorded at 25° by time-lapse Nomarski microscopy using a Zeiss Axioimager microscope. The cross-sectional area of the polar body was measured using Axiovision software (Zeiss) after selecting polar bodies in the best focal plane of the movie.
Co-immunoprecipitation and Western analysis:
Purified rabbit anti-MEI-1 (Srayko et al. 2000), anti-PPH4.1 (Sumiyoshi et al. 2002), purified rabbit IgG (ZYMED, negative control), or anti-cblB protein (Dobson et al. 2002) were crosslinked to protein-A–agarose beads with dimethylpimelimidate (DMP), using a protocol modified from Van Der Wijk et al. (2005). Antibody was incubated with protein-A–agarose beads for 1 hr at room temperature and crosslinked with freshly made 20 mm DMP in 0.1 m Na2B4O7 (pH 9.0) for 30 min at room temperature. To quench excess DMP, the beads were allowed to settle and incubated with 0.1 m Na2B4O7 for 30 min, followed by two 1-hr rounds of incubation with 0.2 m Tris–HCl (pH 8.0). The beads were quickly washed once with 0.1 m glycine (pH 2.3), twice with 0.1 m Tris–HCl (pH 8.0), and resuspended in phosphate-buffered saline (PBS) to make a 1:1 slurry. Crosslinked antibodies were stored at 4° in PBS containing 0.02% NaN3.
C. elegans liquid culture was performed as previously described (Lewis and Fleming 1995). Cytosolic extracts were prepared according to Lee and Schedl (2001). Generally, 1–3 ml of packed worms were washed twice with PBS and twice with ddH2O and resuspended in 5 ml homogenization buffer (HB) [15 mm HEPES, pH 7.6, 10 mm KCl, 1.5 mm MgCl2, 0.1 mm EDTA, 0.5 mm EGTA, 44 mm sucrose with freshly added 1 mm DTT and protease inhibitors (Complete, Mini, EDTA-free, Roche)]. Lysates were sonicated and cleared at 10,000 × g for 10 min at 4°. For each immunoprecipitation (IP), 0.5 ml lysate was incubated with 50 μl antibody coupled beads either at room temperature for 2 hr or at 4° overnight. After washing six times with IP buffer (HB buffer with 100 mm NaCl), proteins were eluted in 0.1 m glycine (pH 2.3), diluted with 2× sodium dodecyl sulfate (SDS) sample buffer and separated by SDS–PAGE. Samples were transferred to PVDF membranes and probed with rabbit anti-MEI-1 (Srayko et al. 2000), mouse anti-α-tubulin (1:1000, Sigma), and/or rabbit anti-PPH-4.1 (Sumiyoshi et al. 2002). Horseradish-peroxidase-conjugated donkey anti-rabbit or anti-mouse sera (Jackson ImmunoResearch Laboratories) were used as the secondaries and were followed by detection using the ECL Plus Detection System (GE Health Care). Bands were quantitated with the Storm 860 Phosphoimager (Molecular Dynamics).
RESULTS
Loss of the PP4R1 subunit suppresses ectopic MEI-1 lethality:
To identify genes that control mei-1 activity, we performed an RNAi feeding screen for suppressors of the ts mei-1(gf) mutation, ct46, which is refractory to post-meiotic MEL-26-mediated degradation (Clark-Maguire and Mains 1994a; Pintard et al. 2003; Xu et al. 2003). Among the 2445 clones in the C. elegans chromosome I feeding RNAi library (Fraser et al. 2000) are the bacterial strains corresponding to mei-1 and mei-2. Consistent with previous genetic results (Mains et al. 1990a), growth on these two bacterial strains suppressed mei-1(gf). Inactivation of one other gene in this library, F16A11.3, also suppressed mei-1(gf) but had no discernible effect on wild type. F16A11.3 encodes a gene with similarity to the R1 regulatory subunit of the PP4 phosphatase complex (BLAST E-value 10−55), and we hereafter refer to F16A11.3 as ppfr-1. ppfr-1(RNAi) increased the hatching rate of mei-1(gf) >10-fold from 1 to 14% at 20° (Table 1, lines 1–4) and thus acts as if the RNAi inhibits ectopic MEI-1 activity. ppfr-1(RNAi) also resulted in a 10-fold increase in the hatching rate of the null allele mel-26(ct61sb4), the post-meiotic inhibitor of mei-1 (lines 5 and 6), indicating that the suppression of ectopic MEI-1 activity was not mediated through mel-26(+).
TABLE 1.
Interaction of mei-1 pathway mutants with ppfr-1
| % hatching at temperature
|
||||
|---|---|---|---|---|
| Line | Genotypea | 15° | 20° | 25° |
| 1 | Wild type | 100 | 99 | 99 |
| 2 | mei-1(gf) | 13 | 1 | 0 |
| 3 | ppfr-1(RNAi) | —b | 98 | — |
| 4 | mei-1(gf); ppfr-1(RNAi) | 51 | 14 | 0 |
| 5 | mel-26 | — | 1 | — |
| 6 | mel-26; ppfr-1(RNAi) | — | 10 | — |
| 7 | ppfr-1(tm2180) | 90 | 86 | 81 |
| 8 | mei-1(gf) ppfr-1(tm2180) | 57 | 7 | 0 |
| 9 | mei-1(gf)/+ | 84 | 31 | 0.2 |
| 10 | mei-1(gf) ppfr-1(tm2180)/+ + | 85 | 45 | 0.4 |
| 11 | mei-1(gf) +/+ ppfr-1(tm2180) | 90 | 69 | 0.4 |
| 12 | mei-1(gf) ppfr-1(tm2180)/ mei-1(gf) + | 30 | 12 | — |
| 13 | mei-2 | — | — | 66 |
| 14 | mei-2 ppfr-1(tm2180) | — | — | 71 |
| 15 | mei-2; tbb-2c | — | 54 | — |
| 16 | mei-2; tbb-2; ppfr-1(RNAi)c | — | 53 | — |
Genetic mutations were as follows: mei-1(gf), ct46; mei-2, ct98, a hypomorph; mel-26, ct61sb4, a null. Either RNAi or a mutation were employed for ppfr-1, as indicated. More than 500 embryos were scored for each genotype.
Not determined.
tbb-2 is sb26, a tubulin allele that renders microtubules refractory to ectopic MEI-1 activity, sensitizing strains with a mei-2 hypomorph (Lu et al. 2004).
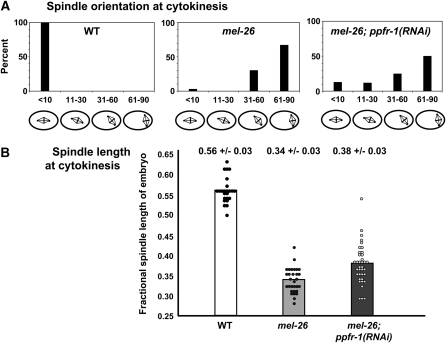
ppfr-1(RNAi) improved spindle morphology in embryos expressing ectopic MEI-1. This was most apparent during cytokinesis at 25°. Wild-type spindles were all aligned along the A–P axis, but almost all mel-26 spindles were inclined >30°. However, substantial fractions were inclined <30° in mel-26; ppfr-1(RNAi) embryos (Figure 1A). Likewise, there was a skewing of the distribution of spindle lengths toward the normal range in mel-26; ppfr-1(RNAi) compared to mel-26, with a slight, but significant, increase in the population's average length (from 0.34 ± 0.03 of egg length to 0.38 ± 0.03, P = 0.03, Figure 1B).
