Abstract
Purpose
To determine the impact of age and health status on adjuvant treatment recommendations for older patients with breast cancer from the perspective of medical oncologists and primary care physicians with geriatric expertise.
Patients and Methods
One hundred fifty-one oncologists and 158 primary care physicians with geriatric expertise participated in an online survey. The survey described hypothetical patients of varying ages (70, 75, 80, and 85 years) and health status (good, average, and poor) who had node-positive, hormone receptor–positive, human epidermal growth factor receptor 2 (HER-2)/neu–negative; and hormone receptor–negative, HER-2/neu–positive breast cancers. The effects of patient age and health status on the survey participants’ adjuvant treatment recommendations were examined using generalized estimation equation methods.
Results
The majority of both oncologists and primary care physicians recommended some form of adjuvant therapy for patients of all ages (70, 75, 80, and 85 years) and health status. Both oncologists and primary care providers were less likely to recommend adjuvant treatment as a patient's age increased or health status declined (P < .0001). There were no significant differences in treatment recommendations among primary care physicians and oncologists for patients with hormone receptor–negative, HER-2/neu–positive tumors (P = .54). However, primary care providers were more likely than oncologists to recommend no adjuvant treatment for patients age 75 years or older with hormone receptor–positive, HER-2/neu–negative tumors (P < .01).
Conclusion
Age and health status influence oncologists’ and primary care providers’ adjuvant treatment recommendations. Evidence-based guidelines for breast cancer treatment in older adults taking into account age and health status are needed.
INTRODUCTION
Despite the fact that aging is the number-one risk factor for breast cancer,1 older women have been under-represented in breast cancer clinical trials.2 As a result of limited data to guide the treatment of older women with breast cancer, variations in treatment patterns are common.3-9 Adjuvant treatment decisions in older adults can also be more complex because factors other than chronologic age, such as functional status and comorbid medical conditions, independently affect morbidity and mortality.10-14
Given the heterogeneity of the older breast cancer population and limited evidence on which to base treatment recommendations, physicians’ perspectives play a critical role in decision making. Previous studies have reported that health care providers serve as the main source of information for patients,15-18 and primary care providers play an active role in referring patients for mammography screening.19 Furthermore, studies show that attitudes toward the risks and benefits of adjuvant treatment for breast cancer in older adults may differ by physician specialty.20 The impact of age versus health status in physician attitudes toward treatment recommendations has also been underexplored. A survey of 28 academic oncologists with expertise in breast cancer demonstrated wide variability in treatment recommendations for older adults with breast cancer, particularly as a patient's age increased and health status declined.21
Because the treatment of older adults with breast cancer can be complex, a multidisciplinary approach is appropriate. The primary care provider with expertise in the care of older adults and the oncologist with expertise in cancer each lend a unique perspective regarding adjuvant therapy in light of a patient's health status. The goal of this study was to understand the impact of patient age and health status on oncologists’ and primary care doctors’ decisions to pursue adjuvant systemic treatment for breast cancer in older adults.
PATIENTS AND METHODS
Study Participants
This research study, conducted in 2007, consisted of an online survey of medical oncologists and primary care providers with expertise in geriatric medicine who treat postmenopausal women for breast cancer. The following inclusion criteria were applied for survey participation: oncologists must have been in practice for at least 2 years and be treating at least 25 postmenopausal patients with breast cancer on adjuvant hormonal therapy; primary care providers had to demonstrate expertise in geriatric medicine. To be eligible to participate in this study, such providers could include general practitioners, family practitioners, internal medicine specialists or geriatricians, but all must have received a Certificate of Added Qualification in geriatric medicine and/or received at least 10 hours of Continuing Medical Education (CME) per year in geriatric health, and have been in practice for at least 2 years. Primary care providers must have treated or currently treat at least five postmenopausal patients with breast cancer. Participants could not work for an advertising agency, market research company, manufacturer or distributor of pharmaceutical products, pharmacy, drug store, or the US Food and Drug Administration.
