Abstract
Purpose
Bevacizumab is an antibody that binds to vascular endothelial growth factor (VEGF) and has activity in metastatic renal cell carcinoma (RCC). Interferon alfa (IFN) is a historic standard first-line treatment for RCC. A prospective, randomized phase III trial of bevacizumab plus IFN versus IFN monotherapy was conducted.
Patients and Methods
Patients with previously untreated, metastatic clear-cell RCC were randomly assigned to receive either bevacizumab (10 mg/kg intravenously every 2 weeks) plus IFN (9 million U subcutaneously three times weekly) or the same dose and schedule of IFN monotherapy in a multicenter phase III trial. The primary end point was overall survival (OS). Secondary end points were progression-free survival (PFS), objective response rate (ORR), and safety.
Results
Between October 2003 and July 2005, 732 patients were enrolled. The prespecified stopping rule for OS has not yet been reached. The median PFS was 8.5 months in patients receiving bevacizumab plus IFN (95% CI, 7.5 to 9.7 months) versus 5.2 months (95% CI, 3.1 to 5.6 months) in patients receiving IFN monotherapy (log-rank P < .0001). The adjusted hazard ratio was 0.71 (95% CI, 0.61 to 0.83; P < .0001). Bevacizumab plus IFN had a higher ORR as compared with IFN (25.5% [95% CI, 20.9% to 30.6%] v 13.1% [95% CI, 9.5% to 17.3%]; P < .0001). Overall toxicity was greater for bevacizumab plus IFN, including significantly more grade 3 hypertension (9% v 0%), anorexia (17% v 8%), fatigue (35% v 28%), and proteinuria (13% v 0%).
Conclusion
Bevacizumab plus IFN produces a superior PFS and ORR in untreated patients with metastatic RCC as compared with IFN monotherapy. Toxicity is greater in the combination therapy arm.
INTRODUCTION
Metastatic renal cell carcinoma (RCC) has long been a chemotherapy-refractory malignancy. The biology of RCC is thought to be influenced by the immune system, and thus interferon alfa (IFN), an immunotherapeutic cytokine, has been investigated. IFN became a standard initial therapy in metastatic RCC, with a 10% to 15% objective response rate (ORR) and a median survival of approximately 12 months.1-3 The addition of interleukin-2, hormonal therapy, or antiproliferative agents such as cis-retinoic acid to IFN has not demonstrated significant advantages over IFN monotherapy in randomized trials.4-6
The pathogenesis of RCC has been further elucidated, resulting in identification of relevant therapeutic targets. Von Hippel-Lindau (VHL) syndrome is an autosomal dominant disorder caused by silencing of the VHL tumor suppressor gene and is associated with increased susceptibility to vascular tumors, including the prominent occurrence of clear-cell RCC. VHL gene silencing also occurs in the majority of noninherited clear-cell RCC, activating the hypoxia-response pathway and inducing transcription of several genes, including vascular endothelial growth factor (VEGF).7-10 VEGF is a potent pro-angiogenic protein, leading to increased vascular permeability and endothelial cell proliferation/migration.11
Therapeutic inhibition of the VEGF pathway thus has strong biologic rationale in RCC. Indeed, two phase III trials have demonstrated substantial clinical benefit from blocking the VEGF receptor with sunitinib or sorafenib.12,13 Bevacizumab (Avastin; Genentech Inc, South San Francisco, CA), an antibody that binds to and neutralizes circulating VEGF protein but does not affect the VEGF receptor, has produced a significant prolongation of time to disease progression compared with placebo in patients with treatment-refractory metastatic RCC in a small randomized trial.14 Thus, on the basis of the biology of RCC and preliminary results with bevacizumab, the clinical benefit of adding bevacizumab to IFN monotherapy was investigated. IFN monotherapy was selected as the comparator arm because, at the time of trial design, it was standard therapy for metastatic RCC based on a demonstrated overall survival (OS) advantage.1,2,15 Although high-dose interleukin-2 also has activity and is an approved therapy in the United States,16-18 the toxicity and small number of patients in whom it can be applied has limited its utility as a building block for combination trials and has precluded its use as a control.
PATIENTS AND METHODS
Patients
The study population consisted of patients 18 years of age and older with metastatic RCC, a clear-cell histologic component confirmed by local pathology review, and no prior systemic therapy for RCC. Patients were required to have a Karnofsky performance status of ≥ 70% and adequate bone marrow, hepatic, and renal function (as defined by granulocytes ≥ 1,500/μL, platelet count ≥ 100,000/μL, AST/ALT ≤ 2.5× upper limit of normal [ULN], alkaline phosphatase ≤ 2.5× ULN, serum bilirubin ≤ 1.5× ULN, urinalysis ≤ 1+ protein [or 24-hour urine protein < 2 g in patients with > 1+ proteinuria], and serum creatinine ≤ 1.5× ULN).
Patients with CNS metastases, New York Heart Association class II to IV heart failure, bleeding (eg, hemoptysis, gastrointestinal bleeding) within 6 months, blood pressure that could not be controlled to less than 160/90 mmHg with medication, history of venous thrombosis within 1 year, or arterial thrombosis (including cerebrovascular accident, unstable angina, myocardial infarction, or claudication with < one block of exertion) within 6 months or who required ongoing therapeutic anticoagulation were excluded. Patients with uncontrolled thyroid function, pregnancy, requirement for systemic corticosteroids greater than physiologic replacement doses, or delayed healing of wounds, ulcers, or bone fractures were excluded. The protocol was approved by the central institutional review board of the National Cancer Institute (NCI) as well as by the institutional review board of each participating site, and all patients provided written informed consent.