Figure 1.—
ppfr-1(RNAi) improves the spindle orientation and morphology of mel-26 embryos at 25°. Spindles in live embryos were scored as described in materials and methods. (A) ppfr-1(RNAi) resulted in an increase in the percentage of mel-26 embryos with spindles inclined <30° from the A–P axis. (B) An increased fraction of mel-26 embryos had longer spindles (expressed as a fraction of egg length ±SD) in combination with ppfr-1(RNAi).
Recently, a deletion of ppfr-1 became available through the National Bioresource Project for the Nematode (Gengyo-Ando and Mitani 2000). tm2180 is a 1027-bp deletion that removes three exons at the 5′-end of the gene, including the start codon, and so likely represents a null allele. tm2180 shows 80–90% hatching at different temperatures (Table 1, line 7; the inviable embryos arrested before morphogenesis but we observed no obvious mitotic defects among 36 one- and two-cell embryos observed by Nomarski microscopy, but see below). As shown on line 8, the suppression of mei-1(gf) by ppfr-1(tm2180) was comparable to that of ppfr-1(RNAi). Notably, loss of ppfr-1 does not suppress mei-1(gf) at 25° (lines 2, 4, and 8), indicating that ppfr-1 is not a bypass suppressor. The ppfr-1 mutation showed weak dominant suppression of mei-1(gf), most evident in mei-1(gf) homozygotes (lines 9–12).
ppfr-1 has subtle meiotic defects:
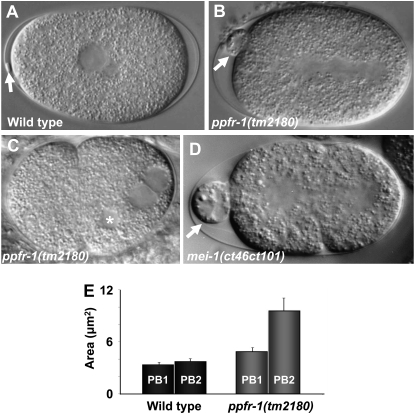
While loss of ppfr-1 decreases ectopic MEI-1 action during mitosis, we asked if there is a similar interaction during meiosis, when MEI-1/MEI-2 normally functions. Although ppfr-1 did not enhance lethality when MEI-1/MEI-2 function is limiting (Table 1, lines 13–16), we found that ppfr-1(tm2180) has meiotic defects consistent with having a role in regulating meiotic MEI-1/MEI-2. Partial loss of MEI-1 activity can lead to polar body defects without accompanying lethality (Mains et al. 1990a; Clandinin and Mains 1993). Indeed, we found that 84% (16/19) of ppfr-1(tm2180) had abnormally large polar bodies (Figure 2, A, B, and E). Polar body extrusion failed in the remaining 16% (3/16) of embryos (Figure 2C), and the resulting aneuploidy likely accounted for the lethality caused by the ppfr-1(tm2180) (Table 1, line 7). Since ppfr-1(RNAi) results in little lethality (Table 1, line 3) and did not display polar body defects (data not shown), RNAi represents an incomplete knockdown.
Figure 2.—
Meiotic defects in ppfr-1(tm2180) mutant embryos. Nomarski images of (A) wild-type and ppfr-1(tm2180) mutants showing (B) an abnormally large polar body (16/19 embryos) or (C) failure of polar body extrusion resulting in a supernumerary maternal pronucleus (asterisk; 3/19 embryos). (D) A mei-1(ct46ct101) null embryo with an abnormally large polar body. Arrows in A, B, and D indicate polar bodies. (E) Quantification of polar body size. Maximal cross-sectional areas of polar bodies were measured during the first cell division. In wild type, both polar bodies were of similar sizes, with averages of 3.4 vs. 3.7 μm2 for an embryo's smaller and larger polar bodies, respectively (n = 4 embryos). In ppfr-1(tm2180), the polar bodies differed in size with an average of 4.8 vs. 9.6 μm2 for the smaller and larger polar bodies, respectively (n = 16 embryos). We do not know which was the first or second polar body, but we arbitrarily refer to the smaller polar body as PB1 and the larger as PB2.
Partial loss of MEI-1 activity leads to a high incidence of male (Him) phenotype (Mains et al. 1990a; Clandinin and Mains 1993), an indication of meiotic abnormalities leading to chromosome loss (Hodgkin et al. 1979). Normally 0.2% of C. elegans self-progeny are XO males due to X chromosome nondisjunction. The ppfr-1 deletion allele is weakly Him, with 0.9% (n = 1150) male offspring.
We conclude that ppfr-1(+) is active during meiosis. The meiotic phenotypes are similar to those seen when MEI-1/MEI-2 is partially limiting, indicating that ppfr-1(+) likely acts as a positive regulator of meiotic MEI-1, similar to what is seen during mitosis.
Loss of other PP4 subunits suppresses ectopic MEI-1 lethality:
We next asked which other PP4 subunits regulate MEI-1. Because mitotic phenotypes could be efficiently assessed using hatching rates, we measured the effects of knockdowns of other PP4 subunits in genetic backgrounds with ectopic MEI-1. RNAi knockdown of pph-4.1 rescued embryonic lethality of mei-1(gf) (Table 2, lines 1–3). Simultaneous RNAi against both pph-4.1 and ppfr-1 showed the same rescuing capacity as pph-4.1 alone (lines 3–5), suggesting that the PPFR-1 regulatory subunit acts only in concert with the PPH-4.1 catalytic subunit to rescue lethality. In contrast, RNAi to the second catalytic subunit, pph-4.2, showed little if any rescue of mei-1(gf) lethality nor did it dramatically alter the ability of either ppfr-1(RNAi) or pph-4.1 to rescue mei-1(gf) (lines 6–9). Similarly, mel-26 was also rescued by RNAi knockdown of pph-4.1 but pph-4.2(RNAi) had little if any effect (lines 10–12).
TABLE 2.
