Abstract
A group of small ventrolateral neurons (s-LNv's) are the principal pacemaker for circadian locomotor rhythmicity of Drosophila melanogaster, and the pigment-dispersing factor (Pdf) neuropeptide plays an essential role as a clock messenger within these neurons. In our comparative studies on Pdf-associated circadian rhythms, we found that daily locomotor activity patterns of D. virilis were significantly different from those of D. melanogaster. Activities of D. virilis adults were mainly restricted to the photophase under light:dark cycles and subsequently became arrhythmic or weakly rhythmic in constant conditions. Such activity patterns resemble those of Pdf01 mutant of D. melanogaster. Intriguingly, endogenous D. virilis Pdf (DvPdf) expression was not detected in the s-LNv-like neurons in the adult brains, implying that the Pdf01-like behavioral phenotypes of D. virilis are attributed in part to the lack of DvPdf in the s-LNv-like neurons. Heterologous transgenic analysis showed that cis-regulatory elements of the DvPdf transgene are capable of directing their expression in all endogenous Pdf neurons including s-LNv's, as well as in non-Pdf clock neurons (LNd's and fifth s-LNv) in a D. melanogaster host. Together these findings suggest a significant difference in the regulatory mechanisms of Pdf transcription between the two species and such a difference is causally associated with species-specific establishment of daily locomotor activity patterns.
PIGMENT-dispersing hormone (Pdh) was initially identified in crustaceans (Ferlund 1976) as a mediator for the dispersion of extraretinal screening pigments to enhance visual sensitivity (reviewed in Aréchiga et al. 1993; Rao 2001). Persistent daily fluctuations in the pigmentation pattern even under a constant condition have suggested that the pigment dispersion rhythm is under the control of an endogenous clock system, thus implicating Pdh as a hormonal factor that channels the central circadian clock functions.
Structural homologs of the crustacean Pdh have been found in diverse insect groups, and these insect peptides are referred to as pigment dispersing factors (Pdf) (Rao and Riehm 1993). Pdf-immunoreactive (ir) neurons in many insects are typically found in the lateral margin of the anterior protocerebrum (accessory medulla) that neighbors the medulla of the optic lobes (Homberg et al. 1991b; Sehadova et al. 2003). Experiments involving surgical extirpation suggested this protocerebral region as a site of circadian pacemaker activity in several insects (Page 1982; reviewed in Saunders 2002). In addition, a dosage- and time-dependent phase shift of locomotive activity in response to injection of Pdf peptide into the accessory medulla in cockroaches and crickets implies that Pdf plays a modulatory function in the insect biological clock system (Stengl and Homberg 1994; Petri and Stengl 1997; Singaravel et al. 2003).
Further understandings of Pdf functions associated with the insect clock came from molecular and neurogenetic studies in the fruit fly, Drosophila melanogaster. Since the identification of the period (per) as the first genetic locus associated with circadian clock functions (Konopka and Benzer 1971), extensive investigations have been undertaken to understand molecular and cellular bases of the endogenous time-keeping system in D. melanogaster. These studies unraveled a group of Per-producing ventrolateral neurons (LNv's) as a strong candidate for the circadian pacemaker (Ewer et al. 1992; Frisch et al. 1994). Subsequently, detection of Pdf immunoreactivity in the LNv's provided an important clue that Pdf functions in the circadian rhythms in this genetically amenable insect (Helfrich-Förster 1995). In line with this, lack of Pdf-ir neurons in behaviorally arrhythmic disconnected (disco) mutants and behavioral alteration by ectopic expression of the locust Pdf gene further supported Pdf as a regulator of circadian rhythms (Dushay et al. 1989; Helfrich-Förster 1998; Helfrich-Förster et al. 2000).
More decisive evidence for the role of Pdf came from genetic studies using a Pdf-null mutation (Pdf01) and selective ablation of Pdf neurons. These types of genetic manipulations caused similar arrhythmic free-running locomotor activity under continuous darkness (Renn et al. 1999). Consistent with these findings, mutants lacking Pdf-receptor functions phenocopy behavioral defects of Pdf01, thus confirming essential roles played by the Pdf-signaling pathway in the regulation of circadian rhythms (Hyun et al. 2005; Lear et al. 2005; Mertens et al. 2005). Multiple lines of evidence also suggest that Pdf is important for intercellular communication between Pdf neurons and non-Pdf clock neurons, and self-sustaining molecular oscillation in both types of neurons, particularly under constant darkness conditions (Peng et al. 2003; Lin et al. 2004; Wu et al. 2008).
Expression of the D. melanogaster Pdf (DmPdf) gene in the protocerebrum is evident in two distinct clusters of ventrolateral neurons, which are classified into large (l)-LNv's and small (s)-LNv's on the basis of their sizes of the somata and in a pair of neurons located in the tritocerebrum (Helfrich-Förster 1997). The latter group appears to be eliminated via programmed cell death shortly after eclosion, thereby leaving only the LNv's in the mature adult brain (Renn et al. 1999). Various lines of evidence demonstrated that the s-LNv neurons are particularly important for the circadian locomotor activity rhythms (Helfrich-Förster 2005; Taghert and Shafer 2006, and references therein). Moreover, DmPdf expression is differentially regulated between the two groups of neurons, as transcription of DmPdf is absent specifically in the s-LNv's but not in the l-LNv's in the ClockJrk (ClkJrk) and cycle0 (cyc0) mutants, suggesting that Clk and Cyc central clock regulators are upstream factors of the DmPdf within the s-LNv's (Park et al. 2000). In addition, circadian fluctuations of the Pdf immunoreactivity at the s-LNv terminals indicate that the rhythmic release of the Pdf peptide from the s-LNv neurons is an important cellular event for the circadian rhythmicity (Park et al. 2000; Nitbach et al. 2006).
Despite well-defined clock functions played by Pdf in D. melanogaster, it has not been determined whether Pdf's function as a circadian regulator is general in other insects. In the hawk moth, Manduca sexta, no Pdf-ir cells were found in the accessory medulla (Homberg et al. 1991a; Wise et al. 2002). Moreover, colocalization of Per and Pdf was not detected in this neuropil in most other insects (Závodská et al. 2003). The same studies also showed that Per immunoreactivity was found mainly in the cytoplasm, which is contrasted to nuclear-cytoplamic shuttling of this protein within the clock neurons of D. melanogaster during the course of a day (e.g., Shafer et al. 2002). These observations raise the possibility that neural and molecular bases of the biological clock system have evolved uniquely among insect species, perhaps to maximize adaptive fitness to their natural environment.
To gain insight into the evolutionary aspects of circadian rhythmicity associated with Pdf, we examined locomotor activity behavior and Pdf expression patterns in D. virilis, a species distantly related to D. melanogaster and diverged from the melanogaster lineage about 63 million years ago (Tamura et al. 2004). We found significantly different circadian locomotor activity patterns between the two species and such dissimilar patterns likely stem from differential regulation of Pdf expression in the key pacemaker neuronal groups.
MATERIALS AND METHODS
Fly strains:
Flies were raised in a food containing yeast-cornmeal-agar medium supplemented with 10% methyl paraben (Tagosept) as a preservative, and kept at room temperature. Canton-S or yellow white (y w) was used as controls, and ClockJrk, cycle0, and Pdf01 mutants were described as previously (Allada et al. 1998; Rutila et al. 1998; Renn et al. 1999; Park et al. 2000). Wild-type D. virilis was obtained from Hall lab (University of Maine).