Study Design
This study was conducted by the Cancer and Leukemia Group B (CALGB) with the support of the Eastern Cooperative Oncology Group, the National Cancer Institute of Canada Clinical Trials Group, and the NCI Cancer Trials Support Unit. Patients were randomly assigned with equal probability to receive either bevacizumab (10 mg/kg given intravenously every 2 weeks) plus IFN (9 million U [MU] subcutaneously three times weekly) or the same dose and schedule of IFN as monotherapy. A stratified random block design was used, with randomization stratified by nephrectomy status (yes v no) and number of adverse prognostic factors (none, one to two, or three or more) which had been previously described for patients with metastatic RCC receiving IFN-based initial systemic therapy.3 These risk factors consisted of Karnofsky performance status less than 80%, lactate dehydrogenase more than 1.5× laboratory ULN, hemoglobin less than laboratory lower limit of normal, serum calcium corrected for albumin more than 10 mg/dL, and time from diagnosis of RCC to start of therapy of less than 1 year.
Bevacizumab was provided by the NCI Cancer Therapy Evaluation Program and was administered at a dose of 10 mg/kg of actual body weight intravenously on days 1 and 15 of each 28-day cycle. No dose adjustments of bevacizumab were permitted, but doses could be held for bevacizumab-related toxicity. IFN-α-2b (Intron; Schering-Plough, Kenilworth, NJ) was provided by the NCI Cancer Therapy Evaluation Program and was administered identically in both arms: subcutaneously at a starting dose of 9 MU on 3 nonconsecutive days per week, with dose reduction to 6 MU and to 3 MU permitted for IFN-related toxicity. One cycle of IFN monotherapy consisted of 28 consecutive days. Treatment was continued until disease progression per investigator assessment according to Response Evaluation Criteria In Solid Tumors (RECIST),19 unacceptable toxicity, or withdrawal of consent.
Efficacy and Safety
Response and progression were assessed according to RECIST and were determined by investigator assessment of radiographs. Tumor assessments were performed at baseline and every 12 weeks. Adverse events were graded according to the NCI Common Terminology Criteria for Adverse Events Version 3.0.
Because there were no safety data on the bevacizumab plus IFN combination, toxicity among the first 60 patients randomly assigned to bevacizumab plus IFN was monitored. If the observed proportion of unacceptable toxicity exceeded 15% by at least one SE, accrual to the trial would be suspended. Unacceptable toxicity was defined as any one of the following treatment-related events: death; grade 4 febrile neutropenia or hypersensitivity; any irreversible (defined as persisting for > 4 weeks) hypertension unable to be controlled to less than 160/90 mmHg with medication or grade 3 or 4 toxicity, excluding nausea, vomiting, and alopecia; or grade 3 or worse cardiovascular, thrombosis/embolism, or CNS hemorrhage/bleeding, regardless of reversibility.
Statistical Design and Data Analysis
The primary end point was OS, defined as the time from registration to death from any cause, with a target sample size of 700 patients. The following assumptions were made: an annual accrual rate of 233 patients accrued over a 3-year enrollment period, 2-year follow-up period, and survival time follows an exponential distribution. The trial was designed with 86% power to detect a 30% improvement in median survival in patients randomly assigned to bevacizumab plus IFN compared with patients randomly assigned to IFN monotherapy, assuming a two-sided significance level of .05. The primary analysis on the overall survival end point was based on the stratified log-rank statistic. Secondary end points were progression-free survival (PFS, defined from the date of randomization to date of progression using RECIST according to the first tumor assessment where disease progression was observed or death from any cause, whichever occurred first), ORR using RECIST, and safety. Patients who discontinued treatment for reasons other than progression were observed for disease progression or death.
The Lan and Demets analog of the O’Brien-Fleming sequential boundary was used to maintain the overall significance level of α = 0.05 while conducting interim analyses of the OS end point. Under the alternative hypotheses, 588 deaths are expected at the end of the trial. According to the protocol, there are eight analyses, including the final, to be performed at 19%, 32%, 46% 61%, 75%, 86%, 94%, and 100% of the information. Six interim analyses have been performed to date. PFS data were also available to the Data Safety Monitoring Board at these analyses, but it was not prespecified in the protocol to use PFS data to determine whether the trial would continue. After public presentation of data from a similar trial (AVOREN) showed benefit to bevacizumab plus IFN,20 the Data Safety Monitoring Board made an independent decision to release the PFS, but not the OS, data.
An intention-to-treat approach was used in the analysis. The primary analysis of the PFS end point was based on a two-sided stratified log-rank test comparing the two arms. The stratification factors used for patient random assignment were prior nephrectomy (yes v no) and number of adverse risk factors (zero v one to two v three or more). In addition, the Kaplan-Meier product-limit method was used to estimate the progression-free survival time and duration of response in the two arms.21 The threshold for significance for the PFS analysis was .05. The χ2 test and Fisher's exact test were used to compare ORRs and adverse events between the two treatment groups, respectively. All analyses were performed using SAS software, version 9.1 (SAS Institute, Cary, NC).