Interaction of PP4 RNAi to subunits with mei-1 pathway mutants at 20°
| Line | Genotypea | % hatching (20°) |
|---|---|---|
| 1 | mei-1(gf) | 1 |
| 2 | pph-4.1 | 97 |
| 3 | mei-1(gf); pph-4.1 | 19 |
| 4 | mei-1(gf); ppfr-1 | 16 |
| 5 | mei-1(gf); pph-4.1; ppfr-1 | 22 |
| 6 | pph-4.2 | 100 |
| 7 | mei-1(gf); pph-4.2 | 3 |
| 8 | mei-1(gf); pph-4.1; pph-4.2 | 11 |
| 9 | mei-1(gf); ppfr-1; pph-4.2 | 13 |
| 10 | mel-26 | 1 |
| 11 | mel-26; pph-4.1 | 14 |
| 12 | mel-26; pph-4.2 | 3 |
| 13 | ppfr-4 | 80 |
| 14 | mei-1(gf); ppfr-4 | 14b |
| 15 | mel-26; ppfr-4 | 8b |
| 16 | ppfr-2 | 100 |
| 17 | mei-1(gf); ppfr-2 | 1 |
| 18 | mei-1(gf); ppfr-1; ppfr-2 | 12 |
| 19 | mel-26; ppfr-2 | 0.1 |
| 20 | smk-1 | 93 |
| 21 | mei-1(gf); smk-1 | 2 |
| 22 | mbk-2 | 60 |
| 23 | mbk-2; ppfr-1 | 31 |
| 24 | mbk-2; ppfr-1(tm2180) | 42 |
| 25 | mbk-2; pph-4.1 | 47 |
| 26 | mbk-2; pphr-1; pph-4.1 | 30 |
| 27 | mbk-2; pph-4.2 | 55 |
| 28 | mbk-2; ppfr-2 | 69 |
Unless otherwise indicated, all PP4 depletions were done by RNAi while mutations were as follows: mei-1(gf), ct46; mel-26, ct61sb4, a null; smk-1, mn156, a hypomorph; mbk-2(dd5ts), a hypomorph at 20°. RNAi to PP4 subunits was done by feeding mel-26 mutant strains and by injection for other strains. More than 500 embryos were scored for each genotype.
Normalized to the hatching rate for ppfr-4(RNAi) feeding.
We also performed RNAi against the two other predicted regulatory subunits of PP4. Y71H2B.3 has the highest BLAST score with the human PP4 α4 subunit (E-value 10−43), and we designate this gene ppfr-4. PPFR-4 interacted with the PPH-4.1 catalytic subunit in a global C. elegans yeast two-hybrid screen (Li et al. 2004). ppfr-4(RNAi) caused high levels of embryonic lethality in the wild-type background, resulting in only 6% survival when injected at high concentrations (we did not investigate the cause of this lethality). However, when ppfr-4(RNAi) was done by feeding, 80% of the embryos hatched, and we were able to use this condition to show suppression of mei-1(gf) and mel-26 (Table 2, lines 13–15).
We did not find genetic interaction with the other predicted C. elegans PP4 regulatory subunits. D2092.2 has the most significant BLAST score to human PP4R2 (E-value 10−8), and we designated this gene ppfr-2. ppfr-2(RNAi) did not suppress either mei-1(gf) or mel-26 nor did it alter the level of suppression of mei-1(gf) by ppfr-1 in double RNAi experiments (Table 2, lines 16–19). The PP4R3 regulatory subunit is encoded by smk-1/rad-2 (Hartman and Herman 1982; Kim et al. 2007), but we found no genetic interaction between mei-1(gf) and an smk-1 hypomorphic mutant (lines 20 and 21). Our results suggest that the PP4 catalytic subunit PPH-4.1 works in concert with the PPFR-1 and PPFR-4 regulatory subunits during its interactions with MEI-1. The other tested PP4 subunits do not seem to be involved in regulating mitotic MEI-1. There is the caveat that RNAi is not equally effective against all genes; however, RNAi is particularly efficient in phenocopying genes expressed in early embryos (Kamath and Ahringer 2003; Sonnichsen et al. 2005).
PP4(+) does not reduce general microtubule stability:
PP4 is involved in regulating spindle formation and/or microtubule stability in both Drosophila (Helps et al. 1998) and C. elegans (Sumiyoshi et al. 2002). The genetic interaction between the PP4 genes and mei-1 might be indirect if loss of PP4 results in microtubules that are generally more stable and thus more resistant to ectopic MEI-1 severing. We tested this model by examining genetic interactions between PP4 genes and mutations that have phenotypes similar to mei-1(gf) but which destabilize microtubules by different means. sb51 is a dominant gf allele of tba-2 (one of two embryonically expressed α-tubulin isotypes) that reduces microtubule stability (Phillips et al. 2004; Lu and Mains 2005). If depletion of PP4 makes microtubules stronger, it should rescue the tba-2(sb51)-compromised microtubule stability. However, ppfr-1(RNAi) did not rescue tba-2(sb51) lethality (Table 3). Similar results were obtained with two other mutants that destabilize microtubules by completely independent mechanisms, including a gf mutant of the kinesin heavy chain unc-116 (Yang et al. 2005) and zyg-9 (Matthews et al. 1998), which encodes a microtubule-stabilizing XMAP215 (Table 3).
TABLE 3.
Interactions of ppfr-1 and mei-1 with microtubule-destabilizing mutations
| % hatching (20°)
|
||
|---|---|---|
| Genotypea | No RNAi | ppfr-1(RNAi) |
| mei-1(gf) | 2 | 12 |
| tba-2(sb51)/+ | 21 | 19 |
| unc-116(rh24) | 17 | 17 |
| zyg-9(b244)b | 43 | 36 |
| % hatching (20°)
|
||
| Genotypea | No RNAi | tbg-1(RNAi) |
| Wild type | 100 | 23 |
| mei-1(gf) | 1 | 1c |
mei-1(gf), ct46; tba-2(sb51) is a neomorph that antagonizes β-tubulins expressed in the early embryo (Lu and Mains 2005); unc-116(rh24) is a neomorph (Yang et al. 2005); zyg-9(b244ts) is a hypomorph at 20° (Kemphues et al. 1986); mel-26, ct61sb4, is null; mbk-2(dd5ts) is a hypomorph at 20° (Quintin et al. 2003); smk-1(mn156) is a hypomorph (Kim et al. 2007). More than 500 embryos were scored for each genotype.
22.5°.
Normalized to the hatching rate for tbg-1(RNAi) feeding.
Both γ-tubulin and MEI-1 are involved in increasing the number of microtubule ends during meiosis to nucleate new microtubules (McNally et al. 2006; Srayko et al. 2006). If this also occurs during mitosis in mei-1(gf), it is possible that loss of PP4 suppresses the effect of too much MEI-1 by reducing microtubule nucleation from the parallel γ-tubulin pathway. If so, then tbg-1(RNAi) should also rescue mei-1(gf). Alternatively, reduction of γ-tubulin may exacerbate mei-1(gf) since there would be fewer microtubules. However, we observed no suppression or enhancement of mei-1(gf) by tbg-1(RNAi) (Table 3).
Taken together, our results suggest that suppression of mei-1(gf) by PP4-regulated microtubule stability/nucleation is unlikely.
