Abstract
Purpose
Imatinib mesylate is standard treatment for patients who have advanced gastrointestinal stromal tumor (GIST), but not all patients benefit equally. In previous studies, GIST genotype correlated with treatment outcome and optimal imatinib dosing.
Patients and Methods
We examined the relationship between kinase genotype and treatment outcome for 428 patients enrolled on the North American phase III study SWOG S0033/CALGB 150105 and treated with either 400 mg or 800 mg daily doses of imatinib.
Results
The presence of KIT exon 11–mutant genotype (n = 283) correlated with improved treatment outcome when compared with KIT exon 9–mutant (n = 32) and wild-type (WT; n = 67) genotypes for objective response (complete response [CR]/partial response [PR], 71.7% v 44.4% [P = .007]; and 44.6% [P = .0002], respectively); time to tumor progression (TTP; median 24.7 months v 16.7 and 12.8 months, respectively); and overall survival (OS; median 60.0 months v 38.4 and 49.0 months, respectively). The survival outcomes for patients with exon 9–mutant, exon 11–mutant or WT GIST were not affected by imatinib dose. However, there was evidence of improved response rates for patients with exon 9–mutant tumors treated with imatinib 800 mg versus 400 mg (CR/PR, 67% v 17%; P = .02). Patients who had CD117-negative GIST had similar TTP but inferior OS compared with patients who had CD117-positive disease, which suggests that patients who have CD117-negative GIST may benefit from imatinib treatment. In addition, we identified novel but rare mutations of the KIT extracellular domain (exons 8 and 9).
Conclusion
We confirmed the favorable impact of KIT exon 11 genotype when compared with KIT exon 9 and wild-type genotype for patients with advanced GIST who are treated with imatinib.
INTRODUCTION
Gastrointestinal stromal tumor (GIST) is the most common mesenchymal tumor of the gastrointestinal tract. In 1998, Hirota et al1 made the seminal discovery that these tumors express the KIT tyrosine kinase and commonly harbor oncogenic mutations in the KIT gene. Subsequently, several investigators reported in vitro evidence of antitumor activity of the small molecule KIT inhibitor imatinib mesylate (Glivec/Gleevec; Novartis Pharma AG, Basel, Switzerland) against KIT mutant cell lines.2,3 These observations led to clinical testing of this agent as a medical therapy for patients who have advanced disease.4-7
When the early trials were underway, laboratory studies revealed significant molecular heterogeneity among GISTs. Notably, 75% to 85% of GISTs had an activating mutation of KIT, 5% to 7% had an activating mutation of the homologous PDGFRA kinase, and approximately 12% to 15% of GISTs did not have a detectable mutation of either kinase.8-11 Correlative molecular studies in phase I to II studies revealed significant differences in objective response, progression-free survival (ie, time to tumor progression [TTP]), and overall survival (OS) between GISTs with different kinase genotypes. Specifically, the outcomes for patients with KIT exon 11–mutant GIST were better than for patients with KIT exon 9–mutant GIST or tumors without a detectable KIT mutation.7,8,12
Prospective studies of the relationship between kinase genotype and imatinib response were incorporated into two pivotal phase III trials that were designed to compare 400 mg and 800 mg daily doses of imatinib.13-15 In this study, we examine the correlation between kinase genotype, imatinib dose, and clinical outcomes in 397 patients with GIST from the North American phase III trial.14 Our findings confirmed that KIT exon 11 mutation is a positive predictive factor for objective response, TTP, and OS. This study also provides prognostic data for other GIST genotypes, including those with KIT exon 9 mutation, PDGFRA mutation, and wild-type (WT) status.
PATIENTS AND METHODS
Eligibility Criteria
Patients were required to have a histologic diagnosis of CD117-positive GIST, as determined by immunohistochemistry with the DAKO polyclonal rabbit antibody (DAKO, Carpinteria, CA), that was deemed incurable (ie, metastatic or unresectable) by expert multimodality management. Institutional review board approval was obtained at each participating center. Each participant signed an institutional review board–approved, protocol-specific informed consent in accordance with federal and institutional guidelines.14 Whenever possible, a tumor sample was collected and sent to the Cancer and Leukemia Group B Pathology Coordinating Office for diagnostic review by a single study pathologist (C.F.), which was followed by tumor genotyping (Appendix Table A1, online only).