CALGB Statistical Center personnel were responsible for patient registration, data collection, and quality assurance for all the data submitted by the participating institutions. Statistical analyses were performed by CALGB statisticians. As part of the quality assurance program of the CALGB, members of the Audit Committee visit all participating CALGB institutions at least once every 3 years to review source documents. The auditors verify compliance with federal regulations and protocol requirements, including those pertaining to eligibility, treatment, adverse events, tumor response, and outcome in a sample of protocols at each institution. Such on-site review of medical records was performed for a subgroup of 177 patients (24.2%) of the 732 patients enrolled onto this study, and no major problems or discrepancies were identified.
RESULTS
Patients
Between October 2003 and July 2005, 732 patients were enrolled at centers in the United States and Canada. Patients were predominantly male, with 85% having previous nephrectomy (Table 1). Twenty-six percent of patients had good-risk disease, 64% had intermediate-risk disease, and 10% had poor-risk disease according to established criteria.3
Table 1.
Baseline Demographics and Clinical Characteristics
| Variable | Bevacizumab Plus Interferon (n = 369)
|
Interferon Monotherapy (n = 363)
|
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Sex | ||||
| Male | 269 | 73 | 239 | 66 |
| Female | 100 | 27 | 124 | 34 |
| Age, years | ||||
| Median | 61 | 62 | ||
| Interquartile range | 56-70 | 55-70 | ||
| ECOG performance status | ||||
| 0 | 230 | 62 | 227 | 62 |
| 1 | 132 | 36 | 133 | 37 |
| 2 | 7 | 2 | 3 | 1 |
| Previous nephrectomy | 312 | 85 | 308 | 85 |
| Previous radiation therapy | 35 | 9 | 38 | 10 |
| Common sites of metastases | ||||
| Lung | 252 | 68 | 254 | 70 |
| Lymph node | 130 | 35 | 129 | 36 |
| Bone | 104 | 28 | 109 | 30 |
| Liver | 74 | 20 | 73 | 20 |
| No. of adverse risk factors | ||||
| 0, favorable | 97 | 26 | 95 | 26 |
| 1-2, intermediate | 234 | 64 | 231 | 64 |
| ≥ 3, poor | 38 | 10 | 37 | 10 |
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
Treatment Administration
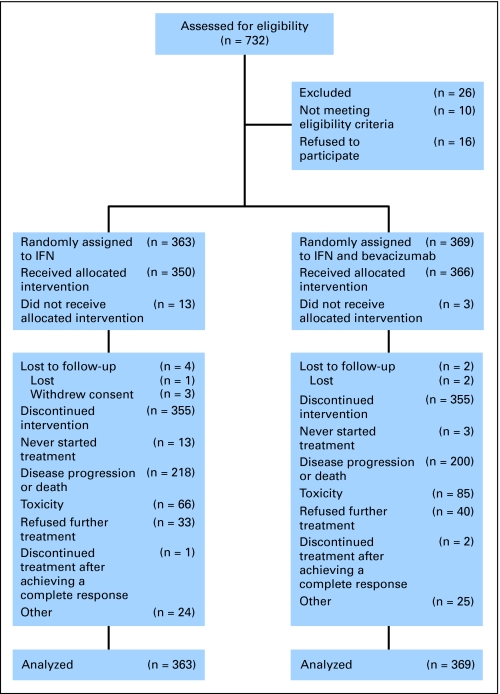
A total of 363 patients were randomly assigned to IFN monotherapy, and 369 patients were randomly assigned to the combination therapy (Fig 1). Patients assigned to IFN monotherapy received a median of three cycles of therapy (range, one to 36 cycles) versus six cycles (range, one to 38 cycles) in patients receiving bevacizumab plus IFN. Dose reductions of IFN to 6 MU and to 3 MU were undertaken in 136 patients (37%) and 37 patients (10%), respectively, on the IFN monotherapy arm and in 170 patients (46%) and 68 patients (18%), respectively, on the bevacizumab plus IFN arm. Treatment delays owing to toxicity (IFN monotherapy v bevacizumab plus IFN) of 4 to 6 days occurred in 24 patients (6.6%) versus 31 patients (8.4%), delays of 7 to 9 days occurred in 31 patients (8.5%) versus 51 patients (13.8%), and delays more than 9 days occurred in 60 patients (8.5%) versus 146 patients (19.9%). The majority of patients discontinued treatment because of disease progression or death (Table 2).
Fig 1.
CONSORT diagram. IFN, interferon alfa.
Table 2.