PP4(+) does not inhibit degradation of post-meiotic MEI-1:
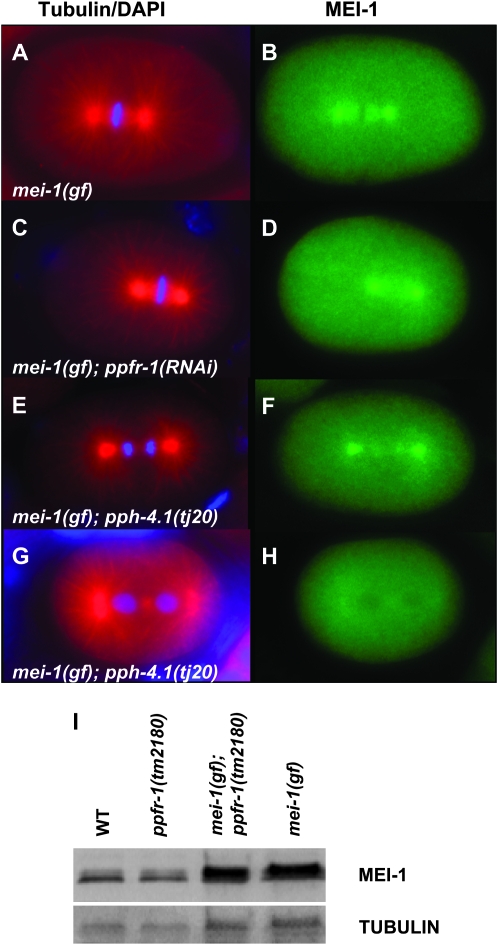
Phosphorylation is often coupled with ubiquitin-mediated protein degradation (Glickman and Ciechanover 2002; Petroski and Deshaies 2005; Hunter 2007), and the kinase MBK-2 is required for MEI-1 degradation (Pellettieri et al. 2003; Quintin et al. 2003; Ming Pang et al. 2004; Stitzel et al. 2006). If PP4 removes a phosphate from MEI-1 that normally marks it for degradation, then loss of PP4 activity would favor degradation, consistent with our genetic observations that loss of PP4 suppresses ectopic MEI-1. We previously found that MEI-1-staining intensity and localization (spindle pole vs. cytoplasm), as revealed by immunofluorescence, are sensitive to small changes in the MEI-1 degradation pathways and correlate well with hatching rates (Lu and Mains 2007). However, we found no reduction in the level or changes in the localization of MEI-1 in one- and two-cell mei-1(gf); ppfr-1(RNAi) embryos at 20° relative to mei-1(gf), even though addition of ppfr-1 increased hatching above control mei-1(gf) levels (Figure 3, A–D; Table 4). Although not sensitive to variations in individual embryos, MEI-1 levels on Western blots of populations of post-meiotic embryos were also not altered (Figure 3I). Similarly, we found no change in MEI-1 levels or localization when mei-1(gf) was suppressed by a pph-4.1 mutation or for RNAi to either PP4 subunit in the mel-26 background (Table 4; Figure 3, E–G).
Figure 3.—
Depletion of PP4 does not affect MEI-1 expression. Embryos were stained with (left) anti-tubulin (red) and DAPI (blue) and (right) anti-MEI-1 (green). Ectopic MEI-1 is found at the center of the centrosome and at lower levels on the spindle microtubules in mei-1(gf) (A and B). The pattern and intensity of ectopic MEI-1 is not altered upon depletion of ppfr-1 by RNAi (C and D) or by a mutation of pph-4.1 (E and F). An example of a rare (3/68) MEI-1 negative mei-1(gf); pph-4.1 embryo is shown for comparison in G and H. MEI-1 does not stain wild-type embryos (methods used for scoring embryos are described in materials and methods). (I) Western blot of post-meiotic embryos probed with anti-MEI-1. Embryos were collected by alkaline hypochlorite treatment of gravid hermaphrodites, a procedure that destroys meiotic-stage embryos because protective eggshells are not yet completely formed. The top panel was probed with anti-MEI-1 while the bottom panel shows the anti-α-tubulin loading control. MEI-1 levels were substantially increased in mei-1(ct46gf) compared to wild type, but addition of ppfr-1(tm2180) did not affect MEI-1 levels either alone or in double mutants with mei-1(gf). The relative pixel intensities for MEI-1 relative to tubulin and normalized to wild type are as follows: wild type, 1.0; ppfr-1, 1.2; mei-1(gf) ppfr-1, 2.4; mei-1(gf), 2.2. The two MEI-1 bands may correspond to the two isoforms encoded by the gene (Clark-Maguire and Mains 1994b) and/or may represent phosphorylation due to MBK-2 or other kinases. However, the ratio between the MEI-1 bands is not altered by ppfr-1.
TABLE 4.
Ectopic MEI-1 in PP4-depleted one- and two-cell embryos at 20°
| Genotypea | % MEI-1 negative (N) | % hatchingb |
|---|---|---|
| mei-1(gf) | 3 (33) | 1 |
| mei-1(gf); ppfr-1(RNAi) | 0 (88) | 13c |
| mei-1(gf); pph-4.1(RNAi) | 3 (68) | 18c |
| mel-26 | 0 (58) | 0.1 |
| mel-26; ppfr-1(RNAi) | 0 (96) | 10d |
| mel-26; pph-4.1(RNAi) | 0 (58) | 8d |
The alleles used included mei-1(gf), ct46; mel-26, ct61sb4, a null; and pph-4.1(tj20), a null.
The percentage of hatching correlated closely with the percentage of MEI-1-negative embryos in a previous study (Lu and Mains 2007).
RNAi was done by injections.
RNAi was done by feeding.
If PP4 phosphatase opposes MBK-2 phosphorylation activity, then we should observe suppression of mbk-2 lethality as is observed when mei-1 or mei-2 activity is decreased (Quintin et al. 2003). Instead, we observed a slight enhancement with depletion of ppfr-1 or pph-4.1 (Table 2, lines 22–26) but not with RNAi to pph-4.2 or ppfr-2 (lines 27 and 28).
To summarize, PP4 appears not to regulate MEI-1 at the level of protein degradation or localization or to genetically interact with MBK-2 kinase to regulate MEI-1.
PP4 physically interacts with MEI-1:
If PP4 regulates MEI-1 via intermediates, reduction of those intermediates may result in a genetic interaction with mei-1(gf). The products of seven genes (in addition to ppfr-4) showed physical interaction with PPH-4.1 in a large yeast two-hybrid interaction screen (Li et al. 2004). Five (cogc-1, ibf-1, Y53F4B.22, F25B3.5, EEED8.3) are included in the RNAi feeding library (Kamath et al. 2003). plk-1 was also tested as a candidate, as centrosomal localization of PLK-1 was altered by RNAi knockdown of PP4 (Sumiyoshi et al. 2002). However, RNAi to none of these genes altered mei-1(gf) hatching at 20° (data not shown).