Cloning and characterization of the Pdf gene in D. virilis:
See supplemental material.
Histology:
Digoxigenin (dig)-labeled antisense DNA probe was produced by asymmetric PCR using full-length DvPdf cDNA template, single primer, and dig-tagged nucleotide, as described (Kim et al. 2006). The probe was incubated with the CNSs, and resulting mRNA-DNA hetroduplex was detected immunologically (Lee et al. 2000). Pdf immunohistochemistry (IHC) of whole-mounted CNS was performed in the same manner as reported previously using rat-derived anti-Pdf (Park et al. 2000). Polyclonal antisera specific to the DvPdf precursor were raised against a synthetic peptide within the PAP region in two rabbits (underlined in Figure 2D). The two antisera produced identical results.
Figure 2.—
Molecular characteristics of the DvPdf gene. (A) Restriction map of the 11.7-kb D. virilis genomic DNA fragment containing DvPdf gene (arrow). A 3.5-kb subfragment defined by KpnI and HindIII (K–H) restriction sites was used for making DvPdf transgenic lines, and K–X upstream fragment (1.9 kb) for DvPdf-gal4 drivers. The second KpnI site designated by a broken line is not present in the phage DNA clone, but predicted to exist in the genome of our D. virilis flies on the basis of the Southern hybridization result. Such a difference is likely to reflect a polymorphic difference between the two genomic sources. E, EcoRI; H, HindIII; K, KpnI; X, XcmI. (B) Northern blotting. Total RNAs (20 μg/lane) were separately purified from D. virilis male (m) or female (f), heads and bodies, and then hybridized to 32P-labeled DvPdf cDNA probe. A probe for the ribosomal protein 49 (rp49) gene was used as a loading control. (C) Southern blot analysis. D. virilis genomic DNA (30 μg/lane) was digested with restriction enzymes as indicated (E, EcoRI; H, HindIII; K, KpnI) and the blot was hybridized to 32P-labeled DvPdf cDNA probe. Numbers on the right indicate size markers (kb). (D) Alignment of dipteran Pdf precursors by using Web-based ClustalW2 software (www.ebi.ac.uk/Tools/clustalw2/index.html). The numbers indicate amino acid length of each precursor. Boldface letters represent mature Pdf peptide, and the underlining indicates residues for producing DvPdf-specific antibody. Consensus prohormone proteolytic cleavage site (KR) and C-terminal amidation signal (GK) are indicated by boxes (cf. Veenstra 2000). Dm, D. melanogaster; Dv, D. virilis; Pr, Phormia regina; Md, Musca domestica; Ag, Anopheles gambiae; Aa, Aedes agypti; Cq, Culex quinquefasciatus. (E) Amino acid identity of the Pdf precursors. Consensus modification sites (boxes) and mature Pdf region are excluded for this comparison.
Analysis of the circadian locomotor activity rhythm:
Flies were entrained for 3–4 cycles of 12-hr light:12-hr dark conditions (12:12 LD) and then proceeded into constant darkness (DD) or constant light (LL). In some experiments, the second LD cycles were provided following constant conditions. Locomotor activity of individual flies was monitored using Drosophila activity monitors (Trikinetics). For the measurement of D. virilis locomotor activity, 7-mm-diameter locomotor monitors equipped with dual detectors were used to allow free moving of the flies (Trikinetics; Rosato and Kyriacou 2006). Data analysis was done with ClockLab software (Actimetrics).
RESULTS
Circadian behavioral rhythms of D. virilis:
Locomotor activity rhythms are one of the best-characterized circadian behaviors in D. melanogaster (Konopka and Benzer 1971; Hamblen et al. 1986; Rosato and Kyriacou 2006). To understand whether the circadian clock system is conserved in other Drosophila species, we analyzed this type of behavior in D. virilis.
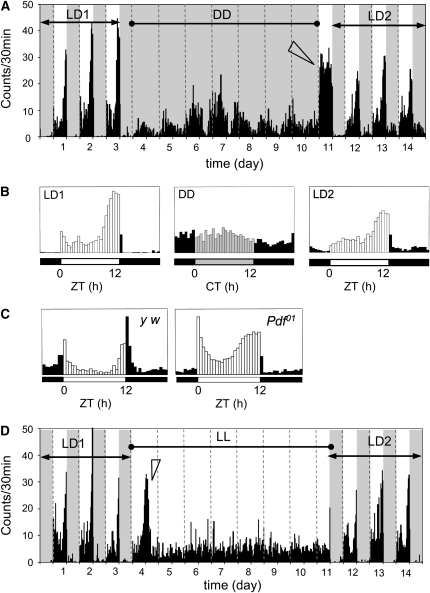
Interestingly, D. virilis adults showed substantial differences in daily activity patterns from those of D. melanogaster. As observed by other studies, under LD cycles, D. melanogaster displayed bimodal activity peaks, each at dawn and dusk, with gradual increase in activity prior to lights on and lights off (e.g., Renn et al. 1999 and Figure 1). By comparison, D. virilis flies were quiescent during the entire nighttime, and a sudden burst of activity followed immediately after lights on. Activity levels gradually rose after midday, reaching the peak at ∼2 hr prior to lights off (Figure 1, A and B). Then, the evening activity vanished rapidly after lights were off and remained inactive throughout the night phase. As a result, 96% of total activity was concentrated in the photophase, indicating that this species is principally diurnal. Similar restriction of activity to the photophase was also observed for other flies (Helfrich et al. 1985; Cymborowski et al. 1996). Such diurnally active patterns are in stark contrast to the crepuscular type of locomotor behavior displayed by wild-type D. melanogaster, which account for 53% of total activity in the dark phase (e.g., Figure 1C).
Figure 1.—
Circadian locomotor activity rhythms in D. virilis. (A) The activities were measured during three 12:12 LD cycles (LD1) and subsequent 7 days of constant darkness (DD), followed by the second three LD cycles (LD2). Under LD cycling condition, flies show prominent evening activity peaks, but no anticipatory increase of activity levels before lights on. Activities are largely restricted to light phase. Arrowhead indicates persistent hyperactivity in the first daytime period following DD. (B) Average activity for each lighting regimen, as described in A. Bars indicate average activity events per 30-min bin per fly. Open bars indicate activities in daytime, solid bars in nighttime, and shaded bars in subjective day. (C) Average activity of D. melanogaster wild-type (y w) and Pdf01 mutants under 12:12 LD cycles. Note that Pdf01 behavior is somewhat comparable to that of D. virilis. (D) Circadian activity in constant light (LL) condition. Activity was monitored for 3 days of LD (LD1), 7 days of LL, followed by 3 days of LD (LD2). Arrowhead indicates gradual decline of activity levels in the first subjective night in LL.
Under DD conditions, a substantial fraction (54%) of D. virilis flies showed arrhythmic free-running locomotor activity, while the remaining flies displayed relatively weak rhythmicity with a short period length (τ = 23.2 hr) (Table 1). This type of free-running rhythms is reminiscent of the Pdf-null mutant flies (Renn et al. 1999). When second LD cycles (LD2) were resumed after 7 days of DD, persistent hyperactivity was observed in the first light phase (arrowhead in Figure 1A), and then LD1-like activity patterns were restored, except for slightly increased night activity (Figure 1, A and B).
TABLE 1.