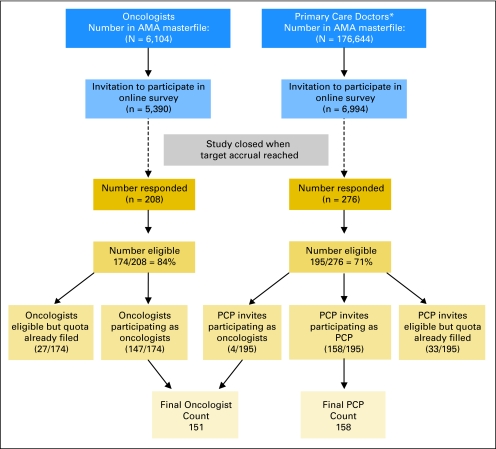
The American Medical Association (AMA) Physician Masterfile and distribution of physicians by specialty according to number of years in practice, sex, and region were used to generate a random list of medical oncologists and geriatricians/primary care doctors who met the target demographic characteristics. Letters of invitation to participate in the online survey were sent to potential participants: 5,390 oncologists (of 6,104 in the AMA Masterfile) and 6,994 primary care providers (of 176,644 in the AMA Masterfile). Of those who responded, 84% of oncologists and 71% of primary care providers were eligible to participate (Fig 1). Participants were enrolled consecutively into the study until the target sample size was reached in each group. Respondents received a $150 honorarium for their participation. This study was approved by the Institutional Review Boards of City of Hope National Medical Center and the University of California, Los Angeles.
Fig 1.
Subject flow for entry into the study. (*) Primary care providers (PCP) include geriatricians, general practice, internal medicine, family practice. AMA, American Medical Association.
Procedure
A total of 151 qualified oncologists and 158 primary care providers with expertise in geriatric medicine completed the online survey. All 151 oncologists indicated oncology as their primary medical specialty. The specialty of the 158 primary care providers included general practice (n = 3), internal medicine (n = 89), family practice (n = 16), and geriatrics (n = 50). The survey had previously been tested in a study of 28 medical oncologists with expertise in breast cancer.21 Minor modifications to the survey were made on the basis of feedback from this pilot study, and new data were integrated regarding breast cancer treatment options.21 The power calculation determined that with 150 participants in each group, the study would have 80% power to detect a minimal difference of 0.097 between oncologists and geriatricians in the percentages of physicians who would treat (ie, 90% v 99.7% as response, at type I error of .05).
The survey consisted of cases describing patients with node-positive breast cancer (T2 [4 cm] N2 [four positive lymph nodes]) that was either hormone receptor positive and HER-2/neu negative or hormone receptor negative and HER-2/neu positive (case descriptions are provided in the Appendix, online only). The hypothetical case patients were of varying ages (70, 75, 80, and 85 years) and were assigned to three different health states (good, average, and poor), with corresponding predicted longevity ranging from 6.1 to 15.8 years for patients with good health and either 5.9 to 14.8 or 4.5 to 8.6 years for those with average or poor health, respectively. A patient in “good health” was described as someone who was active, exercised 3 days a week, and had no other medical problems. A patient in “average health” was described as having non–insulin-dependent diabetes and hypertension, and who lived independently but required assistance with housework. A patient in “poor health” was described as someone with a history of transient ischemic attack, coronary artery bypass graft, and severe osteoarthritis who lived independently but had a home health aide who provided assistance with dressing, housework, and shopping.
Cases were presented to participating physicians in order of patient health condition, from “good” to “poor.” Within each health category, physicians were asked to choose the treatment they would recommend for the hypothetical patient age 70 to 85 years. Treatment choices included chemotherapy, trastuzumab (if HER-2/neu positive), endocrine therapy (if hormone receptor positive), “other,” or no therapy. Specific responses for “other” that corresponded to other given choices were recoded appropriately; otherwise they were considered inappropriate responses and were recoded as missing. These responses were dichotomized as either recommending some form of adjuvant treatment (recoded as “1”) or recommending no adjuvant treatment (recoded as “0”).