Treatment Arms
Patients were randomly allocated to receive either the conventional dose (400 mg once daily) or a high dose (800 mg daily, given as 400 mg twice daily) of imatinib. Patients received treatment until disease progression or unacceptable toxicity occurred. Complete details and results from this study were reported recently.14
RESULTS
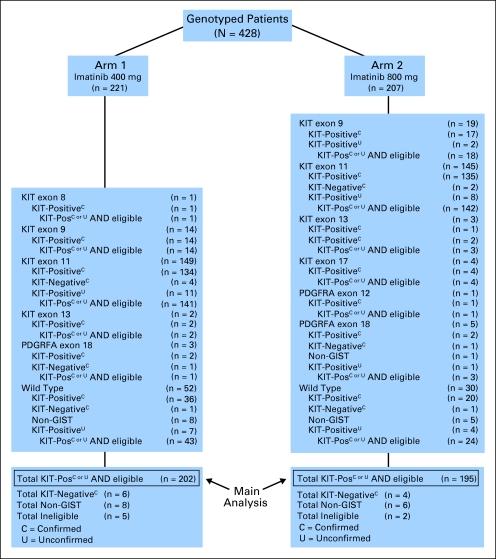
The main clinical study enrolled 746 patients who had advanced GIST between December 15, 2000 and September 1, 2001. Median follow-up was 4.5 years for patients who remained on study at the time of this report.14 Tumor samples were obtained from 447 consenting patients, 428 of whom (95.7%) were successfully genotyped (Table 1; Fig 1). Of the 428 samples analyzed, central pathology review was performed on all but 36 patient cases, and it confirmed 368 (93.9%) of 392 as CD117-positive GIST. Another 10 were diagnosed as CD117-negative GIST, and 14 were non-GIST sarcoma. The 14 patient cases of non-GIST sarcoma included nine patient cases of leiomyosarcoma, one patient case of monophasic synovial sarcoma, one patient case of malignant peripheral-nerve sheath tumor, one patient case of well-differentiated liposarcoma (spindle cell type), one patient case of undifferentiated sarcoma with epitheloid morphology, and one patient case of epitheloid malignancy not otherwise specified (NOS). Patient cases not centrally reviewed were categorized as CD117-positive GIST on the basis of immunohistochemical staining performed at the enrolling institution.
Table 1.
Tumor Genotype Versus Tumor Pathology Status
| Genotype | Pathology Status
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Confirmed CD117+*
|
Confirmed CD117−†
|
Confirmed Non-GIST Sarcoma†
|
Unconfirmed CD117+‡
|
|||||
| No. | % | No. | % | No. | % | No. | % | |
| KIT 8 | 1 | 0.3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| KIT 9 | 31 | 8.4 | 0 | 0.0 | 0 | 0.0 | 2 | 5.6 |
| KIT 11 | 269 | 73.1 | 6 | 60.0 | 0 | 0.0 | 19 | 52.7 |
| KIT 13 | 3 | 0.8 | 0 | 0.0 | 0 | 0.0 | 2 | 5.6 |
| KIT 17 | 4 | 1.1 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| PDGFRA 12 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 2.8 |
| PDGFRA 18 | 4 | 1.1 | 2 | 20.0 | 1 | 7.1 | 1 | 2.8 |
| WT | 56 | 15.2 | 2 | 20.0 | 13 | 92.9 | 11 | 30.6 |
| Total | 368 | 10 | 14 | 36 | ||||
Abbreviations: GIST, gastrointestinal stromal tumor; KIT 8, mutation of KIT exon 8; WT, wild type (no mutation of KIT or PDGFRA).
Five patients were ineligible (3, KIT 11; 1, KIT 9; 1, PDGFRA 18).
All patients were otherwise eligible except for pathology review.
Two patients were ineligible (both with KIT 11 mutations).
Fig 1.
CONSORT diagram of Cancer and Leukemia Group B study 150105. GIST, gastrointestinal stromal tumor; Pos, positive.
Similar to previous reports, mutations in KIT exon 11 were the most common imatinib-target mutation found among the confirmed and unconfirmed CD117-positive GISTs (71.3% of patient cases), followed by mutations in KIT exon 9 (8.2%), KIT exon 13 (1.2%), PDGFRA exon 18 (1.2%), and KIT exon 17 (approximately 1%). One of 14 tumors judged to be a non-GIST sarcoma was found to have a PDGFRA mutation. On the basis of our experience and the published literature, intragenic PDGFRA gain-of-function mutations do not occur in other human sarcomas, so this was likely a GIST with unusual immunophenotypic (CD117-negative) and morphologic features.9,16-21 However, on the basis of central pathology review and the study protocol, this case was classified as a non-GIST sarcoma (ie, epitheloid malignancy, NOS). Notably, this tumor had epitheloid morphology and had no immunohistochemical staining for CD117, CD34, desmin, smooth muscle actin, S100, or cytokeratin.