Locomotor activity rhythms in DD condition
| Genotype | N | R (%) | WR (%) | AR (%) | Period (hr) (mean ± SEM) | Powera(mean ± SEM) |
|---|---|---|---|---|---|---|
| y w | 53 | 45 (84) | 4 (8) | 4 (8) | 24.0 ± 0.5 | 59.4 ± 28.4 |
| D. virilis | 55 | 8 (15) | 17 (31) | 30 (54) | 23.2 ± 3.3 | 8.3 ± 7.5 |
| y w; Pdf01 | 55 | 18 (33) | 15 (27) | 22 (40) | 22.8 ± 1.8 | 20.7 ± 24.5 |
| DvPdfS1a | 37 | 32 (86) | 5 (14) | 0 | 23.6 ± 0.5 | 71.9 ± 45.9 |
| DvPdfS1a; Pdf01 | 25 | 19 (76) | 5 (20) | 1 (4) | 24.4 ± 1.1 | 45 ± 40.6 |
| DvPdfS2/CyO | 44 | 40 (91) | 3 (7) | 1 (2) | 23.9 ± 2.3 | 60.2 ± 39.0 |
| DvPdfS2/CyO; Pdf01 | 30 | 28 (94) | 1 (3) | 1 (3) | 23.9 ± 0.5 | 75.0 ± 29.8 |
| D. virilis (LL) | 58 | 6 (10) | 21 (36) | 31 (54) | 26.5 ± 7.2 | 6.1 ± 4.3 |
N, number of lines tested; R, rhythmic (power ≥10); WR, weak rhythmic (0 < power < 10); AR, arrhythmic; LL, locomotor activity in constant light condition.
Power was defined as the amplitude of the peak above the significant line (α = 0.025) in the chi-square periodogram (Liu et al. 1991).
We also measured free-running locomotor activity in a constant light (LL) condition. Instead of a precipitous decrease of activity in response to lights off shown in LD condition, the activity levels gradually declined in the first subjective night (arrowhead in Figure 1D), and then became largely arrhythmic, which was similar to previous observations in D. melanogaster (Konopka et al. 1989). When flies were subsequently exposed to LD2, they were mostly inactive in the first night, and then typical diurnal activity patterns were resumed. In summary, locomotor activity of D. virilis is highly sensitive to lighting conditions, such that the flies seem to be motivated to move actively during daytime. Moreover, endogenous clock functions governing free-running activity rhythms are not as robust as D. melanogaster ones.
Characterization of the D. virilis Pdf (DvPdf) gene:
The foregoing behavioral data of D. virilis showed strong resemblance to those of Pdf-null (Pdf01) mutant flies of D. melanogaster: the lack of lights-on anticipation, slight phase advance of evening activity peak, and significantly arrhythmic free-running behavior in DD (Figure 1C; Renn et al. 1999). Such similarity led us to wonder whether the genome of our wild-type D. virilis carries a spontaneous Pdf loss-of-function mutation, as was the case for Pdf01 mutation that we described previously in D. melanogaster (Renn et al. 1999). Thus we first cloned and characterized DvPdf sequence (Figure 2A). The sequence data matched perfectly with the open reading frame (ORF) later published in the genome database for D. virilis (Dvir\GJ23022; Drosophila 12 Genomes Consortium 2007).
Results from RACE defined a 614-bp DvPdf transcriptional unit (supplemental Figure 1). Northern blot analysis agreed with this result, as it revealed ∼0.8-kb DvPdf transcript expressed mainly in the head of male or female flies (Figure 2B). Comparison of the cDNA sequence with genomic sequence showed that the DvPdf is a single-exon gene, as is the case for the DmPdf gene (Park and Hall 1998). The Southern blot result suggests that the DvPdf gene is present in a single copy per haploid genome (Figure 2C).
Conceptual 99-amino-acid DvPdf peptide precursor consists of three distinct domains: signal sequence (24 aa) at the N terminus for secretory pathway, followed by Pdf-associated peptide (PAP, 53 aa), then by Pdf at the C terminus (Figure 2D). This tripartite structure is typical of Pdf precursors identified in other arthropod species (e.g., Rao 2001). The presence of dibasic consensus cleavage site (KR) just before the Pdf indicates that the precursors are processed to produce mature 18-amino-acid-long Pdf peptide. Alignment of several dipteran Pdf precursors shows that the mature Pdf sequences are highly conserved, which points to the physiological significance of this domain (Figure 2D). In contrast, PAP regions are markedly diverged; for instance, a mere 22% of identity is observed in the precursor region between housefly (Musca domestica) and D. virilis (Figure 2E).
In two species outside the Drosophila genus, we found that the Pdf gene contains an intron within the open reading frame. Comparison of cDNA sequence (Matsushima et al. 2004) with PCR-amplified genomic DNA one revealed a 63-bp phase-0 intron (i.e., between two codons) in the M. domestica Pdf (MdPdf) gene (GenBank accession no. FJ043031). Anopheles gambiae Pdf (AgPdf) also contains a larger 278-bp intron (also phase-0) at a comparable position on the basis of the reported genome database (GenBank accession no. FJ154750). Thus it seems that Pdf gene structure and sequence have changed significantly during the course of dipteran evolution, as was observed for another neuropeptide gene Corazonin (Choi et al. 2005).
Lack of Pdf expression in the s-LNv-like neurons in D. virilis adults:
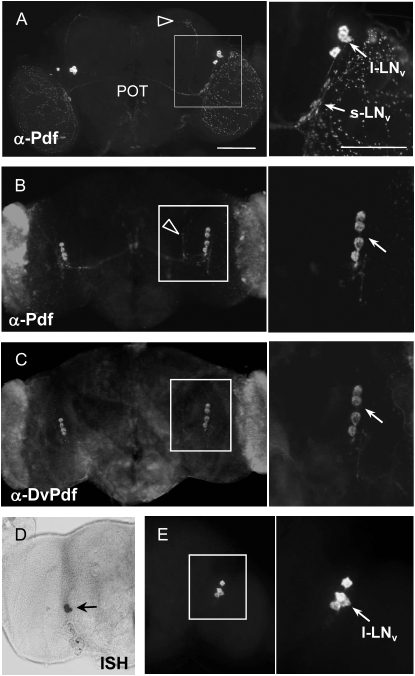
Expression of the D. melanogaster Pdf (DmPdf) is limited to two neuronal groups, s-LNv's and l-LNv's, in the ventrolateral margin of the anterior protocerebrum of mature adults. These two groups are easily recognizable due to distinct sizes of their somata and axonal projection patterns unique to each group (Figure 3A; Park et al. 2000; Helfrich-Förster et al. 2007). DmPdf production from the s-LNv's is particularly important for the circadian rhythms in this species.
Figure 3.—
Pdf expression patterns in D. virilis. (A) Pdf-ir patterns in D. melanogaster adult brain. Small and large ventrolateral neurons (s-LNv and l-LNv) are indicated by arrows and dorsally oriented projections from the s-LNv's, by an arrowhead. Area marked by a box is enlarged in the right. (B and C) Pdf-ir patterns observed in D. virilis adult brain. IHC was done (B) with anti-Pdf or (C) anti-DvPdf raised against DvPdf specific region (Figure 2D). (D and E) In situ hybridization (ISH) of DvPdf mRNA in the D. virilis adult brain. ISH signal was detected (D) by a colorimetric reaction, or (E) fluorescence using tyramide signal amplification system (TSA). Both methods confirmed localization of the DvPdf mRNA only in l-LNv-like neurons (arrows). Bar, 100 μm.