Reason for Treatment Discontinuation
| Reason | Bevacizumab Plus Interferon (n = 355)
|
Interferon Monotherapy (n = 355)
|
Total (n = 710)
|
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Never started treatment | 3 | < 1 | 13 | 4 | 16 | 2 |
| Disease progression or death | 200 | 56 | 218 | 61 | 394 | 56 |
| Toxicity | 85 | 24 | 66 | 19 | 151 | 21 |
| Refused further treatment | 40 | 11 | 33 | 9 | 73 | 10 |
| Discontinued treatment after achieving a complete response | 2 | < 1 | 1 | < 1 | 3 | < 1 |
| Other | 25 | 7 | 24 | 7 | 49 | 7 |
PFS
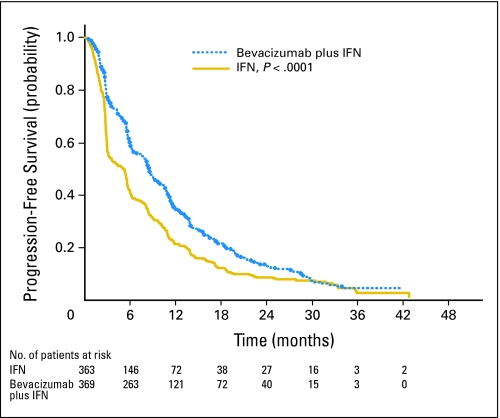
At the time of this report, 657 patients have experienced disease progression or have died (331 patients assigned to IFN monotherapy and 326 patients assigned to bevacizumab plus IFN), and 499 deaths have been observed. No additional interim analysis on the OS end point was performed based on the 499 deaths observed at the time of manuscript submission. A multivariable proportional hazards model of baseline variables predicting PFS was constructed including lactate dehydrogenase, hemoglobin, number of adverse risk factors (≥ three v none), and platelets (Table 3).22 The hazard ratio (HR) for treatment arm in this model is 0.67 (95% CI, 0.57 to 0.79; P < .0001). The median PFS was 8.5 months in patients receiving bevacizumab plus IFN (95% CI, 7.5 to 9.7 months) versus 5.2 months (95% CI, 3.1 to 5.6 months) for IFN monotherapy (Fig 2; P < .0001). The unstratified estimate of HR is 0.72 (95% CI, 0.61 to 0.83; P < .0001) and the estimate of HR adjusting for stratification factors is 0.71 (95% CI, 0.61 to 0.83; P < .0001).
Table 3.
Multivariable Proportional Hazards Model of Treatment as a Predictor of Progression-Free Survival
| Factor | Progression
|
P | |
|---|---|---|---|
| HR | 95% CI | ||
| Treatment arm, bevacizumab + interferon v interferon monotherapy | 0.67 | 0.57 to 0.79 | < .0001 |
| Measurable disease, yes v no | 1.30 | 1.00 to 1.69 | .054 |
| LDH* | 1.06 | 1.03 to 1.09 | .0002 |
| Alkaline phosphatase† | 0.99 | 0.97 to 1.01 | .324 |
| Site of disease | |||
| Lung, yes v No | 1.14 | 0.95 to 1.35 | .158 |
| Lymph node, yes v no | 1.15 | 0.98 to 1.37 | .092 |
| Hemoglobin | 0.94 | 0.90 to 0.99 | .018 |
| Nephrectomy, yes v no | 1.12 | 0.89 to 1.42 | .329 |
| MSKCC risk factors | |||
| 1-2 v 0 | 1.07 | 0.87 to 1.30 | .535 |
| ≥ 3 v 0 | 1.43 | 1.01 to 2.02 | .044 |
| Platelets* | 1.15 | 1.08 to 1.24 | < .0001 |
Abbreviations: HR, hazard ratio; MSKCC, Memorial Sloan-Kettering Cancer Center.
HR based on 100-unit change in the continuous variable.
HR based on 200-unit change in the continuous variable.
Fig 2.
Kaplan-Meier progression-free survival probability curves by the two treatment arms. Progression-free survival in patients receiving bevacizumab plus interferon alfa (IFN) was 8.5 months (95% CI, 7.5 to 9.7 months) compared with 5.2 months (95% CI, 3.1 to 5.6 months) in patients receiving IFN monotherapy (P < .0001).
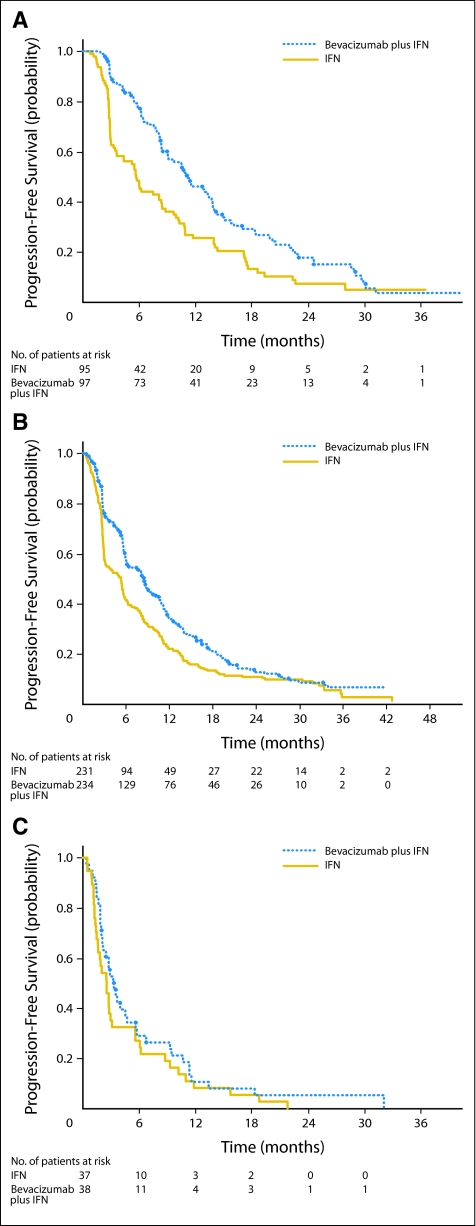
PFS was also examined in an exploratory subset analysis according to the number of adverse risk factors.3 Patients with no risk factors (good risk, 26% of all patients) had a median PFS of 11.1 months for bevacizumab plus IFN (95% CI, 9.0 to 13.8 months) versus 5.7 months (95% CI, 3.6 to 8.3 months) for IFN monotherapy. Patients with one to two risk factors (intermediate risk, 64% of all patients) had a median PFS of 8.4 months (95% CI, 6.1 to 9.9 months) for bevacizumab plus IFN versus 5.3 months (95% CI, 3.1 to 5.7 months) for IFN monotherapy. Patients with three or more risk factors (poor risk, 10% of all patients) had a median PFS of 3.3 months (95% CI, 2.2 to 4.7 months) for bevacizumab plus IFN versus 2.6 months (95% CI, 1.6 to 3.1 months) for IFN monotherapy (Appendix Fig A1, online only).