Thirty-three patients had KIT exon 9 mutations, of which 31 were the usual AY502-503 internal tandem duplication that has been reported previously.8,22-24 However, two patients had variant exon 9 mutations. One was a tandem reduplication of codons 506 to 508 (FAF) after F508, which we have observed only once before in our series of greater than 1,500 genotyped GISTs.8 The second was a novel homozygous deletion of codons 484 to 487 (KHNG). Imatinib response for these two variant exon 9–mutant cases was not assessed, but the TTP was 10.6 and 46.9 months for the patients with the 506 to 508 FAF tandem duplication and the deletion KHNG 484 to 487, respectively. In addition, we found one GIST with a KIT exon 8 deletion/substitution (TYD417-419Y). The only previous report of an exon 8 mutation in GIST was in a familial GIST kindred (deletion codon 419).25 Germline DNA from surrounding normal tissue in our patient case was found to be WT; therefore, this patient represents the first example of a sporadic GIST with a KIT exon 8 mutation. The patient had an unconfirmed partial response to standard-dose imatinib (TTP, 8.1 months) and a censored OS of 59.3 months.
Eight patients had tumors with a PDGFRA exon 18 mutation. These mutations included the deletion/substitution IMHDS 843-847M (one patient case) and the deletion DIMH842-845 (three patient cases). As expected from in vitro data and previous clinical trials, the overall survival was more than 12 months for all four of these patients (mean 40.8 months).8,26 There were four patients whose tumors harbored the substitution D842V, which has in vitro resistance to imatinib, including the case classified as epitheloid malignancy, NOS.8,11,26 Three of these patients had a progression-free survival less than 2 months, while the fourth patient had not progressed as of 34 months of follow-up. The mean overall survival time was 9.7 months for these patients. A PDGFRA exon 12 V561D was found in the tumor from one patient, who had not progressed or died as of 31 months of follow-up.
Correlation of Tumor Genotype With Clinical Outcome (All Doses)
The primary objective of the correlative studies was to determine the effects of tumor genotype and/or imatinib dose on clinical outcome. For this analysis, we included all genotyped cases that met clinical eligibility criteria, except those that were categorized as CD117-negative or non-GIST sarcoma. The total was 397 of the 428 genotyped patients (Fig 1).
The best clinical response to imatinib was classified as complete response (CR), partial response (PR), stable disease (SD), PD (progressive disease), or not assessable (NA) using RECIST criteria. After patient cases with unknown response (NA) were omitted, patients whose tumor had a KIT exon 11 mutation were significantly more likely to achieve a CR/PR than patients whose tumor had a KIT exon 9 mutation (71.7% v 44.4%; P = .007; Table 2), or WT genotype (71.7% v 44.6%, P = .0002). There was no statistically significant difference in the likelihood of achieving a CR/PR for patients with KIT exon 9–mutant GIST compared with WT GIST (P = 1.00).
Table 2.
Tumor Genotype Versus Objective Clinical Response for All CD117+ Tumors
| Response | Genotype
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
KIT 8
|
KIT 9
|
KIT 11
|
WT
|
KIT 13
|
KIT 17
|
PDGFRA 12
|
PDGFRA 18
|
|||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| CR | 1 | 3.1 | 18 | 6.4 | 3 | 4.5 | 0 | 0.0 | ||||||||
| PR | 1 | 100.0 | 11 | 34.4 | 162 | 57.2 | 22 | 32.8 | 2 | 40.0 | 1 | 25.0 | 1 | 100.0 | 1 | 25.0 |
| SD | 12 | 37.5 | 53 | 18.7 | 19 | 28.4 | 1 | 20.0 | 2 | 50.0 | 2 | 50.0 | ||||
| PD | 3 | 9.4 | 18 | 6.4 | 12 | 17.9 | 1 | 20.0 | 1 | 25.0 | 1 | 25.0 | ||||
| NA | 5 | 15.6 | 32 | 11.3 | 11 | 16.4 | 1 | 20.0 | ||||||||
| Total | 1 | 32 | 283 | 67 | 5 | 4 | 1 | 4 | ||||||||
NOTE. Responses were reported for both confirmed and unconfirmed tumors from eligible patients.
Abbreviations: KIT 8, mutation of KIT exon 8; WT, wild type (no mutation of KIT or PDGFRA); CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; NA, not assessable.