To investigate the causal relationship between DvPdf expression and Pdf01-like activity patterns displayed by D. virilis, DvPdf expression was examined in the brain of D. virilis adults using anti-Pdf that detects a mature peptide. Surprisingly, Pdf immunoreactivity was found exclusively in a cluster of four neurons that gave rise to contralateral projections through the posterior optic tract (POT) and extensive arborization in the medulla of the optic lobes (Figure 3B, n = 20). These neuroanatomical features are comparable to those of the l-LNv's of D. melanogaster, suggesting that DvPdf-expressing neurons are equivalent to the l-LNv's. Furthermore, the absence of s-LNv-like Pdf immunoreactivity as well as short and dorsal fibers deviated from the POT in the medial region (arrowhead in Figure 3B), are remarkably similar to the Pdf-ir patterns described for the ClkJrk and cyc02 mutants of D. melanogaster (Park et al. 2000).
It is possible that s-LNv-like neurons process DvPdf precursors differentially to contain PAP as a functional peptide, thereby lacking Pdf immunoreactivity. To test this, we performed IHC using antisera raised against the PAP region of the DvPdf precursor (anti-DvPdf), as indicated in Figure 2D. The results from this experiment were identical to those obtained with anti-Pdf (Figure 3, B vs. C, n = 18). In addition, in situ hybridization also produced signals only in the l-LNv-like neurons, verifying a limited transcriptional activity of the DvPdf gene to these neurons (Figure 3, D and E, n = 18). We also confirmed the lack of s-LNv Pdf immunoreactivity in a different D. virilis strain that we obtained from the University of California San Diego Drosophila Stock Center (15010–1051.09) (data not shown, n = 5). Therefore, transcriptional regulatory mechanisms of the Pdf gene seem to have evolved differentially between the two species. Taking all of these data into consideration, we propose that the lack of DvPdf expression in the s-LNv-like pacemaker neurons is responsible for the Pdf01-like behavior phenotype of D. virilis.
Expression of the D. virilis Pdf gene in D. melanogaster:
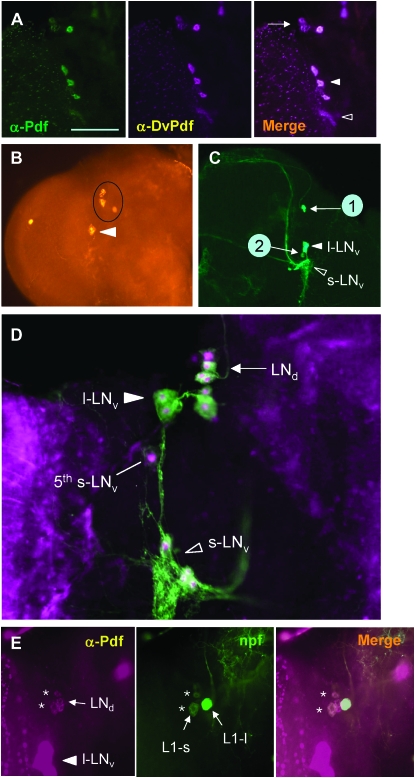
Since tissue-specific expression of a gene is regulated by an interaction between cis-acting elements and their cognate trans-acting factors, the loss of DvPdf expression in the s-LNv-like neurons could result from nonfunctional cis-acting elements within the DvPdf regulatory region. If so, then DvPdf cis-acting elements would likely be inactive in the s-LNv's of D. melanogaster host as well. To test this hypothesis, we employed a heterologous transgenic assay in which a 3.5-kb genomic fragment, spanning from 1.9-kb 5′ upstream to 0.9-kb downstream of the DvPdf gene, was introduced into the D. melanogaster genome (Figure 2A). To detect DvPdf expression unambiguously in the D. melanogaster CNS, the transgene was recombined with the Pdf01 allele. In this genetic context, only DvPdf transgene would produce Pdf-ir materials, as Pdf01 mutant CNSs are devoid of Pdf completely (Renn et al. 1999). IHC using either anti-Pdf or anti-DvPdf revealed robust DvPdf immunoreactivity in both l-LNv and s-LNv groups (Figure 4A, arrowheads) as well as in the abdominal ganglionic neurons (n = 8, data not shown). These data suggest that DvPdf's regulatory sequence is recognized by host transcription factors, thus capable of directing DvPdf expression in all endogenous neuronal groups in D. melanogaster.
Figure 4.—
Expression of the DvPdf transgene in D. melanogaster. (A) Expression of the DvPdfS1a transgene in the Pdf-null (Pdf01) mutant. Both anti-Pdf (green) and anti-DvPdf (magenta) detect all l-LNv's (solid white arrowhead) and s-LNv's (open arrowhead). In addition to these normal Pdf neurons, a group of Pdf-ir neurons are found in a region dorsal to the l-LNv's (arrow). (B) Pdf immunoreactivity of the progeny from {DmPdf-gal4; UAS-rpr × DvPdfS1a; DvPdfT3}. This type of transgenic manipulation kills all endogenous Pdf neurons. Only two groups of ectopic DvPdf neurons are stained (circle and arrowhead). (C) DvPdf-promoted GFP expression. Progeny from {DvPdf-gal4 × UAS-mCD8GFP} cross was processed to visualize GFP signals in adult brains. Two groups of ectopic neurons are designated by numbers. (D) Immunostaining of GFP-labeled DvPdf neurons with anti-Tim at ZT20 (lights are off at ZT12 in 12:12 LD cycles). Nuclear anti-TIM immunoreactivity (magenta) is clearly observed within all of the DvPdf-producing neurons (green). (E) Double labeling of the LNd's with npf and DvPdf. Progeny from {UAS-mCD8GFP; npf-gal4 × DvPdfS1a; DvPdfT3} were immunostained with anti-Pdf. On average, 1.9 of 2.4 npf neurons (i.e., L1-s) are positive for DvPdf (n = 10 brain hemisphere), as indicated by asterisks. L1-l is another npf neuron, which is not part of LNd's.
Interestingly, we found transgenic DvPdf expression in two additional groups of neurons. One of them, consisting of three to four neurons per brain lobe, located in a region dorsal to the l-LNv's (arrow in Figure 4A), while the other single neuron with relatively weak immunoreactivity was observed in the vicinity of the l-LNv's. Due to weak staining intensities, the latter neuron was not always clearly distinguishable. These ectopic neurons were consistently identified in two independent transgenic lines, DvPdf S1a and DvPdf T3 (Figure 4A, and data not shown, n > 10 for each transgene). To distinguish more clearly these ectopic neuronal groups from the l-LNv's and s-LNv's, DvPdf expression was examined in the CNS lacking the endogenous Pdf neurons. For this, selective ablation of the l-LNv's and s-LNv's was achieved through the expression of a proapoptotic gene, reaper (rpr) by using DmPdf-gal4 driver (cf. Renn et al. 1999). The result confirmed two distinct groups of ectopic Pdf-ir neurons, while two groups of endogenous neurons were successfully removed (Figure 4B).
To further investigate whether such ectopic expression reflects transcriptional activity of the DvPdf transgene, DvPdf-gal4 drivers using ∼1.9-kb upstream sequence were employed to express GFP reporter gene. As a result, GFP expression was clearly detected in the s-LNv's, l-LNv's, as well as in the extra sets of neurons (numbered in Figure 4C). Thus it is apparent that DvPdf expression reflects bona fide transcriptional activity regulated by the DvPdf cis-acting sequences in the heterologous host.