Statistical Analysis
We examined the effects of patient age and health status on whether physicians would recommend treatment using the generalized linear mixed-effects model, also known as multilevel or hierarchical models, for binomial outcomes to account for correlations among responses within an individual respondent. Whether to recommend treatment or not (1 or 0) was modeled for a respondent as a function of patient age (70, 75, 80, or 85 years old) and health status (good, average, or poor), both of which were treated as categoric variables. The covariates shown in Table 1 were included in the model for adjustment. Because the results for patient age and health status remained consistent regardless of covariate adjustments, we present the unadjusted results. Proc GENMOD (SAS/STAT, version 9.1, SAS Institute Inc, Cary, NC) was used to obtain estimates of the generalized linear mixed-effects models, using the exchangeable correlation matrix. Significance tests were conducted using the Wald test results based on type I error of .05.
Table 1.
Characteristics of Oncologists and Primary Care Study Responders
| Physician Characteristic | Oncologists (n = 151)
|
Primary Care Physicians (n = 158)
|
P* | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age, years | |||||
| 30-39 | 23 | 15.2 | 71 | 44.9 | < .0001 |
| 40-49 | 39 | 25.8 | 45 | 28.5 | |
| 50-59 | 70 | 46.4 | 38 | 24.1 | |
| 60+ | 19 | 12.6 | 4 | 2.5 | |
| Mean | 50.1 | 42.9 | < .0001 | ||
| Sex | |||||
| Male | 123 | 81.5 | 105 | 66.5 | .0027 |
| Female | 28 | 18.5 | 53 | 33.5 | |
| Region† | |||||
| Northeast | 32 | 21.2 | 47 | 29.8 | .17 |
| Midwest | 35 | 23.2 | 29 | 18.4 | |
| South | 47 | 31.1 | 54 | 34.2 | |
| West | 37 | 24.5 | 28 | 17.7 | |
| Primary specialty | |||||
| Internal medicine | 0 | 0 | 89 | 56.3 | |
| General/family practice | 0 | 0 | 19 | 12.0 | |
| Geriatrics | 0 | 0 | 50 | 31.7 | |
| Oncology | 151 | 100 | 0 | 0 | |
| Practice type | |||||
| Primarily office | 133 | 88.1 | 91 | 57.6 | < .0001 |
| Primarily hospital | 17 | 11.3 | 39 | 24.7 | |
| Other | 1 | 0.1 | 28 | 17.7 | |
| No. years in practice | |||||
| < 10 | 29 | 19.2 | 85 | 53.8 | < .0001 |
| 10-19 | 51 | 33.8 | 38 | 24.0 | |
| ≥ 20 | 71 | 47.0 | 35 | 22.2 | |
| Mean | 17.3 | 12.1 | < .0001 | ||
| No. patients treated in the last month | |||||
| < 200 | 26 | 17.2 | 30 | 19.0 | .0015 |
| 200-299 | 47 | 31.1 | 28 | 17.7 | |
| 300-399 | 36 | 23.8 | 26 | 16.5 | |
| ≥ 400 | 42 | 27.8 | 74 | 46.8 | |
| Mean | 300.8 | 357.7 | .0043 | ||
P values are based on χ2 test (categorical) or t test (continuous).
Northeast, CT, MA, ME, NH, NJ, NY, PA, RI, VT; Midwest, IA, IL, IN, KS, MI, MN, MO, ND, NE, OH, SD, WI; South, AL, AR, DC, DE, FL, GA, KY, LA, MD, MS, NC, OK, SC, TN, TX, VA, WV; West, AK, AZ, CA, CO, HI, ID, MT, NM, NV, OR, UT, WA, WY.