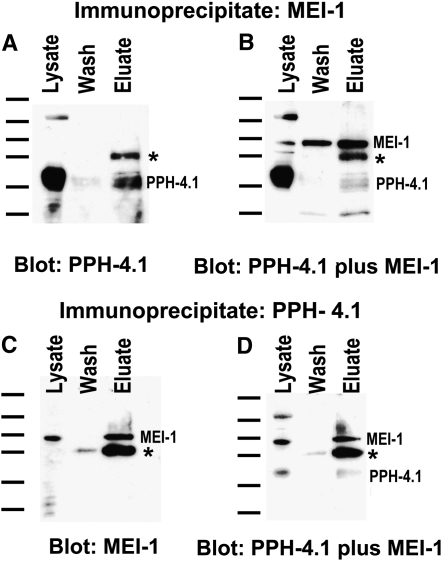
The worm PPH-4.1 catalytic subunit localizes to the spindle poles (Sumiyoshi et al. 2002), one of the locations where ectopic MEI-1 is found in mei-1(gf). This suggests that their interaction might be via direct binding. To address this possibility, we performed reciprocal co-immunoprecipitation experiments. The Western blot in Figure 4A shows that PPH-4.1 was present in both a wild-type lysate and an anti-MEI-1 immunoprecipitate of that lysate. The presence of MEI-1 in the lysate was confirmed when the blot was reprobed with anti-MEI-1, demonstrating the efficacy of the immunoprecipitation (Figure 4B). The reciprocal co-immunoprecipitation detected MEI-1 in an anti-PPH-4.1 immunoprecipitate (Figure 4, C and D). Specificity was evident because the band corresponding to MEI-1 was not present when the MEI-1 immunoprecipitate was probed only with PPH-4.1 (Figure 4A) and vice versa (Figure 4C). Neither band was observed in lysates or immunoprecipitates using nonspecific IgG or an unrelated antibody, anti-cblB protein (data not shown). Furthermore, the immunoprecipitated bands are near the predicted molecular weights (52 and 37 kDa for MEI-1 and PPH-4.1, respectively) and comigrated with the corresponding lysate bands. Finally, the MEI-1 band was absent from lysates of mei-1(null) worms (data not shown), and the PPH-4.1 band was not detected when the antibody was preincubated with a blocking protein (Sumiyoshi et al. 2002). These data suggest that the pph-4.1/mei-1 genetic interactions are direct, mediated either by direct binding of the corresponding proteins or through bridging by another PP4 subunit, or perhaps MEI-2. This predicts that PPH-4.1 removes an inhibitory phosphate from MEI-1/MEI-2.
Figure 4.—
MEI-1 and PPH-4.1 co-immunoprecipitate. Wild-type worm lysates were immunoprecipitated followed by Western blotting. (A and B) Blots of lysates, washes, and anti-MEI-1 immunoprecipitates were probed with anti-PPH-4.1 (A) or anti-PPH-4.1 plus anti-MEI-1 (B). PPH-4.1 (A and B) and MEI-1 (B) were present in the anti-MEI-1 immunoprecipitates. Multiple PPH-4.1 bands were detected as previously observed (Sumiyoshi et al. 2002). (C and D) In the reciprocal experiment, lysates were immunoprecipitated with anti-PPH-4.1 and blots were probed with anti-MEI-1 (C) and anti-MEI-1 plus anti-PPH-4.1 (D). Both MEI-1 (C and D) and PPH-4.1 (D) were immunoprecipitated by anti-PPH-4.1. The asterisk denotes that a fraction of the IgG heavy chain was eluted from the beads and detected by the anti-IgG secondary. Molecular weight markers, corresponding to 175, 83, 62, 47.5, 32.5, and 25 kDa, are shown to the left of each panel.
DISCUSSION
mei-1 and mei-2 encode the subunits of the worm “katanin” microtubule-severing protein. Katanin regulates the shape and dynamics of female meiotic spindles but must be degraded before mitosis (Clark-Maguire and Mains 1994a; Srayko et al. 2000, 2006; McNally et al. 2006). Degradation of post-meiotic MEI-1 is carried out by two independent pathways (Lu and Mains 2007), one involving MEL-26/CUL-3 ubiquitin ligase (Dow and Mains 1998; Furukawa et al. 2003; Pintard et al. 2003; Xu et al. 2003) and another that includes MBK-2 kinase (Pellettieri et al. 2003; Quintin et al. 2003; Ming Pang et al. 2004; Stitzel et al. 2006). Here we report a further level of MEI-1 regulation involving phosphorylation independent of the known protein degradation pathways. Although this interaction was initially detected with a mei-1(gf) allele, we also saw interactions with mei-1(+): PP4 mutants genetically interact with mel-26, which results in ectopic MEI-1(+); the PP4 physical interactions shown in Figure 4 are with MEI-1(+); and meiotic defects are consistent with ppfr-1 regulating MEI-1(+) (Figure 2). Although the suppression of lethality caused by ectopic MEI-1 is not as strong as that described previously for other suppressors of mei-1(gf) (Mains et al. 1990a; Clandinin and Mains 1993; Lu et al. 2004; Lu and Mains 2005), levels of suppression were consistently observed in the 15 genotypes tested in Tables 1 and 2, increasing hatching by ∼10-fold.
PP4 subunit composition for MEI-1 regulation:
PP4 has many other functions in C. elegans, including centrosome maturation in the early embryo, sperm meiotic spindle function, oocyte chiasma formation (Sumiyoshi et al. 2002), embryonic DNA repair checkpoint function (Hartman and Herman 1982; Kim et al. 2007), and regulation of longevity (Panowski et al. 2007). Diverse PP4 functions are also found in other organisms (Cohen et al. 2005). Loss of distinct PP4 subunits results in different C. elegans phenotypes, indicating that PP4 subunit composition varies with function. Gingras et al. (2005) used tandem affinity purification and mass spectrometry to determine the subunit composition of mammalian and yeast PP4 complexes. The catalytic subunit PP4c was found in several distinct complexes, including one with PP4R1, a second with α4 and the TRiC/CCT chaperonin complex, and a third with PP4R2 and PP4R3. Although the authors did not find a complex containing both PP4R1 and α4, our genetic evidence indicates that the corresponding C. elegans products, PPFR-1 and PPFR-4, likely act in concert. Hastie et al. (2000) and Kloeker and Wadzinski (1999) reported that the mammalian regulatory subunit PP4R1 inhibits PP4 enzymatic activity, but they suggested that the in vitro substrates used might not have been physiologically relevant. Instead, PP4R1 may serve to limit substrate specificity. Our work is consistent with this idea since genetically the PPFR-1/PP4R1 regulatory subunit acted in concert with, rather than in opposition to, the PPH-4.1/PP4c catalytic subunit. Simultaneous RNAi against both pph-4.1 and ppfr-1 showed the same rescuing capacity as either alone (Table 1), again consistent with the two subunits working together.
The mechanism of PP4 suppression of ectopic MEI-1:
Depletion of PP4 subunits suppresses ectopic MEI-1 in mel-26(null), suggesting that PP4 is involved in a pathway parallel to mel-26: if the genes acted sequentially, knocking down PP4 when mel-26 was completely absent would have no effect. MBK-2 is known to directly phosphorylate MEI-1 (Stitzel et al. 2006) and acts in parallel to MEL-26 and so was an attractive candidate for the kinase that counteracts PP4. In this model, depletion of PP4 would suppress the mbk-2(ts) mutant under semipermissive conditions as do other suppressors of ectopic MEI-1 (Quintin et al. 2003), but we instead found a slight enhancement (Table 2). The model that MBK-2 and PP4 counteract one another predicts that loss of PP4 suppresses lethality from ectopic MEI-1 by increasing the level of MEI-1 phosphorylated by MBK-2, leading to increased degradation of ectopic MEI-1. However, we found no changes in the levels (or localization) of mitotic MEI-1 in mei-1(gf) or mel-26 embryos rescued by PP4 depletion (Table 4, Figure 3). This suggests that PP4 is not involved in MEI-1 degradation induced by MBK-2 (or any other kinase). Although subtle changes in protein levels may not be detectable in our assay, previous work has shown that the percentage of MEI-1 negative embryos closely correlates with hatching rates in all genetic backgrounds scored by Lu and Mains (2007).