The ectopic DvPdf neurons showed anatomical resemblance to those described for non-Pdf clock neurons, dorsolateral neurons (LNd), and the fifth s-LNv. To verify this, we carried out a double-labeling experiment in which DvPdf-gal4-driven GFP-marked neurons were labeled with anti-Timeless (Tim). Tim, as a clock protein, has been shown to be a marker of the LNd and the fifth s-LNv neurons, let alone s-LNv's and l-LNv's (Kaneko and Hall 2000; Rieger et al. 2006; Helfrich-Förster et al. 2007). Indeed, all GFP-positive neurons marked “1” (Figure 4C) were also labeled with anti-Tim (Figure 4D, n = 12), confirming that these neurons are a subset of the LNd neurons. The other extra single neuron marked “2” in the vicinity of the l-LNv's (Figure 4C) was also Tim-ir (Figure 4D), thus this neuron is comparable to the one previously described as the fifth s-LNv (Helfrich-Förster et al. 2007).
We previously demonstrated that two or three neurons out of six LNd's per brain lobe (designated as L1-s) produce neuropeptide-F (npf) in the male brain (Lee et al. 2006). To examine whether DvPdf-LNd's overlap with npf-expressing ones, npf-gal4-driven GFP-labeled L1-s neurons were immunostained with anti-Pdf. As a result, approximately two L1-s neurons were found to produce DvPdf (Figure 4E). Unlike npf, however, DvPdf expression is common to both sexes. These findings support functional diversity among the six LNd's, as proposed previously (Lee et al. 2006; Rieger et al. 2006; Helfrich-Förster et al. 2007).
Regulation of the DvPdf gene by central clock factors:
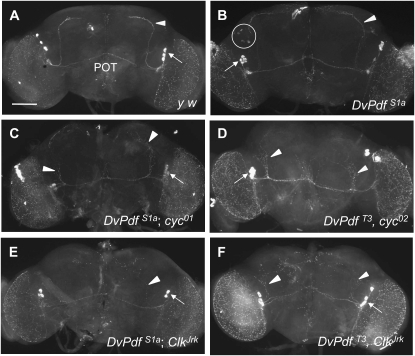
Selective loss of DmPdf expression in the s-LNv's was observed in the brain of arrhythmic ClkJrk and cyc0 mutants, suggesting that Clk:Cyc heterodimer transcription factors are required for DmPdf transcription particularly in this neuronal group (Park et al. 2000). This prompted us to examine whether the Clk and Cyc similarly control the DvPdf transgene. To test this, the DvPdf transgene was recombined with ClkJrk or cyc0 alleles and DvPdf expression was examined in these mutants. As a result, the DvPdf expression was detected only in the l-LNv's in ClkJrk, cyc01, and cyc02 mutant brains (Figure 5, C–F vs. A and B), suggesting that the mutant alleles abolish expression of endogenous DmPdf as well as DvPdf transgene in the s-LNv's. Of interest, the ClkJrk and cyc0 mutations also eliminated DvPdf expression in the LNd's and fifth s-LNv (Figure 5, B vs. C–F). Thus we concluded that Clk and Cyc are essential upstream components for the expression of DmPdf as well as DvPdf transgene in neurons outside the l-LNv's.
Figure 5.—
Regulation of the DvPdf transgene expression in ClkJrk and cyc0 mutant. (A) Control (y w). Dorsally projected fibers stemming from the s-LNv's are indicated by an arrowhead and l-LNv's, by an arrow. POT, posterior optic tract. (B) DvPdfS1a transgenic line. Ectopic LNd neurons are in the circle. (C and D) Pdf immunoreactivity in cyc0 mutant CNS, and (E and F) in ClkJrk mutant CNS. Two transgenic lines (S1a and T3) produce signals only in l-LNv's (arrows). Short processes deviated from the POT are projected dorsally (arrowheads in C–F). At least 10 specimens per genotype were observed with consistent results.
Rescue of arrhythmic Pdf01 mutant behavior by the DvPdf transgene:
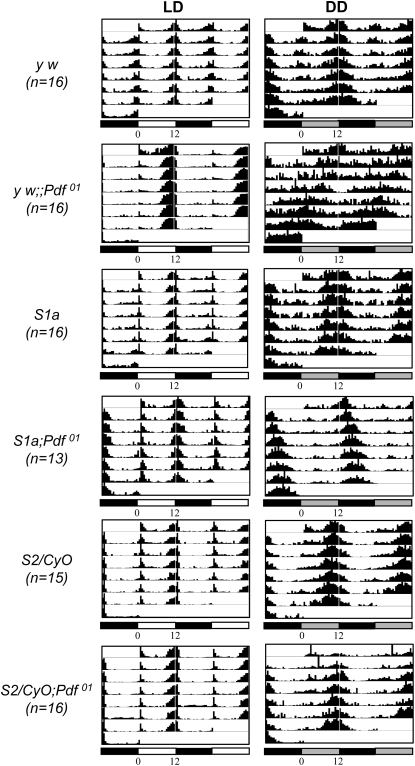
Since Pdf is essential for normal circadian locomotor activity rhythms in D. melanogaster (Renn et al. 1999), we investigated whether expression of the DvPdf transgene can rescue Pdf01 mutant phenotypes. While ∼67% of the Pdf01 flies showed arrhythmic or weakly rhythmic locomotor activity in DD condition, two independent transgenic lines carrying both the endogenous DmPdf gene and the DvPdf transgene (i.e., wild-type background) or only DvPdf (i.e., Pdf01 mutant background) showed normal locomotor activity rhythms (Figure 6 and Table 1). These results suggest that DvPdf precursors are appropriately processed to produce the functional Pdf peptide in a D. melanogaster host and deliver clock information properly, despite significant sequence divergence in the PAP regions (Figure 2). Ectopic production of the Pdf in the LNd's and fifth s-LNv from the DvPdf transgene does not seem to influence normal circadian activity rhythms.
Figure 6.—
Average actograms for indicated genotypes (n, number of flies). Open and solid bars, respectively, designate day and night phases in 12:12 LD, and shaded bars, subjective day in the constant dark (DD). See also Table 1 for the quantitative data analysis. Arrhythmic circadian locomotor activities displayed by Pdf01 mutant flies are restored by two independent DvPdf transgenes (S1a and S2).
DISCUSSION
Circadian behavior and Pdf expression of D. virilis:
In this study, we have characterized circadian locomotor activity rhythms of D. virilis, a species that has radiated from the melanogaster linage ∼63 million years ago, which is approximately the beginning of the Cenozoic era. During this geological period, major paleoclimatic changes are proposed to drive speciation of Drosophila (Tamura et al. 2004). In addition, studies indicate diversification of native habitats of Drosophila species, as D. virilis is suggested to be indigenous to eastern Asia (Throckmorton 1982), whereas D. melanogaster is believed to be African origin (Keller 2007). Therefore, it is possible that D. virilis and D. melanogaster may have evolved unique biological clock systems that suit their endemic environment. This is supported by our behavioral data that revealed substantially different daily and circadian locomotor activity patterns between D. virilis and D. melanogaster.