ORR
Among the 639 patients with measurable disease, the ORR was higher in patients treated with bevacizumab plus IFN (25.5%; 95% CI, 20.9% to 30.6%) than for those treated with IFN monotherapy (13.1%; 95% CI, 9.5% to 17.3%; P < .0001). The median duration of response was 8.7 months (95% CI, 5.6 to 11.4 months) for IFN monotherapy and 11.9 months (95% CI, 8.3 to 14.8 months; P = .977) for bevacizumab plus IFN.
Adverse Events
In patients assessable for toxicity (n = 349 for IFN and n = 366 for bevacizumab plus IFN), 79% of patients receiving bevacizumab plus IFN experienced grade 3 or worse toxicity as compared with 61% of patients receiving IFN monotherapy (P < .0001; Table 4). Bevacizumab plus IFN resulted in significantly more grade 3 toxicities, including hypertension (9% v 0%), anorexia (17% v 8%), fatigue (35% v 28%), and proteinuria (13% v 0%). The incidence of grade 4 neutropenia and anemia was low in each arm (1% v 0% for each), and there were no differences in the rate of febrile neutropenia or requirement for RBC transfusion. There were four treatment-related deaths on the IFN monotherapy arm and three treatment-related deaths on the bevacizumab plus IFN arm.
Table 4.
Grade 3 or 4 Treatment-Related Adverse Events for Patients Treated With Interferon or Bevacizumab Plus Interferon
| Event | Bevacizumab Plus Interferon (n = 366)
|
Interferon Monotherapy (n = 349)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Grade 3
|
Grade 4
|
Grade 3
|
Grade 4
|
|||||
| No. | % | No. | % | No. | % | No. | % | |
| Hematologic adverse events | ||||||||
| Anemia | 12 | 3 | 2 | 1 | 12 | 3 | 1 | 0 |
| Low neutrophils/granulocytes | 29 | 8 | 4 | 1 | 29 | 8 | 1 | 0 |
| Thrombocytopenia | 7 | 2 | 1 | 0 | 2 | 1 | 0 | 0 |
| Febrile neutropenia | 0 | 0 | 0 | 0 | 4 | 1 | 0 | 0 |
| Nonhematologic adverse events | ||||||||
| Cardiovascular | ||||||||
| Cardiac ischemia/infarction | 1 | 0 | 4 | 1 | 0 | 0 | 0 | 0 |
| Left ventricular dysfunction | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 |
| Hypertension | 34 | 9 | 2 | 1 | 0 | 0 | 0 | 0 |
| Thrombosis/embolism | 3 | 1 | 3 | 1 | 1 | 0 | 2 | 1 |
| Constitutional symptoms | ||||||||
| Fatigue | 127 | 35 | 7 | 2 | 98 | 28 | 6 | 2 |
| Weight loss | 15 | 4 | 0 | 0 | 5 | 1 | 0 | 0 |
| Endocrine | ||||||||
| Thyroid dysfunction | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gastrointestinal | ||||||||
| Anorexia | 63 | 17 | 0 | 0 | 28 | 8 | 0 | 0 |
| Nausea | 26 | 7 | 0 | 0 | 16 | 4 | 0 | 0 |
| Perforation, GI | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Hemorrhage/bleeding | ||||||||
| Genitourinary | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| GI | 2 | 1 | 2 | 1 | 0 | 0 | 1 | 0 |
| CNS | ||||||||
| Cerebrovascular ischemia | 2 | 1 | 3 | 1 | 1 | 0 | 0 | 0 |
| Pulmonary | ||||||||
| Dyspnea | 19 | 5 | 4 | 1 | 10 | 3 | 1 | 0 |
| Pneumonitis/pulmonary infiltrates | 1 | 0 | 0 | 0 | 2 | 1 | 1 | 0 |
| Renal | ||||||||
| Proteinuria | 47 | 13 | 9 | 2 | 1 | 0 | 0 | 0 |
| Maximum overall adverse events | 243 | 66 | 46 | 13 | 197 | 56 | 16 | 5 |
Secondary Treatment
No cross-over was permitted for patients randomly assigned to IFN monotherapy. Nonetheless, considering patients who stopped therapy for any reason other than death, a substantial percentage of patients on both arms received systemic anticancer therapy subsequent to progression; 57% of patients on IFN monotherapy and 49% of patients on bevacizumab plus IFN (Table 5). The majority of patients assigned to IFN monotherapy received further therapy, including VEGF-targeted agents such as sunitinib and sorafenib, which emerged during the conduct of this trial.