RESULTS
Primary care doctors with a specialty in geriatrics (n = 50) and those without a specialty in geriatrics (n = 108) were compared in terms of age, sex, region, practice type, and number of years in practice, all of which were not significantly different between groups. The response patterns for treatment recommendations according to breast cancer hormone-receptor status also did not vary among primary care doctors who did or did not have a specialty in geriatrics (P = .12 for hormone-positive tumor; P = .56 for hormone-negative tumor; data not shown). As such, for analysis, the responses for primary care doctors with a specialty in geriatrics and those without a specialty in geriatrics were grouped together and collectively referred to as primary care doctors. The characteristics of the oncologists and primary care doctors who completed the survey are summarized in Table 1. Primary care doctors were more likely to be younger (P < .0001) and female (P = .003), compared with participating oncologists, consistent with the national distribution of physician characteristics in these specialties based on the AMA Physician Masterfile (data not shown). The majority of oncologists and primary care doctors had office-based practices; however, a higher proportion of primary care doctors were hospital based (P < .0001). Among primary care doctors, 56% described themselves as internists, 12% as general/family practitioners, and 32% as geriatricians. There was no significant difference in the geographic distribution between primary care doctors and oncologists (P = .14). Oncologists, on average, were in practice longer than primary care doctors (mean, 17.3 v 12.1 years, respectively; P < .0001), consistent with the national distribution of physician characteristics in these specialties based on the AMA Physician Masterfile (data not shown). However, primary care doctors had treated more patients in the 3 months before the survey, than oncologists (mean, 358 v 301 patients, respectively; P = .004).
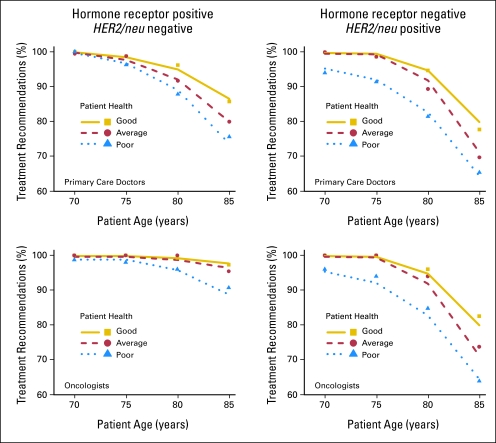
Figure 2 summarizes the percentage of primary care doctors and oncologists that recommended adjuvant systemic therapy for patients ages 70, 75, 80, and 85 years of varying health status and with tumors that were either hormone receptor positive and HER-2/neu negative (left panels), or hormone receptor negative and HER-2/neu positive (right panels). The majority of both oncologists and primary care doctors recommended some form of adjuvant therapy for patients of all ages (70, 75, 80, and 85 years) and health status. For either tumor type, both oncologists and primary care doctors were less likely to recommend adjuvant treatment as the patient's age increased or health status declined (P < .0001). Age and health status had a more prominent effect on adjuvant treatment decisions for hormone receptor–negative tumors.
Fig 2.
Percentage of physicians recommending adjuvant breast cancer treatment for theoretical patients of varying age and health status. Treatment choice included chemotherapy, trastuzumab (if HER2/neu positive), endocrine therapy (if hormone receptor positive), “other,” or no therapy.
Table 2 shows the recommendations for adjuvant therapy for hormone receptor–positive and HER-2/neu–negative breast cancer by the two groups of physicians. In addition to patient age and health status, which significantly influenced treatment recommendations, primary care doctors were less likely than oncologists to recommend adjuvant therapy (P < .0001). Moreover, physician difference was more apparent for patients age 75 years or older, in that primary care doctors were less likely than oncologists to recommend adjuvant therapy for these older patients (P = .01). For example, for an 85-year-old patient in average health, 80% of primary care doctors and 95% of oncologists recommended treatment. For an 85-year-old patient in poor health, 76% of primary care doctors and 91% of oncologists recommended adjuvant therapy. On the other hand, for patients age 70 years and in either good or average health, there were no significant differences in treatment recommendations between primary care doctors and oncologists. Although the difference is slight, a smaller percentage (98.7%) of oncologists recommended treatment for 70-year-old patients in poor health compared with primary care doctors (100%; P = .03). Among oncologists, the percentage recommending treatment decreased more precipitously for patients of poor health than for patients of good or average health (P = .026), notably for patients age 80 and 85 years old.