The first insight that Pdf might be responsible for behavioral characteristics of D. virilis comes from the uncanny resemblance in locomotor activity patterns between D. virilis and Pdf01 mutant flies of D. melanogaster. Under LD condition, activities of the Pdf01 flies are largely restricted to the daytime; such diurnally shifted activity of the Pdf01 flies is likely to be attributed to both prominently reduced morning anticipatory behavior and the slight phase advance of evening activity peaks to the photophase (Renn et al. 1999). Moreover, like D. virilis, Pdf01 flies are largely arrhythmic or weakly rhythmic in DD condition. Therefore, it is reasonable to suggest that the lack of Pdf expression in the s-LNv equivalent neurons is intimately associated with behavioral characteristics of D. virilis. These results are also consistent with morning oscillator functions of s-LNv's (Grima et al. 2004; Stoleru et al. 2004), and further support the importance of Pdf's role within the s-LNv neurons for lights-on anticipatory behavior.
Daily locomotor activity patterns described for the housefly, M. domestica are notably similar to those of D. virilis (Helfrich et al. 1985), as both species display day-phase-restricted activity without lights-on anticipation. IHC using anti-Pdf showed both large and small LNv-equivalent neuronal groups in the adult brain of M. domestica (Pyza and Meinertzhagen 1997; Pyza et al. 2003). In contrast to this result, in situ hybridization revealed MdPdf mRNA expression only in the l-LNv-like neurons (Matsushima et al. 2004). We confirmed these results independently (data not shown, n = 6). A plausible explanation for this discrepancy is that the s-LNv-like neurons contain materials that cross-react with the anti-Pdf. From these data, it is tempting to propose that lack of Pdf expression in s-LNv-like neurons is also responsible for diurnally active locomotion displayed by M. domestica.
Regulation of DvPdf expression:
In the heterologous host, expression of the DvPdf gene is evident in all of DmPdf-positive groups, suggesting that donor DvPdf regulatory elements are capable of interacting with host trans-acting factors to activate its expression in a manner similar to that of DmPdf. Although no useful information about potential elements emerged from simple sequence alignment between 0.5-kb upstream sequence of DmPdf (Park et al. 2000) and 1.9-kb of DvPdf, the cis-regulatory element(s) responsible for s-LNv Pdf expression is likely conserved in both DmPdf and DvPdf. An important question raised from these studies is, then, Why do D. virilis flies lack DvPdf expression particularly in the s-LNv-like neurons? It could be that D. virilis does not possess the s-LNv-like neurons. However, this is unlikely, because a fly species (M. domestica), which is even more remotely related to D. melanogaster, contains Pdf-ir s-LNv-like neurons (Pyza and Meinertzhagen 1997; Pyza et al. 2003), although such immunoreactivity likely originated from cross-reactivity, as mentioned earlier. We tried to confirm the presence of s-LNv's in the D. virilis CNS using anti-Tim, as was done for D. melanogaster (Figure 4D). However, no immunosignals were detectable even in the l-LNv's at two different time points. Although similarity of the Tim between the two species is substantial (76% overall amino acid identity; Ousley et al. 1998), perhaps diversity between the two proteins does not allow anti-Tim to detect virilis Tim protein.
In D. melanogaster, DmClk and DmCyc proteins are well-defined upstream positive factors responsible for DmPdf expression specifically in the s-LNv's (Park et al. 2000). Our present study shows that these factors are also essential for the DvPdf transgenic expression in the s-LNv's, LNd's and fifth s-LNv, suggesting that DvClk and DvCyc likely act as positive regulators for the DvPdf in the s-LNv-like neurons in D. virilis brain. Thus the lack of DvPdf expression in the s-LNv-like neurons might be due to a loss of function of these proteins in D. virilis.
According to the genome database, DvClk gene (Dvir\GJ11427) predicts to encode a protein of 988 amino acids. Our RT–PCR result suggests that DvClk from our flies encodes a 987-amino-acid product and has differences from the Dvir\GJ11427 at three sites (data not shown). Two of them are within polyglutamate (Q) stretches, missing two Q's at one position and having the addition of one Q at another position. The other one is a homologous substitution of leucine to isoleucine (data not shown). Amino acid composition of the DvClk shows 70% identity to DmClk. For Cyc, our sequence of DvCyc deduced from RT–PCR matches perfectly to that from genome database (Dvir\GJ14003), and amino acid residues of the DvCyc share 85% identity with the DmCyc (data not shown). In other words, we did not find any significant mutations within the ORFs of both DvClk and DvCyc that might alter their functions. Moreover, robust activity rhythms displayed by D. virilis under LD cycles, in contrast to significantly abnormal LD behavior of D. melanogaster ClkJrk and cyc0 mutants (Allada et al. 1998; Rutila et al. 1998), suggest that functions of the two clock proteins are unlikely defective in D. virilis.
Absence of s-LNv-specific DvPdf expression could be accomplished through negative regulation. According to Helfrich-Förster et al. (2007), l-LNv's and LNd's in D. melanogaster appear to be originated from the common precursor cells, as clusters of these cells are mixed without clear anatomical distinction in the ventral region of early pupal brain. As the pupal development progresses, presumptive LNd's are separated from l-LNv's, migrate dorsally, and start to develop their characteristic projections. Shortly after this stage, l-LNv's become Pdf-positive, while LNd's remain Pdf-negative. However, the authors found one exceptional specimen in which the migration of the LNd's is impaired; interestingly, these neurons are Pdf-positive. We interpret these findings as follows: activation of the DmPdf in both l-LNv's and LNd's during pupal development is a default pathway, and then the suppression of the DmPdf is acquired during the maturation of the LNd neurons, perhaps through the activation of repressors. These studies provide an interesting possibility of the transcriptional suppression of DmPdf in the LNd's and fifth s-LNv. This notion is supported by the ectopic DvPdf transgene expression in these neurons, as negative trans-acting factors might be unable to interact with DvPdf's regulatory region due to sequence incompatibility, thus allowing ectopic expression of the DvPdf. As an extrapolation of these results, it would be interesting to investigate whether negative factors suppress DvPdf expression in the s-LNv-like (and perhaps LNd- and fifth s-LNv-like) neurons in D. virilis. Transgenic dissection of the 1.9-kb DvPdf upstream region (e.g., Choi et al. 2008) will help reveal specific cis-acting elements that are necessary for such negative DvPdf regulation.
Acknowledgments
We are grateful to J. Hogsette (United States Department of Agriculture–Agricultural Research Service) for kind provision of the house flies, Jeffrey Hall (University of Maine) for wild-type D. virilis, P. Valvo for a kind gift of a fly food ingredient, and R. Blackman and T. Kaufman (Indiana University) for D. virilis genomic library. We thank Y.-J. Kim (University of California at Riverside) for the in situ hybridization protocols. We also thank Jim Hall, B. McKee, and T. Dockendorff for their comments on the manuscript. This work was supported by a National Institutes of Health grant (MH-66197) (to J.H.P.).