Table 5.
Second-Line Systemic Therapy Received in Patients Who Discontinued Protocol Therapy for Any Reason Other Than Death*
| Therapy | Bevacizumab Plus Interferon (n = 340)
|
Interferon Monotherapy (n = 332)
|
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Any second-line therapy | 166 | 49 | 188 | 57 |
| VEGF-targeted therapy as second-line therapy | 119 | 35 | 160 | 48 |
| Bevacizumab as second-line therapy | 21 | 6 | 50 | 15 |
| Chemotherapy as second-line therapy | 46 | 14 | 47 | 14 |
| Investigational treatment as second-line therapy | 12 | 4 | 29 | 9 |
| Cytokines as second-line therapy | 27 | 8 | 10 | |
Abbreviation: VEGF, vascular endothelial growth factor.
Patients may have received multiple drugs from different classes in combination as second-line therapy.
DISCUSSION
This prospective randomized trial demonstrates that addition of bevacizumab to IFN significantly prolongs PFS and increases ORR in previously untreated patients with metastatic clear-cell RCC compared with IFN monotherapy. This trial validates antibody-mediated inhibition of the VEGF ligand as a clinically relevant strategy in RCC. This is the one of the first demonstrations of the benefit of combining multiple nonchemotherapy agents in cancer systemic therapy. Previous attempts in metastatic RCC of combining other agents with immunotherapy have not demonstrated benefit over monotherapy.4-6 It is noteworthy that the mechanism of these two agents may not be entirely independent, as IFN has demonstrated antiangiogenic effects23 and antibody-mediated VEGF inhibition has antitumor effects through improvement in dendritic cell function.24
A similarly designed multicenter international trial has also been reported. That trial randomly assigned 649 untreated patients with metastatic RCC to treatment with IFN-α-2a (Roferon; Hoffmann-La Roche, Nutley, NJ) plus placebo infusion or to IFN-α-2a plus bevacizumab 10 mg/kg administered intravenously every 2 weeks.20 A significant difference in favor of the bevacizumab-containing arm for investigator-assessed ORR (31% v 13%; P < .0001) and PFS (10.2 months v 5.4 months; P < .0001) was demonstrated. These findings further validate the benefits of this approach. The slightly lower absolute value of PFS and ORR in the present trial may be a reflection of the worse risk group distribution of treated patients, the requirement for only a component of clear-cell histology as compared with clear-cell predominant in the international trial, and the lack of nephrectomy in a substantial proportion of patients in the present trial. The consistent PFS and ORR advantage observed in both studies strengthens the overall conclusion that there is clinical benefit to adding bevacizumab to IFN.
Demonstration of an OS advantage has appropriately been considered a gold standard in oncology drug development. However, the simultaneous emergence of multiple active therapies in metastatic RCC has lead to the adoption of PFS as a viable end point. That is, patients who experienced disease progression on the control arm of a clinical trial receive subsequent active therapy that may obscure OS benefit. This effect has been observed in a phase III trial of sorafenib versus placebo in cytokine-refractory RCC in which an OS advantage was apparent with censoring before cross-over of placebo patients to sorafenib, but no advantage could be demonstrated in an intent-to-treat analysis.25 The viability of PFS in the present trial is limited by being a secondary end point and lack of placebo control.
Bevacizumab monotherapy has also been investigated in untreated patients with metastatic RCC in a small randomized trial with a median PFS of 8.5 months and an ORR of 13%.26 The potential benefits of the combination of bevacizumab and IFN versus either as monotherapy must be balanced against the increased toxicity observed with the combination regimen. That is, an increased ORR and/or a delay of tumor progression, and perhaps a subsequent reduction/delay of tumor-related symptoms, is balanced against increased toxicity. The extent to which IFN contributes to the activity of the combination is unclear at present. It is possible that treatment of RCC with single-agent bevacizumab may produce a benefit similar to that of the combination with less toxicity, although this hypothesis requires prospective testing. Additional efforts to identify patients most likely to benefit, such as the laboratory parameters identified in the present analysis, are warranted.
This study has several limitations. There was no placebo infusion in this nonblinded trial and no independent review of radiographs. As such, investigator bias in interpretation of radiographs could potentially have contributed to the improved PFS and ORR. Although comparisons across trials are imperfect, the similarity of PFS and ORR of the present trial and the blinded, placebo-controlled international trial of bevacizumab and IFN20 make it seem unlikely that there was substantial investigator bias in the present study.