Table 2.
Treatment Recommendations for Hypothetical Patient With Hormone Receptor–Positive and HER2/neu–Negative Breast Cancer
| Patient Age (years) | Physicians Recommending Adjuvant Treatment
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oncologists (n = 151)
|
Primary Care Physicians (n = 158)
|
|||||||||||
| Good Health
|
Average Health
|
Poor Health
|
Good Health
|
Average Health
|
Poor Health
|
|||||||
| No.* | % | No.* | % | No.* | % | No.* | % | No.* | % | No.* | % | |
| 70 | 150 | 100 | 149 | 100 | 148 | 98.7 | 153 | 100 | 152 | 99.3 | 155 | 100 |
| 75 | 151 | 100 | 151 | 100 | 148 | 98 | 152 | 98.7 | 152 | 98.7 | 150 | 96.2 |
| 80 | 150 | 99.3 | 151 | 100 | 145 | 96 | 149 | 96.1 | 141 | 91.6 | 137 | 87.8 |
| 85 | 146 | 97.3 | 144 | 95.4 | 137 | 90.7 | 132 | 85.7 | 123 | 79.9 | 118 | 75.6 |
NOTE. Cancer was T2 (4 cm) N2 (four postitive lymph nodes), estrogen receptor positive, HER2/neu negative. Treatment choice included chemotherapy, trastuzumab (if HER2/neu positive), endocrine therapy (if hormone receptor positive), “other,” or no therapy.
Denominators for some cells are less than the number of physicians surveyed because of missing response.
Table 3 summarizes physician recommendations for tumors that are hormone receptor negative and HER-2/neu positive. There were no significant differences between oncologists’ and primary care providers’ recommendations for adjuvant therapy, regardless of patient age or health status (P = .54). However, for both oncologists and primary care physicians, treatment recommendations for adjuvant therapy were influenced by patient age (P < .0001) and health status (P < .0001). There was little change in the percentage of physicians recommending treatment at age 70 and 75 years, but the change grew larger for patients older than 75 years. In general, oncologists and primary care doctors were less likely to recommend adjuvant treatment for patients in poor health than for patients in good or average health. For patients age 70 and 75 years with good or average health, there was practically no difference in the percent of physicians recommending adjuvant treatment. However, for patients age 80 and 85 years, physicians were less likely to recommend treatment for patients in average health, compared with those in good health. Table 4 provides the P value for the effects of patient age, patient health, and physician on treatment recommendations described in Tables 2 and 3.
Table 3.
Treatment Recommendations for Hypothetical Patient With Hormone Receptor–Negative and HER2/neu–Positive Breast Cancer
| Patient Age (years) | Physicians Recommending Adjuvant Treatment
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oncologists (n = 151)
|
Primary Care Physicians (n = 158)
|
|||||||||||
| Good Health
|
Average Health
|
Poor Health
|
Good Health
|
Average Health
|
Poor Health
|
|||||||
| No.* | % | No.* | % | No.* | % | No.* | % | No.* | % | No.* | % | |
| 70 | 151 | 100 | 151 | 100 | 145 | 96 | 154 | 100 | 154 | 100 | 144 | 94.1 |
| 75 | 151 | 100 | 151 | 100 | 142 | 94 | 152 | 98.7 | 152 | 98.7 | 140 | 91.5 |
| 80 | 144 | 96 | 141 | 94 | 128 | 84.8 | 145 | 94.8 | 137 | 89.5 | 124 | 81.6 |
| 85 | 123 | 82.6 | 110 | 73.8 | 96 | 64 | 120 | 77.9 | 107 | 69.9 | 101 | 65.6 |
NOTE. Cancer was T2 (4 cm) N2 (four positive lymph nodes), estrogen receptor negative, HER2/neu positive. Treatment choice included chemotherapy, trastuzumab (if HER2/neu negative), endocrine therapy (if hormone receptor negative), “other,” or no therapy.