References
- Allada, R., N. E. White, W. V. So, J. C. Hall and M. Rosbash, 1998. A mutant Drosophila homolog of mammalian clock disrupts circadian rhythms and transcription of period and timeless. Cell 93 791–804. [DOI] [PubMed] [Google Scholar]
- Aréchiga, H., F. Fernandez-Quiroz, F. Fernandez de Miguel and L. Rodriguez-Sosa, 1993. The circadian system of crustaceans. Chronobiol. Int. 10 1–19. [DOI] [PubMed] [Google Scholar]
- Choi, Y. J., G. Lee, J. C. Hall and J. H. Park, 2005. Comparative analysis of corazonin-encoding genes (Crz's) in Drosophila species and functional insights into Crz-expressing neurons. J. Comp. Neurol. 482 372–385. [DOI] [PubMed] [Google Scholar]
- Choi, S-H., G. Lee, P. Monahan and J. H. Park, 2008. Spatial regulation of Corazonin neuropeptide expression requires multiple cis-acting elements in Drosophila melanogaster. J. Comp. Neurol. 507 1184–1195. [DOI] [PubMed] [Google Scholar]
- Cymborowski, B., S-F. Hong, H. G. McWatters and D. S. Saunders, 1996. S-antigen antibody partially blocks entrainment and the effects of constant light on the circadian rhythm of locomotor activity in the adult blow fly, Calliphora vicina. J. Biol. Rhythms 11 68–74. [DOI] [PubMed] [Google Scholar]
- Drosophila 12 Genomes Consortium, 2007. Evolution of genes and genomes on the Drosophila phylogeny. Nature 450 203–218. [DOI] [PubMed] [Google Scholar]
- Dushay, M. S., M. Rosbash and J. C. Hall, 1989. The disconnected visual system mutations in Drosophila melanogaster drastically disrupt circadian rhythms. J. Biol. Rhythms. 4 1–27. [DOI] [PubMed] [Google Scholar]
- Ewer, J., B. Frisch, M. J. Hamblen-Coyle, M. Rosbash and J. C. Hall, 1992. Expression of the period clock gene within different cell types in the brain of Drosophila adults and mosaic analysis of these cells' influence on circadian behavioral rhythms. J. Neurosci. 12 3321–3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlund, P., 1976. Structure of a light adapting hormone from the shrimp Pandalus borealis. Biochim. Biophys. Acta 439 17–25. [DOI] [PubMed] [Google Scholar]
- Frisch, B., P. E. Hardin, M. J. Hamblen-Coyle, M. Rosbash and J. C. Hall, 1994. A promoterless period gene mediates behavioral rhythmicity and cyclical per expression in a restricted subset of the Drosophila nervous system. Neuron 12 555–570. [DOI] [PubMed] [Google Scholar]
- Grima, B., E. Chelot, R. Xia and F. Rouyer, 2004. Morning and evening peaks of activity rely on different clock neurons of the Drosophila brain. Nature 431 869–873. [DOI] [PubMed] [Google Scholar]
- Hamblen, M., W. A. Zehring, C. P. Kyriacou, P. Reddy, Q. Yu et al., 1986. Germ-line transformation involving DNA from the period locus in Drosophila melanogaster: overlapping genomic fragments that restore circadian and ultradian rhythmicity to per0 and per- mutants. J. Neurogenet. 3 249–291. [DOI] [PubMed] [Google Scholar]
- Helfrich, C., B. Cymborowski and W. Engelmann, 1985. Circadian activity rhythm of the house fly continues after optic tract severance and lobectomy. Chronobiol. Int. 2 19–32. [DOI] [PubMed] [Google Scholar]
- Helfrich-Förster, C., 1995. The period clock gene is expressed in central nervous system neurons which also produce a neuropeptide that reveals the projections of circadian pacemaker cells within the brain of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 92 612–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich-Förster, C., 1997. Development of pigment-dispersing hormone-immunoreactive neurons in the nervous system of Drosophila melanogaster. J. Comp. Neurol. 380 335–354. [DOI] [PubMed] [Google Scholar]
- Helfrich-Förster, C., 1998. Robust circadian rhythmicity of Drosophila melanogaster requires the presence of lateral neurons: a brain-behavioral study of disconnected mutants. J. Comp. Physiol. 182 435–453. [DOI] [PubMed] [Google Scholar]
- Helfrich-Förster, C., 2005. Neurobiology of the fruit fly's circadian clock. Genes Brain Behav. 4 65–76. [DOI] [PubMed] [Google Scholar]
- Helfrich-Förster, C., M. Täuber, J. H. Park, M. Mühlig-Versen, S. Schneuwly et al., 2000. Ectopic expression of the neuropeptide pigment-dispersing factor alters the rhythm of locomotor activity in Drosophila melanogaster. J. Neurosci. 20 3339–3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich-Förster, C., O. T. Shafer, C. Wülbeck, E. Grieshaber, D. Rieger et al., 2007. Development and morphology of the clock-gene-expressing lateral neurons of Drosophila melanogaster. J. Comp. Neurol. 500 47–70. [DOI] [PubMed] [Google Scholar]
- Homberg, U., N. T. Davis and J.G. Hildebrand, 1991. a Peptide-immunocytochemistry of neurosectetory cells in the brain and retrocerebral complex of the sphinx moth Manduca sexta. J. Comp. Neurol. 303 35–52. [DOI] [PubMed] [Google Scholar]
- Homberg, U., S. Würden, H. Dircksen and K. R. Rao, 1991. b Comparative anatomy of pigment-dispersing hormone-immunoreactive neurons in the brain of orthopteroid insects. Cell Tissue Res. 266 343–357. [Google Scholar]
- Hyun, S., Y. Lee, S. T. Hong, S. Bang, D. Paik et al., 2005. Drosophila GPCR Han is a receptor for the circadian clock neuropeptide PDF. Neuron 48 267–278. [DOI] [PubMed] [Google Scholar]
- Kaneko, M., and J. C. Hall, 2000. Neuroanatomy of cell expressing clock genes in Drosophila: transgenic manipulation of the period and timeless genes to mark the perikarya of circadian pacemaker neurons and their projections. J. Comp. Neurol. 422 66–94. [DOI] [PubMed] [Google Scholar]
- Keller, A., 2007. Drosophila melanogaster's history as a human commensal. Curr. Biol. 17 R77–R81. [DOI] [PubMed] [Google Scholar]
- Kim, Y-J., D. Zitnan, K. H. Cho, D. A. Schooley, A. Mizoguchi et al., 2006. Central peptidergic ensembles associated with organization of an innate behavior. Proc. Natl. Acad. Sci. USA 103 14211–14216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka, R. J., and S. Benzer, 1971. Clock mutants of Drosophila melanogaster. Proc. Natl. Acad. Sci. U.S.A. 68 2112–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka, R. J., C. Pittendrigh and D. Orr, 1989. Reciprocal behaviour associated with altered homeostasis and photosensitivity of Drosophila clock mutants. J. Neurogenet. 6 1–10. [DOI] [PubMed] [Google Scholar]
- Lear, B. C., C. E. Merrill, J. M. Lin, A. Schroeder, L. Zhang et al., 2005. A G protein-coupled receptor, groom-of-PDF, is required for PDF neuron action in circadian behavior. Neuron 48 221–227. [DOI] [PubMed] [Google Scholar]
- Lee, G., M. Foss, S. F. Goodwin, T. Carlo, B. J. Taylor et al., 2000. Spatial, temporal, and sexually dimorphic expression patterns of the fruitless gene in the Drosophila central nervous system. J. Neurobiol. 43 404–426. [DOI] [PubMed] [Google Scholar]