An alternative model invokes a role for PP4 in general microtubule stability. Consistent with this, PP4 localizes to the microtubule-organizing centers in mammals, flies, and worms and mutations in these systems disrupt spindle function (Brewis et al. 1993; Helps et al. 1998; Sumiyoshi et al. 2002; Toyo-Oka et al. 2008). Arguing against this indirect model, we found no interactions with ppfr-1(RNAi) in genetic backgrounds that have phenotypes similar to mei-1(gf) but which interfere with microtubule stability by three independent mechanisms [a lf mutation of a gene encoding a microtubule-stabilizing protein (zyg-9) and gf alleles of a tubulin (tba-2) and of a kinesin (unc-116) (Table 3)]. Toyo-Oka et al. (2008) recently reported that in cultured cells PP4 counteracts CDK1 phosphorylation of NDEL, a protein that recruits katanin to the centrosome when phosphorylated. This phosphorylation is unlikely to be relevant to the MEI-1–PP4 interaction because loss of PP4 increases katanin function in the other system, which is the opposite of what we observed. Our results suggest that the PP4 phosphatase directly activates MEI-1 rather than indirectly decreasing microtubule stability. Finally, we tested five other candidates that show yeast two-hybrid interactions with PPH-4.1, but again found no genetic interactions, arguing against the possibility that the interaction between PP4 and MEI-1 is mediated by an intermediate.
The interaction between PP4 and MEI-1 is likely direct. By reciprocal co-IP experiments (Figure 4), we demonstrated that PPH-4.1 binds MEI-1 (or is at least in the same complex). This suggests that there is a kinase that inactivates MEI-1 microtubule-severing after meiosis, and by removing PP4, the equilibrium is shifted toward the inactive form of MEI-1. Known kinases regulate katanin microtubule-severing activity in Xenopus extracts, but these activate rather than inhibit severing (McNally et al. 2002).
PPFR-1 functions during meiosis:
The ppfr-1 deletion allele has incompletely penetrant meiotic defects, including chromosome segregation defects and either formation of larger-than-normal polar bodies or failures in polar body extrusion (Figure 2). These phenotypes are also characteristic of mei-1 and mei-2 hypomorphs (Mains et al. 1990a; Clandinin and Mains 1993). The relative weakness of the ppfr-1 meiotic phenotypes may stem from regulatory redundancy with MEI-1 degradation systems, which we recently found to be active at low levels during meiosis to prevent excess MEI-1/MEI-2 activity at that time (J. L. Johnson, C. Lu, E. Raharjo, K. McNally, F. J. McNally and P. E. Mains, unpublished results).
PP4(+) appears to continue to activate MEI-1/MEI-2 upon completion of meiosis, when the microtubule-severing complex is being degraded. This seemingly unexpected activity of PP4 may be unavoidable since PP4 must be active at this time for centrosome maturation in preparation for the first mitotic cleavage (Sumiyoshi et al. 2002). A likely way for the embryo to prevent PP4 from slowing MEI-1/MEI-2 downregulation is for the activity of the MEI-1/MEI-2 inhibitor kinase to be sufficiently high to overcome the effects of PP4. In any case, by screening for suppressors in a genetic background with compromised post-meiotic degradation, we uncovered an inhibitory phosphorylation event that can potentially inactivate MEI-1 more quickly than degradation. Because of the redundant layers of MEI-1 regulation, this system would have been difficult to find by other means.
The three partially redundant systems together ensure that microtubule-severing activity remains high during meiosis and then decreases to low levels with appropriate kinetics during the 15-min transition to mitosis. The three pathways may have subtly different properties; for example, the MBK-2 pathway is activated immediately after meiosis (Stitzel et al. 2007) while the MEL-26 pathway activation occurs later (Lu and Mains 2007). Regulation by an inhibitory phosphorylation system differs from the protein degradation pathways in that it is likely very rapid but reversible. Katanin functions in a wide variety of organisms, including in mitotic spindle dynamics in mammals (McNally et al. 2006; Zhang et al. 2007), cilia and flagellar function in unicellular organisms (Lohret et al. 1998; Dymek et al. 2004; Sharma et al. 2007), axonal outgrowth (Karabay et al. 2004), and in organizing plant cell walls (Burk et al. 2007). These systems might also employ a reversible inhibitory system similar to what we have described here.
Acknowledgments
We thank M. A. Srayko, J. D. McGhee, J. Gaudet, D. Hansen, and S. Childs for their valuable input during this work and E. Raharjo and B. Gavinolla for help with some experiments. Some of the strains were obtained from the Caenorhabditis Genetics Center, funded by the National Institutes of Health Center for Research Resources. This work was supported by grants from the Canadian Institute of Health Research and the Alberta Heritage Foundation for Medical Research to P.E.M.; from the Fundacai para a Ceinca e a Tecnologia (SFRH/BPD/21510/2005) to J.-E.G.; from the Centre National de la Recherche Scientifique, Fondation pour la Recherche Médicale, Association pour la Recherche sur le Cancer, and the City of Paris to L.P.; and from the Ministry of Education, Culture, Sports, Science and Technology of Japan (KAKENHI on Priority Areas “Systems Genomics”) to A.S.