Denominators for some cells are less than the number of physicians surveyed because of missing response.
Table 4.
P for Effects of Patient Age, Patient Health, and Physician on Treatment Recommendations for Hormone Receptor–Positive and Hormone Receptor–Negative Breast Cancer
| Model | Age | Health | Physician | Age × Health | Age × Physician | Health × Physician |
|---|---|---|---|---|---|---|
| Hormone receptor positive | ||||||
| Age, health, physician | < .0001 | < .0001 | < .0001 | — | — | — |
| Age, health, physician, age × physician, health × physician | < .0001 | .0003 | .79 | — | .010* | .026* |
| Hormone receptor negative | ||||||
| Age, health, physician | < .0001 | < .0001 | .54 | — | — | — |
| Age, health, age × health | < .0001 | < .0001 | — | < .0001 | — | — |
NOTE. Multiplication indicates interactions between factors.
df = 1.
DISCUSSION
Treatment decisions for older adults with breast cancer are complex for several reasons. First, most clinical trials, which set the standard for oncology care, enroll a low proportion of older adults.2 Second, patients with poor health are often excluded from these trials. Therefore, there are little evidence-based data on which to base treatment recommendations for patients over the age of 70 years or those with poor health. Third, the majority of older adults are likely to have comorbid medical conditions at the time of presentation with breast cancer. Such comorbid medical conditions may introduce competing risk factors for mortality other than from breast cancer and influence patients’ ability to tolerate cancer therapy. In these instances, physicians must carefully weigh the potential risks and benefits of adjuvant therapy for breast cancer.
Balancing benefits against the risks of adjuvant breast cancer treatment in the older patient population is challenging. Tools to aid in decision-making, such as Adjuvant! Online (www.adjuvantonline.com/index.jsp), are limited by a dearth of evidence-based data in women older than 75 years. Although this computer-based program for estimation of the benefits from adjuvant treatments has the ability to incorporate variations of health status (ie, perfect health, minor problems, medical problems average for age, and major medical problems), the impact or severity of specific medical problems is not covered. In addition, assessment of patient functional status is not included. Lastly, although estimates of therapy efficacy are provided, age-related risks of adjuvant therapy are not. These additional data are needed to help physicians and patients weigh the risks and benefits of adjuvant therapy.
Our study focused on the attitudes of physicians in decision making. Primary care physicians with expertise in geriatrics routinely manage a multitude of comorbid conditions, and are skilled at weighing the interaction of treatment for one illness against concurrent comorbid diseases and overall functional capacity. Their insight in this regard, coupled with their perception of the benefits and toxicity of cancer therapy, influence whether an older adult is referred to an oncologist. This served as the rationale for studying and comparing the views of primary care physicians with expertise in geriatric medicine with those of oncologists regarding systemic adjuvant breast cancer therapy.
This study demonstrates that although both primary care doctors and oncologists are influenced by patient age and health status when deciding whether adjuvant treatment should be administered, the influence of these factors is more pronounced in primary care doctors, especially in our hypothetical cases involving patients age 75 years and older with hormone receptor–positive, HER-2/neu–negative disease. There are several possible reasons for this finding. One possibility is that the uncertainty related to treatment efficacy in these ages has a greater impact on primary care providers. Alternatively, primary care providers and oncologists may differ in their assessment of life expectancy and overall benefit from adjuvant treatment within the context of advanced age and declining health status. Lastly, primary care providers may not be as skilled as oncologists in weighing the risk and benefits of various cancer therapies in light of the risk of relapse based on the tumor characteristics.