In conclusion, bevacizumab plus IFN produces significantly prolonged PFS and a higher ORR compared with IFN monotherapy in patients with untreated metastatic RCC.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Brian I. Rini, Genentech (C); Walter M. Stadler, Genentech (C); Janice Dutcher, Genentech (C) Stock Ownership: None Honoraria: None Research Funding: Brian I. Rini, Genentech; Walter M. Stadler, Genentech; Daniel A. Vaena, Genentech Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Brian I. Rini, Susan Halabi, Walter M. Stadler, Eric J. Small
Administrative support: Susan Halabi
Provision of study materials or patients: Brian I. Rini, Jonathan E. Rosenberg, Walter M. Stadler, Daniel A. Vaena, James N. Atkins, Joel Picus, Piotr Czaykowski, Janice Dutcher, Eric J. Small
Collection and assembly of data: Brian I. Rini, Susan Halabi, Walter M. Stadler, San-San Ou, Laura Archer
Data analysis and interpretation: Brian I. Rini, Susan Halabi, Walter M. Stadler, San-San Ou, Laura Archer, Eric J. Small
Manuscript writing: Brian I. Rini, Susan Halabi, Jonathan E. Rosenberg, Walter M. Stadler, Eric J. Small
Final approval of manuscript: Brian I. Rini, Susan Halabi, Jonathan E. Rosenberg, Walter M. Stadler, Daniel A. Vaena, San-San Ou, Laura Archer, James N. Atkins, Joel Picus, Piotr Czaykowski, Janice Dutcher, Eric J. Small
Appendix
The following institutions participated in this study: University of Oklahoma, Oklahoma, OK–Howard Ozer, MD, supported by CA37447; Christiana Care Health Services, Inc Community Clinical Oncology Program (CCOP), Wilmington, DE–Stephen Grubbs, MD, supported by CA45418; Dana-Farber Cancer Institute, Boston, MA–Eric P. Winer, MD, supported by CA32291; Duke University Medical Center, Durham, NC–Jeffrey Crawford, MD, supported by CA47577; Evanston Northwestern Healthcare CCOP, Evanston, IL–Gershon Y. Locker, MD; Georgetown University Medical Center, Washington, DC–Minetta C. Liu, MD, supported by CA77597; Grand Rapids Clinical Oncology Program, Grand Rapids, MI–Marianne Lange, MD; Greenville CCOP, see above Cancer Center of Carolinas; Hematology-Oncology Associates of Central New York, Syracuse, NY–Jeffrey Kirshner, MD, supported by CA45389; Illinois Oncology Research Association, Peoria, IL–John W. Kugler, MD, supported by CA35113; Kansas City Community Clinical Oncology Program CCOP, Kansas City, MO–Jorge C. Paradelo, MD; Massachusetts General Hospital, Boston, MA–Michael L. Grossbard, MD, supported by CA12449; Missouri Baptist Medical Center, St. Louis, MO–Alan P. Lyss, MD, supported by CA114558-02; Missouri Valley Cancer Consortium, Omaha, NE–Gamini S. Soori, MD; Mount Sinai Medical Center, Miami, FL–Rogerio Lilenbaum, MD, supported by CA45564; Mount Sinai School of Medicine, New York, NY–Lewis R. Silverman, MD, supported by CA04457; Nevada Cancer Research Foundation CCOP, Las Vegas, NV–John A. Ellerton, MD, supported by CA35421; New Hampshire Oncology-Hematology PA, Hooksett, NH–Douglas J. Weckstein, MD; Northern Indiana Cancer Research Consortium CCOP, South Bend, IN–Rafat Ansari, MD, supported by CA86726; Rhode Island Hospital, Providence, RI–William Sikov, MD, supported by CA08025; Roswell Park Cancer Institute, Buffalo, NY–Ellis Levine, MD, supported by CA02599; Southeast Cancer Control Consortium Inc. CCOP, Goldsboro, NC–James N. Atkins, MD, supported by CA45808; State University of New York Upstate Medical University, Syracuse, NY–Stephen L. Graziano, MD, supported by CA21060; The Ohio State University Medical Center, Columbus, OH–Clara D. Bloomfield, MD, supported by CA77658; University of California at San Diego, San Diego, CA–Joanne Mortimer, MD, supported by CA11789; University of California at San Francisco, San Francisco, CA–Alan P. Venook, MD, supported by CA60138; University of Chicago, Chicago, IL–Gini Fleming, MD, supported by CA41287; University of Illinois MBCCOP, Chicago, IL–Lawrence E. Feldman, MD, supported by CA74811; University of Iowa, Iowa City, IA–Gerald Clamon, MD, supported by CA47642; University of Maryland Greenebaum Cancer Center, Baltimore, MD–Martin Edelman, MD, supported by CA31983; University of Massachusetts Medical School, Worcester, MA–William V. Walsh, MD, supported by CA37135; University of Minnesota, Minneapolis, MN–Bruce A. Peterson, MD, supported by CA16450; University of Missouri/Ellis Fischel Cancer Center, Columbia, MO–Michael C. Perry, MD, supported by CA12046; University of Nebraska Medical Center, Omaha, NE–Anne Kessinger, MD, supported by CA77298; University of North Carolina at Chapel Hill, Chapel Hill, NC–Thomas C. Shea, MD, supported by CA47559; University of Texas Southwestern Medical Center, Dallas, TX–Debasish Tripathy, MD; Wake Forest University School of Medicine, Winston-Salem, NC–David D. Hurd, MD, supported by CA03927; Washington University School of Medicine, St. Louis, MO–Nancy Bartlett, MD, supported by CA77440; and Western Pennsylvania Cancer Institute, Pittsburgh, PA–Richard K. Shadduck, MD.
Fig A1.
Kaplan-Meier progression-free survival probability curves by the two treatment arms according to the number of adverse risk features. (A) Kaplan-Meier progression-free survival in patients with zero adverse risk features receiving bevacizumab plus interferon alfa (IFN) versus IFN monotherapy; (B) Kaplan-Meier progression-free survival in patients with one to two adverse risk features; (C) Kaplan-Meier progression-free survival in patients with three or more adverse risk features.
published online ahead of print at www.jco.org on October 20, 2008.