References
- Albertson, D. G., 1984. Formation of the first cleavage spindle in nematode embryos. Dev. Biol. 101 61–72. [DOI] [PubMed] [Google Scholar]
- Albertson, D. G., and J. N. Thomson, 1993. Segregation of holocentric chromosomes at meiosis in the nematode, Caenorhabditis elegans. Chromosome Res. 1 15–26. [DOI] [PubMed] [Google Scholar]
- Brenner, S., 1974. The genetics of Caenorhabditis elegans. Genetics 77 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewis, N. D., A. J. Street, A. R. Prescott and P. T. Cohen, 1993. PPX, a novel protein serine/threonine phosphatase localized to centrosomes. EMBO J. 12 987–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burk, D. H., R. Zhong and Z. H. Ye, 2007. The katanin microtubule severing protein in plants. J. Integr. Plant Biol. 49 1174–1182. [Google Scholar]
- Chen, J., R. T. Peterson and S. L. Schreiber, 1998. Alpha 4 associates with protein phosphatases 2A, 4, and 6. Biochem. Biophys. Res. Commun. 247 827–832. [DOI] [PubMed] [Google Scholar]
- Clandinin, T. R., and P. E. Mains, 1993. Genetic studies of mei-1 gene activity during the transition from meiosis to mitosis in Caenorhabditis elegans. Genetics 134 199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Maguire, S., and P. E. Mains, 1994. a Localization of the mei-1 gene product of Caenorhabditis elegans, a meiotic-specific spindle component. J. Cell Biol. 126 199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Maguire, S., and P. E. Mains, 1994. b mei-1, a gene required for meiotic spindle formation in Caenorhabditis elegans, is a member of a family of ATPases. Genetics 136 533–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, P. T., A. Philp and C. Vazquez-Martin, 2005. Protein phosphatase 4: from obscurity to vital functions. FEBS Lett. 579 3278–3286. [DOI] [PubMed] [Google Scholar]
- Dobson, C. M., T. Wai, D. Leclerc, H. Kadir, M. Narang et al., 2002. Identification of the gene responsible for the cblB complementation group of vitamin B12-dependent methylmalonic aciduria. Hum. Mol. Genet. 11 3361–3369. [DOI] [PubMed] [Google Scholar]
- Dow, M. R., and P. E. Mains, 1998. Genetic and molecular characterization of the Caenorhabditis elegans gene, mel-26, a postmeiotic negative regulator of mei-1, a meiotic-specific spindle component. Genetics 150 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dymek, E. E., P. A. Lefebvre and E. F. Smith, 2004. PF15p is the Chlamydomonas homologue of the Katanin p80 subunit and is required for assembly of flagellar central microtubules. Eukaryot. Cell 3 870–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley, M. L., D. L. Baillie, D. L. Riddle and A. M. Rose, 2006. Genetic balancers in WormBook, edited by The C. elegans Research Community. WormBook, http://www.wormbook.org. [DOI] [PMC free article] [PubMed]
- Fire, A., S. Xu, M. K. Montgomery, S. A. Kostas, S. E. Driver et al., 1998. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391 806–811. [DOI] [PubMed] [Google Scholar]
- Fraser, A. G., R. S. Kamath, P. Zipperlen, M. Martinez-Campos, M. Sohrmann et al., 2000. Functional genomic analysis of C. elegans chromosome I by systematic RNA interference. Nature 408 325–330. [DOI] [PubMed] [Google Scholar]
- Furukawa, M., Y. J. He, C. Borchers and Y. Xiong, 2003. Targeting of protein ubiquitination by BTB-Cullin 3-Roc1 ubiquitin ligases. Nat. Cell Biol. 5 1001–1007. [DOI] [PubMed] [Google Scholar]
- Gengyo-Ando, K., and S. Mitani, 2000. Characterization of mutations induced by ethyl methanesulfonate, UV, and trimethylpsoralen in the nematode Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 269 64–69. [DOI] [PubMed] [Google Scholar]
- Gingras, A. C., M. Caballero, M. Zarske, A. Sanchez, T. R. Hazbun et al., 2005. A novel, evolutionarily conserved protein phosphatase complex involved in cisplatin sensitivity. Mol. Cell. Proteomics 4 1725–1740. [DOI] [PubMed] [Google Scholar]
- Glickman, M. H., and A. Ciechanover, 2002. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol. Rev. 82 373–428. [DOI] [PubMed] [Google Scholar]
- Gomes, J. E., S. E. Encalada, K. A. Swan, C. A. Shelton, J. C. Carter et al., 2001. The maternal gene spn-4 encodes a predicted RRM protein required for mitotic spindle orientation and cell fate patterning in early C. elegans embryos. Development 128 4301–4314. [DOI] [PubMed] [Google Scholar]
- Hartman, P. S., and R. K. Herman, 1982. Radiation-sensitive mutants of Caenorhabditis elegans. Genetics 102 159–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie, C. J., G. K. Carnegie, N. Morrice and P. T. Cohen, 2000. A novel 50 kDa protein forms complexes with protein phosphatase 4 and is located at centrosomal microtubule organizing centres. Biochem. J. 347(Pt. 3): 845–855. [PMC free article] [PubMed] [Google Scholar]
- Helps, N. R., N. D. Brewis, K. Lineruth, T. Davis, K. Kaiser et al., 1998. Protein phosphatase 4 is an essential enzyme required for organisation of microtubules at centrosomes in Drosophila embryos. J. Cell Sci. 111(Pt. 10): 1331–1340. [DOI] [PubMed] [Google Scholar]
- Hodgkin, J., H. R. Horvitz and S. Brenner, 1979. Nondisjunction mutants of the nematode Caenorhabditis elegans. Genetics 91 67–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter, T., 2007. The age of crosstalk: phosphorylation, ubiquitination, and beyond. Mol. Cell 28 730–738. [DOI] [PubMed] [Google Scholar]
- Kamath, R. S., and J. Ahringer, 2003. Genome-wide RNAi screening in Caenorhabditis elegans. Methods 30 313–321. [DOI] [PubMed] [Google Scholar]
- Kamath, R. S., A. G. Fraser, Y. Dong, G. Poulin, R. Durbin et al., 2003. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421 231–237. [DOI] [PubMed] [Google Scholar]
- Karabay, A., W. Yu, J. M. Solowska, D. H. Baird and P. W. Baas, 2004. Axonal growth is sensitive to the levels of katanin, a protein that severs microtubules. J. Neurosci. 24 5778–5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemphues, K. J., N. Wolf, W. B. Wood and D. Hirsh, 1986. Two loci required for cytoplasmic organization in early embryos of Caenorhabditis elegans. Dev. Biol. 113 449–460. [DOI] [PubMed] [Google Scholar]
- Kim, S. H., A. H. Holway, S. Wolff, A. Dillin and W. M. Michael, 2007. SMK-1/PPH-4.1-mediated silencing of the CHK-1 response to DNA damage in early C. elegans embryos. J. Cell Biol. 179 41–52. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kloeker, S., and B. E. Wadzinski, 1999. Purification and identification of a novel subunit of protein serine/threonine phosphatase 4. J. Biol. Chem. 274 5339–5347. [DOI] [PubMed] [Google Scholar]
- Lee, M. H., and T. Schedl, 2001. Identification of in vivo mRNA targets of GLD-1, a maxi-KH motif containing protein required for C. elegans germ cell development. Genes Dev. 15 2408–2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, J. A., and J. T. Fleming, 1995. Basic culture methods. Methods Cell Biol. 48 3–29. [PubMed] [Google Scholar]
- Li, S., C. M. Armstrong, N. Bertin, H. Ge, S. Milstein et al., 2004. A map of the interactome network of the metazoan C. elegans. Science 303 540–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohret, T. A., F. J. McNally and L. M. Quarmby, 1998. A role for katanin-mediated axonemal severing during Chlamydomonas deflagellation. Mol. Biol. Cell 9 1195–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, C., and P. E. Mains, 2005. Mutations of a redundant α-tubulin gene affect Caenorhabditis elegans early embryonic cleavage via MEI-1/katanin-dependent and -independent pathways. Genetics 170 115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, C., and P. E. Mains, 2007. The C. elegans anaphase promoting complex and MBK-2/DYRK kinase act redundantly with CUL-3/MEL-26 ubiquitin ligase to degrade MEI-1 microtubule-severing activity after meiosis. Dev. Biol. 302 438–447. [DOI] [PubMed] [Google Scholar]