There are several limitations to this study. First, the study was closed after the target accrual goal was met. Therefore, we cannot be sure of the exact response rate. Second, this survey was deployed electronically, which could explain the preponderance of younger physicians who responded to our survey, although, because of the study design, we were unable to compare the characteristics of participants with those of the nonparticipants. Third, these were hypothetical cases, so we cannot be certain that the responses reflect actual practice patterns of the participating physicians. Fourth, we did not assess the physicians’ knowledge of breast cancer treatment. In addition, given the shortage of geriatricians in the United States, we surveyed primary care doctors with geriatric expertise, as well as geriatricians. Some may look at this as a limitation whereas others may view this as a strength because the sample may be more representative of providers in the community. Lastly, these results apply only to patients with breast cancer and cannot be extrapolated to other tumors.
These limitations notwithstanding, this research has important strengths. To our knowledge, it is the first study to examine the perspective of both oncologists and primary care physicians with geriatric expertise regarding breast cancer treatment, taking into account the age and the health status of patients. These results highlight the need for evidence-based guidelines and decision aides for breast cancer treatment in older adults taking into account age, health status, and age-related toxicities of therapy. Collaboration between oncologists and primary care physicians with geriatric expertise can help to accomplish this goal.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Arti Hurria, Genentech (C) Stock Ownership: None Honoraria: Arash Naeim, Pfizer Research Funding: Arti Hurria, Pfizer, Abraxis Biosciences, Sanofi-Aventis; Arash Naeim, Pfizer, Genentech, Amgen Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Arti Hurria, Arash Naeim
Financial support: Arti Hurria, Arash Naeim
Administrative support: Arti Hurria
Provision of study materials or patients: Arti Hurria, Arash Naeim
Collection and assembly of data: Arti Hurria, Arash Naeim
Data analysis and interpretation: Arti Hurria, F. Lennie Wong, Doojduen Villaluna, Smita Bhatia, Cathie T. Chung, Joanne Mortimer, Arash Naeim
Manuscript writing: Arti Hurria, F. Lennie Wong, Smita Bhatia, Cathie T. Chung, Joanne Mortimer, Sara Hurvitz, Arash Naeim
Final approval of manuscript: Arti Hurria, Doojduen Villaluna, F. Lennie Wong, Smita Bhatia, Cathie T. Chung, Sara Hurvitz, Arash Naeim
Appendix
Cases 1, 2, and 3
A woman is diagnosed with a 4-cm infiltrating ductal carcinoma, four of 12 positive lymph nodes, estrogen and progesterone receptor negative, HER-2/neu 3+ (fluorescent in situ hybridization amplified). She is status post–lumpectomy and axillary lymph node dissection. Staging workup reveals no distant disease. Multiple gated acquisition scan reveals a left ventricular ejection fraction of 65%.
Cases 4, 5, and 6
A woman is diagnosed with a 4-cm infiltrating ductal carcinoma, four of 12 positive lymph nodes, estrogen and progesterone receptor positive, HER-2/neu 1+ (fluorescent in situ hybridization not amplified). She is status post lumpectomy and axillary lymph node dissection. Staging workup reveals no distant disease. Multiple gated acquisition scan reveals a left ventricular ejection fraction of 65%.
Case Discussion
For cases 1 to 6, the respondent was asked to make adjuvant breast cancer treatment decisions presuming the patient was age 70, 75, 80, or 85 years and either in good, average, or poor health. Health status is defined according to the following: good health—the patient has no other medical problems, exercises regularly, and lives independently without assistance; average health—she has non–insulin-dependent diabetes and hypertension, she lives independently, but she receives assistance with housework; poor health—she has a history of a TIA, CABG, and severe osteoarthritis, she lives independently, but she has a home health aide who provides assistance with dressing, housework, and shopping.