- Lee, G., J. H. Bahn and J. H. Park, 2006. Sex- and clock-controlled expression of the neuropeptide F gene in Drosophila. Proc. Natl. Acad. Sci. USA 103 12580–12585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, Y., G. D. Stormo and P. H. Taghert, 2004. The neuropeptide pigment-dispersing factor coordinates pacemaker interactions in the Drosophila circadian system. J. Neurosci. 24 7951–7957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X., Q. Yu, Z. Huang, L. Zwiebel, J.C. Hall et al., 1991. The strength and periodicity of D. melanogaster circadian rhythms are differentially affected by alterations in period gene expression. Neuron 6 753–766. [DOI] [PubMed] [Google Scholar]
- Matsushima, A., S. Sato, Y. Chuman, Y. Takeda, S. Yokotani et al., 2004. cDNA cloning of the housefly pigment-dispersing factor (PDF) precursor protein and its peptide comparison among the insect circadian neuropeptides. J. Pept. Sci. 10 82–91. [DOI] [PubMed] [Google Scholar]
- Mertens, I., A. Vandingenen, E. C. Johnson, O. T. Shafer, W. Li et al., 2005. PDF receptor signaling in Drosophila contributes to both circadian and geotactic behaviors. Neuron 48 213–219. [DOI] [PubMed] [Google Scholar]
- Nitbach, M. N., Y. Wu, V. Sheeba, W. C. Lemon, J. Strumbos et al., 2006. Electrical hyperexcitation of lateral ventral pacemakerbneurons desynchronizes downstream circadian oscillators in the fly circadian circuit and induces multiplebbehavioral periods. J. Neurosci. 26 479–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ousley, A., K. Zafarullah, Y. Chen, M. Emerson, L. Hickman et al., 1998. Conserved regions of the timeless (tim) clock gene in Drosophila analyzed through phylogenetic and functional studies. Genetics 148 815–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page, T. L., 1982. Transplantation of the cockroach circadian pacemaker. Science 216 73–75. [DOI] [PubMed] [Google Scholar]
- Park, J. H., and J. C. Hall, 1998. Isolation and chronobiological analysis of a neuropeptide pigment-dispersing factor gene in Drosophila melanogaster. J. Biol. Rhythms 13 219–228. [DOI] [PubMed] [Google Scholar]
- Park, J. H., C. Helfrich-Förster, G. Lee, L. Li, M. Rosbash et al., 2000. Differential regulation of circadian pacemaker output by separate clock genes in Drosophila. Proc. Natl. Acad. Sci. USA 97 3608–3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, Y., D. Stoleru, J. D. Levine, J. C. Hall and M. Rosbash, 2003. Drosophila free-running rhythms require intercellular communication. PLoS Biol. 1 E13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri, B., and M. Stengl, 1997. Pigment-dispersing hormone shifts the phase of the circadian pacemaker of the cockroach Leucophaea maderae. J. Neurosci. 17 4087–4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyza, E., and I. A. Meinertzhagen, 1997. Neurites of period-expressing PDH cells in the fly's optic lobe exhibit circadian oscillations in morphology. Eur. J. Neurosci. 9 1784–1788. [DOI] [PubMed] [Google Scholar]
- Pyza, E., T. Siuta and T. Tanimura, 2003. Development of PDF-immunoreactive cells, possible clock neurons, in the housefly Musca domestica. Microsc. Res. Tech. 62 103–113. [DOI] [PubMed] [Google Scholar]
- Rao, K. R., 2001. Crustacean pigmentary effector hormones: chemistry and functions of RPCF, PDH, and related peptides. Am. Zool. 41 364–379. [Google Scholar]
- Rao, K. R., and J. P. Riehm, 1993. The circadian system of crustaceans. Ann. NY Acad. Sci. 680 78–88. [DOI] [PubMed] [Google Scholar]
- Renn, S. C., J. H. Park, M. Rosbash, J. C. Hall and P. H. Taghert, 1999. A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell 99 791–802. [DOI] [PubMed] [Google Scholar]
- Rieger, D., O. T. Shafer, K. Tomioka and C. Helfrich-Förster, 2006. Functional analysis of circadian pacemaker neurons in Drosophila melanogaster. J. Neurosci. 26 2531–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosato, E., and C. P. Kyriacou, 2006. Analysis of locomotor activity rhythms in Drosophila. Nat. Protocols 1 559–568. [DOI] [PubMed] [Google Scholar]
- Rutila, J. E., V. Suri, M. Le, W. V. So, M. Rosbash et al., 1998. CYCLE is a second bHLH-PAS clock protein essential for circadian rhythmicity and transcription of Drosophila period and timeless. Cell 93 805–814. [DOI] [PubMed] [Google Scholar]
- Saunders, D. S., 2002. Insect Clocks, Ed. 3. Elsevier, Amsterdam, The Netherlands.
- Sehadova, H., I. Sauman and F. Sehnal, 2003. Immunocytochemical distribution of pigment-dispersing hormone in the cephalic ganglia of polyneopteran insects. Cell Tissue Res. 312 113–125. [DOI] [PubMed] [Google Scholar]
- Shafer, O. T., M. Rosbash and J. W. Truman, 2002. Sequential nuclear accumulation of the clock proteins period and timeless in the pacemaker neurons of Drosophila melanogaster. J. Neurosci. 22 5946–5954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singaravel, M., Y. Fujisawa, M. Hisada, A. S. Saifullah and K. Tomioka, 2003. Phase shifts of the circadian locomotor rhythm induced by pigment-dispersing factor in the cricket Gryllus bimaculatus. Zool. Sci. 11 1347–1354. [DOI] [PubMed] [Google Scholar]
- Stengl, M., and U. Homberg, 1994. Pigment-dispersing hormone-immunoreactive neurons in the cockroach Leucophaea maderae share properties with circadian pacemaker neurons. J. Comp. Physiol. 175 203–213. [DOI] [PubMed] [Google Scholar]
- Stoleru, D., Y. Peng, J. Agusto and M. Rosbash, 2004. Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature 431 862–868. [DOI] [PubMed] [Google Scholar]
- Taghert, P. H., and O. T. Shafer, 2006. Mechanisms of clock output in the Drosophila circadian pacemaker system. J. Biol. Rhythms 21 445–457. [DOI] [PubMed] [Google Scholar]
- Tamura, K, S. Subramanian and S. Kumar, 2004. Temporal patterns of fruit fly (Drosophila) evolution revealed by mutation clocks. Mol. Biol. Evol. 21 36–44. [DOI] [PubMed] [Google Scholar]
- Throckmorton, L. H., 1982. The virilis species group, pp. 227–296 in The Genetics and Biology of Drosophila, Vol. 3b, edited by M. Ashburner, H. L. Carson and J. N. Thompson, Jr. Academic Press, New York.
- Veenstra, J. A., 2000. Mono- and dibasic proteolytic cleavage sites in insect neuroendocrine peptide precursors. Arch. Insect Biochem. Physiol. 43 49–63. [DOI] [PubMed] [Google Scholar]
- Wise, S., N. T. Davis, E. Tyndale, J. Noveral, M. G. Folwell et al., 2002. Neuroanatomical studies of period gene expression in the Hawkmoth, Manduca sexta. J. Comp. Neurol. 447 366–380. [DOI] [PubMed] [Google Scholar]
- Wu, Y., G. Cao and M. N. Nitabach, 2008. Electrical silencing of PDF neurons advances the phase of non-PDF clock neurons in Drosophila. J Biol. Rhythms 23 117–128. [DOI] [PubMed] [Google Scholar]
- Závodská, R, I. Sauman and F. Sehnal, 2003. Distribution of PER protein, pigment-dispersing hormone, prothoracicotropic hormone, and eclosion hormone in the cephalic nervous system of insects. J. Biol. Rhythms 18 106–122. [DOI] [PubMed] [Google Scholar]