- Lu, C., M. Srayko and P. E. Mains, 2004. The Caenorhabditis elegans microtubule-severing complex MEI-1/MEI-2 katanin interacts differently with two superficially redundant beta-tubulin isotypes. Mol. Biol. Cell 15 142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mains, P. E., K. J. Kemphues, S. A. Sprunger, I. A. Sulston and W. B. Wood, 1990. a Mutations affecting the meiotic and mitotic divisions of the early Caenorhabditis elegans embryo. Genetics 126 593–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mains, P. E., I. A. Sulston and W. B. Wood, 1990. b Dominant maternal-effect mutations causing embryonic lethality in Caenorhabditis elegans. Genetics 125 351–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews, L. R., P. Carter, D. Thierry-Mieg and K. Kemphues, 1998. ZYG-9, a Caenorhabditis elegans protein required for microtubule organization and function, is a component of meiotic and mitotic spindle poles. J. Cell Biol. 141 1159–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarter, J., B. Bartlett, T. Dang and T. Schedl, 1999. On the control of oocyte meiotic maturation and ovulation in Caenorhabditis elegans. Dev. Biol. 205 111–128. [DOI] [PubMed] [Google Scholar]
- McNally, K., A. Audhya, K. Oegema and F. J. McNally, 2006. Katanin controls mitotic and meiotic spindle length. J. Cell Biol. 175 881–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally, K. P., D. Buster and F. J. Mcnally, 2002. Katanin-mediated microtubule severing can be regulated by multiple mechanisms. Cell Motil Cytoskeleton 53 337–349. [DOI] [PubMed] [Google Scholar]
- Ming Pang, K., T. Ishidate, K. Nakamura, M. Shirayama, C. Trzepacz et al., 2004. The minibrain kinase homolog, mbk-2, is required for spindle positioning and asymmetric cell division in early C. elegans embryos. Dev. Biol. 265 127–139. [DOI] [PubMed] [Google Scholar]
- Nanahoshi, M., Y. Tsujishita, C. Tokunaga, S. Inui, N. Sakaguchi et al., 1999. Alpha4 protein as a common regulator of type 2A-related serine/threonine protein phosphatases. FEBS Lett. 446 108–112. [DOI] [PubMed] [Google Scholar]
- Panowski, S. H., S. Wolff, H. Aguilaniu, J. Durieux and A. Dillin, 2007. PHA-4/Foxa mediates diet-restriction-induced longevity of C. elegans. Nature 447 550–555. [DOI] [PubMed] [Google Scholar]
- Pellettieri, J., V. Reinke, S. K. Kim and G. Seydoux, 2003. Coordinate activation of maternal protein degradation during the egg-to-embryo transition in C. elegans. Dev. Cell 5 451–462. [DOI] [PubMed] [Google Scholar]
- Petroski, M. D., and R. J. Deshaies, 2005. Function and regulation of cullin-RING ubiquitin ligases. Nat. Rev. Mol. Cell Biol. 6 9–20. [DOI] [PubMed] [Google Scholar]
- Phillips, J. B., R. Lyczak, G. C. Ellis and B. Bowerman, 2004. Roles for two partially redundant alpha-tubulins during mitosis in early Caenorhabditis elegans embryos. Cell Motil. Cytoskeleton 58 112–126. [DOI] [PubMed] [Google Scholar]
- Pintard, L., J. H. Willis, A. Willems, J. L. Johnson, M. Srayko et al., 2003. The BTB protein MEL-26 is a substrate-specific adaptor of the CUL-3 ubiquitin-ligase. Nature 425 311–316. [DOI] [PubMed] [Google Scholar]
- Quintin, S., P. E. Mains, A. Zinke and A. A. Hyman, 2003. The mbk-2 kinase is required for inactivation of MEI-1/katanin in the one-cell Caenorhabditis elegans embryo. EMBO Rep. 4 1175–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatten, G., 1994. The centrosome and its mode of inheritance: the reduction of the centrosome during gametogenesis and its restoration during fertilization. Dev. Biol. 165 299–335. [DOI] [PubMed] [Google Scholar]
- Sharma, N., J. Bryant, D. Wloga, R. Donaldson, R. C. Davis et al., 2007. Katanin regulates dynamics of microtubules and biogenesis of motile cilia. J. Cell Biol. 178 1065–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnichsen, B., L. B. Koski, A. Walsh, P. Marschall, B. Neumann et al., 2005. Full-genome RNAi profiling of early embryogenesis in Caenorhabditis elegans. Nature 434 462–469. [DOI] [PubMed] [Google Scholar]
- Srayko, M., D. W. Buster, O. A. Bazirgan, F. J. McNally and P. E. Mains, 2000. MEI-1/MEI-2 katanin-like microtubule severing activity is required for Caenorhabditis elegans meiosis. Genes Dev. 14 1072–1084. [PMC free article] [PubMed] [Google Scholar]
- Srayko, M., E. T. O'Toole, A. A. Hyman and T. Müller-Reichert, 2006. Katanin disrupts the microtubule lattice and increases polymer number in C. elegans meiosis. Curr. Biol. 16 1944–1949. [DOI] [PubMed] [Google Scholar]
- Stitzel, M. L., J. Pellettieri and G. Seydoux, 2006. The C. elegans DYRK kinase MBK-2 marks oocyte proteins for degradation in response to meiotic maturation. Curr. Biol. 16 56–62. [DOI] [PubMed] [Google Scholar]
- Stitzel, M. L., K. C. Cheng and G. Seydoux, 2007. Regulation of MBK-2/Dyrk kinase by dynamic cortical anchoring during the oocyte-to-zygote transition. Curr. Biol. 17 1545–1554. [DOI] [PubMed] [Google Scholar]
- Sulston, J. E., E. Schierenberg, J. G. White and J. N. Thomson, 1983. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev. Biol. 100 64–119. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi, E., A. Sugimoto and M. Yamamoto, 2002. Protein phosphatase 4 is required for centrosome maturation in mitosis and sperm meiosis in C. elegans. J. Cell Sci. 115 1403–1410. [DOI] [PubMed] [Google Scholar]
- Timmons, L., D. L. Court and A. Fire, 2001. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene 263 103–112. [DOI] [PubMed] [Google Scholar]
- Toyo-Oka, K., D. Mori, Y. Yano, M. Shiota, H. Iwao et al., 2008. Protein phosphatase 4 catalytic subunit regulates Cdk1 activity and microtubule organization via NDEL1 dephosphorylation. J. Cell Biol. 180 1133–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Wijk, T., C. Blanchetot and J. Den Hertog, 2005. Regulation of receptor protein-tyrosine phosphatase dimerization. Methods 35 73–79. [DOI] [PubMed] [Google Scholar]
- Wada, T., T. Miyata, R. Inagi, M. Nangaku, M. Wagatsuma et al., 2001. Cloning and characterization of a novel subunit of protein serine/threonine phosphatase 4 from mesangial cells. J. Am. Soc. Nephrol. 12 2601–2608. [DOI] [PubMed] [Google Scholar]
- Xu, L., Y. Wei, J. Reboul, P. Vaglio, T. H. Shin et al., 2003. BTB proteins are substrate-specific adaptors in an SCF-like modular ubiquitin ligase containing CUL-3. Nature 425 316–321. [DOI] [PubMed] [Google Scholar]
- Yang, H., K. McNally and F. J. McNally, 2003. MEI-1/katanin is required for translocation of the meiosis I spindle to the oocyte cortex in C. elegans. Dev. Biol. 260 245–259. [DOI] [PubMed] [Google Scholar]
- Yang, H. Y., P. E. Mains and F. J. McNally, 2005. Kinesin-1 mediates translocation of the meiotic spindle to the oocyte cortex through KCA-1, a novel cargo adapter. J. Cell Biol. 169 447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, D., G. C. Rogers, D. W. Buster and D. J. Sharp, 2007. Three microtubule severing enzymes contribute to the “Pacman-flux” machinery that moves chromosomes. J. Cell Biol. 177 231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]