published online ahead of print at www.jco.org on October 27, 2008
Supported by the Paul Beeson Career Development Award in Aging Research (Grant No. K23 AG026749-01) and American Society of Clinical Oncology Association of Specialty Professors Junior Development Award in Geriatric Oncology (both to A.H.); National Institutes of Health Grant No. 1K23CA102149 (A.N.); and Pfizer.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Armstrong K, Eisen A, Weber B: Assessing the risk of breast cancer. N Engl J Med 342:564-571, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Lewis JH, Kilgore ML, Goldman DP, et al: Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol 21:1383-1389, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Newcomb PA, Carbone PP: Cancer treatment and age: Patient perspectives. J Natl Cancer Inst 85:1580-1584, 1993 [DOI] [PubMed] [Google Scholar]
- 4.Busch E, Kemeny M, Fremgen A, et al: Patterns of breast cancer care in the elderly. Cancer 78:101-111, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Newschaffer CJ, Penberthy L, Desch CE, et al: The effect of age and comorbidity in the treatment of elderly women with nonmetastatic breast cancer. Arch Intern Med 156:85-90, 1996 [PubMed] [Google Scholar]
- 6.Bergman L, Dekker G, van Leeuwen FE, et al: The effect of age on treatment choice and survival in elderly breast cancer patients. Cancer 67:2227-2234, 1991 [DOI] [PubMed] [Google Scholar]
- 7.Hillner BE, Penberthy L, Desch CE, et al: Variation in staging and treatment of local and regional breast cancer in the elderly. Breast Cancer Res Treat 40:75-86, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Ballard-Barbash R, Potosky AL, Harlan LC, et al: Factors associated with surgical and radiation therapy for early stage breast cancer in older women. J Natl Cancer Inst 88:716-726, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Hurria A, Brogan K, Panageas KS, et al: Patterns of toxicity in older patients with breast cancer receiving adjuvant chemotherapy. Breast Cancer Res Treat 92:151-156, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Reuben DB, Rubenstein LV, Hirsch SH, et al: Value of functional status as a predictor of mortality: Results of a prospective study. Am J Med 93:663-669, 1992 [DOI] [PubMed] [Google Scholar]
- 11.Narain P, Rubenstein LZ, Wieland GD, et al: Predictors of immediate and 6-month outcomes in hospitalized elderly patients: The importance of functional status. J Am Geriatr Soc 36:775-783, 1988 [DOI] [PubMed] [Google Scholar]
- 12.Maione P, Perrone F, Gallo C, et al: Pretreatment quality of life and functional status assessment significantly predict survival of elderly patients with advanced non-small-cell lung cancer receiving chemotherapy: A prognostic analysis of the multicenter Italian lung cancer in the elderly study. J Clin Oncol 23:6865-6872, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Lee SJ, Lindquist K, Segal MR, et al: Development and validation of a prognostic index for 4-year mortality in older adults. JAMA 295:801-808, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Walter LC, Brand RJ, Counsell SR, et al: Development and validation of a prognostic index for 1-year mortality in older adults after hospitalization. JAMA 285:2987-2994, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Ciambrone D: Treatment decision-making among older women with breast cancer. J Women Aging 18:31-47, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Kreling B, Figueiredo MI, Sheppard VL, et al: A qualitative study of factors affecting chemotherapy use in older women with breast cancer: Barriers, promoters, and implications for intervention. Psychooncology 15:1065-1076, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Maly RC, Leake B, Silliman RA: Breast cancer treatment in older women: Impact of the patient-physician interaction. J Am Geriatr Soc 52:1138-1145, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Oskay-Ozcelik G, Lehmacher W, Konsgen D, et al: Breast cancer patients’ expectations in respect of the physician-patient relationship and treatment management results of a survey of 617 patients. Ann Oncol 18:479-484, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Sabatino SA, McCarthy EP, Phillips RS, et al: Breast cancer risk assessment and management in primary care: Provider attitudes, practices, and barriers. Cancer Detect Prev 31:375-383, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Malek K, Fink AK, Thwin SS, et al: The relationship among physicians’ specialty, perceptions of the risks and benefits of adjuvant tamoxifen therapy, and its recommendation in older patients with breast cancer. Med Care 42:700-706, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Hurria A, Naeim A, Elkin E, et al: Adjuvant treatment recommendations in older women with breast cancer: A survey of oncologists. Crit Rev Oncol Hematol 61:255-260, 2007 [DOI] [PubMed] [Google Scholar]