Supported in part by grants from the National Cancer Institute to the Cancer and Leukemia Group B (Richard L. Schilsky, MD, Chair; Grant No. CA31946); to the Cancer and Leukemia Group B Statistical Center (Stephen George, PhD; Grant No. CA33601); and by Grants No. CA33601 (S.H.), CA60138 (J.E.R.), CA41287 (W.M.S.), CA47642 (D.A.V.), CA45808 (J.N.A.), and CA77440 (J.P.).
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00072046.
REFERENCES
- 1.Interferon-alpha and survival in metastatic renal carcinoma: Early results of a randomised controlled trial—Medical Research Council Renal Cancer Collaborators. Lancet 353:14-17, 1999 [PubMed] [Google Scholar]
- 2.Pyrhönen S, Salminen E, Ruutu M, et al: Prospective randomized trial of interferon alfa-2a plus vinblastine versus vinblastine alone in patients with advanced renal cell cancer. J Clin Oncol 17:2859-2867, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Motzer RJ, Bacik J, Murphy BA, et al: Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol 20:289-296, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Negrier S, Escudier B, Lasset C, et al: Recombinant human interleukin-2, recombinant human interferon alfa-2a, or both in metastatic renal-cell carcinoma. Groupe Francais d’Immunotherapie. N Engl J Med 338:1272-1278, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Porzsolt F, Messerer D, Hautmann R, et al: Treatment of advanced renal cell cancer with recombinant interferon alpha as a single agent and in combination with medroxyprogesterone acetate: A randomized multicenter trial. J Cancer Res Clin Oncol 114:95-100, 1988 [DOI] [PubMed] [Google Scholar]
- 6.Motzer RJ, Murphy BA, Bacik J, et al: Phase III trial of interferon alfa-2a with or without 13-cis-retinoic acid for patients with advanced renal cell carcinoma. J Clin Oncol 18:2972-2980, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Gnarra JR, Tory K, Weng Y, et al: Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat Genet 7:85-90, 1994 [DOI] [PubMed] [Google Scholar]
- 8.Shuin T, Kondo K, Torigoe S, et al: Frequent somatic mutations and loss of heterozygosity of the von Hippel-Lindau tumor suppressor gene in primary human renal cell carcinomas. Cancer Res 54:2852-2855, 1994 [PubMed] [Google Scholar]
- 9.Wiesener MS, Munchenhagen PM, Berger I, et al: Constitutive activation of hypoxia-inducible genes related to overexpression of hypoxia-inducible factor-1alpha in clear cell renal carcinomas. Cancer Res 61:5215-5222, 2001 [PubMed] [Google Scholar]
- 10.Herman JG, Latif F, Weng Y, et al: Silencing of the VHL tumor-suppressor gene by DNA methylation in renal carcinoma. Proc Natl Acad Sci U S A 91:9700-9704, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dvorak HF: Vascular permeability factor/vascular endothelial growth factor: A critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol 20:4368-4380, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Escudier B, Eisen T, Stadler WM, et al: Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 356:125-134, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Motzer RJ, Hutson TE, Tomczak P, et al: Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 356:115-124, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Yang JC, Haworth L, Sherry RM, et al: A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 349:427-434, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coppin C, Porzsolt F, Awa A, et al: Immunotherapy for advanced renal cell cancer. Cochrane Database Syst Rev 1:CD001425, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Yang JC, Sherry RM, Steinberg SM, et al: Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J Clin Oncol 21:3127-3132, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDermott DF, Regan MM, Clark JI, et al: Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol 23:133-141, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Fyfe GA, Fisher RI, Rosenberg SA, et al: Long-term response data for 255 patients with metastatic renal cell carcinoma treated with high-dose recombinant interleukin-2 therapy. J Clin Oncol 14:2410-2411, 1996 [DOI] [PubMed] [Google Scholar]
- 19.Therasse P, Arbuck SG, Eisenhauer EA, et al: New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205-216, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Escudier B, Pluzanska A, Koralewski P, et al: Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: A randomised, double-blind phase III trial. Lancet 370:2103-2111, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Kaplan E, Meier P: Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457-481, 1958 [Google Scholar]
- 22.Cox DR: Regression models and life tables. J R Stat Soc B 74:187-220, 1972 [Google Scholar]
- 23.Slaton JW, Perrotte P, Inoue K, et al: Interferon-alpha-mediated down-regulation of angiogenesis-related genes and therapy of bladder cancer are dependent on optimization of biological dose and schedule. Clin Cancer Res 5:2726-2734, 1999 [PubMed] [Google Scholar]
- 24.Gabrilovich DI, Ishida T, Nadaf S, et al: Antibodies to vascular endothelial growth factor enhance the efficacy of cancer immunotherapy by improving endogenous dendritic cell function. Clin Cancer Res 5:2963-2970, 1999 [PubMed] [Google Scholar]
- 25.Bukowski RM, et al: Final results of the randomized phase III trial of sorafenib in advanced renal cell carcinoma: Survival and biomarker analysis. J Clin Oncol 25:240s, 2007. (suppl; abstr 5023) [Google Scholar]
- 26.Bukowski RM, Kabbinavar FF, Figlin RA, et al: Randomized phase II study of erlotinib combined with bevacizumab compared with bevacizumab alone in metastatic renal cell cancer. J Clin Oncol 25:4536-4541, 2007 [DOI] [PubMed] [Google Scholar]