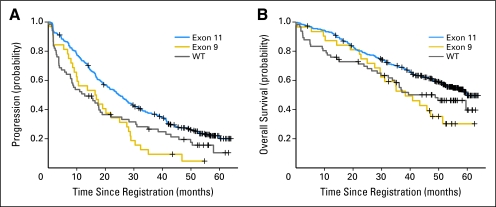
TTP and OS for the entire study were reported previously.14 There were no significant differences in TTP or OS between genotyped and nongenotyped patients (n = 299). Kaplan-Meier plots (Fig 2) demonstrated significantly longer TTP for patients whose GISTs contained a KIT exon 11 mutation compared with those whose GISTs had a KIT exon 9 mutation or no kinase mutation (ie, WT; P = .0013 and P = .005, respectively). In contrast, there was no significant difference in TTP between patients whose GIST had a KIT exon 9 mutation or WT genotype (P = .46). The median TTP was 24.7, 16.7, and 12.8 months for KIT exon 11–mutant, KIT exon 9–mutant, and WT GISTs, respectively.
Fig 2.
Correlation of gastrointestinal stromal tumor (GIST) genotype and time to progression or overall survival for patients with CD117-positive GISTs.
OS was analyzed for these GIST subgroups: median OS was 60.0, 38.4, and 49.0 months for KIT exon 11–mutant, KIT exon 9–mutant, and WT GISTs, respectively (Fig 2). Patients whose GIST had a KIT exon 11–mutant kinase had a significantly longer OS than patients whose GIST had an exon 9–mutation or no kinase mutation (ie, WT; P = .011 and P = .049, respectively). In contrast, there was no significance difference in OS for patients whose GISTs had a KIT exon 9 mutation compared with those who had WT genotype (P = .46).
Correlation of Tumor Genotype and Imatinib Dose With Clinical Outcome
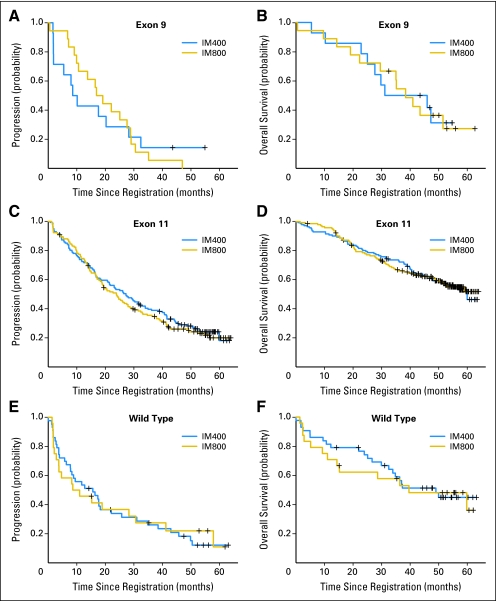
We examined whether there was any interaction of imatinib dose, GIST genotype, and clinical outcomes. There is borderline evidence that the degree of association between response and treatment arm depends on genotype (P = .05). In particular, patients with KIT exon–9 mutant GISTs had a significantly higher response rate when treated with IM800 compared with IM400 (CR/PR 17% v 67% for 400 mg and 800 mg, respectively; odds ratio [OR], 9.05; P = .02; Appendix Table A2, online only). In contrast, there were no differences in objective response rates for patient with KIT exon 11–mutant or WT GISTs treated with either dose of imatinib.
We also examined the effect of the assigned imatinib dose and genotype on TTP (Table 3; Fig 3). The differences between the treatment arms were not significant for the patients with KIT exon 11–mutant or WT GISTs (P = .53 and P = .94, respectively). Previously, Debiec-Rychter et al15 reported that patients who had KIT exon 9–mutant GIST had a significantly increased median TTP when treated with imatinib 800 mg compared with imatinib 400 mg. However, in this study, the difference in TTP between the two treatment arms was not significant (9.4 months and 18.0 months for 400 mg and 800 mg, respectively; P = .97).
Table 3.
Correlation of Imatinib Dose and Tumor Genotype With TTP and OS
| Genotype | Treatment Arm | No. of Patients | TTP (months) | OS (months) |
|---|---|---|---|---|
| Exon 11 | IM400 | 141 | 27.2 | 60.0 |
| Exon 11 | IM800 | 142 | 23.9 | NR |
| KIT exon 9 | IM400 | 14 | 9.4 | 38.6 |
| KIT exon 9 | IM800 | 18 | 18.0 | 38.4 |
| WT | IM400 | 43 | 15.6 | 49.0 |
| WT | IM800 | 24 | 9.8 | 39.5 |
Abbreviations: TTP, time to progression; OS, overall survival; IM400, imatinib 400 mg daily; IM800, imatinib 800 mg daily; NR, not reached; WT, wild type.
Fig 3.
Correlation of tumor genotype (KIT exon 9–mutant, KIT exon 11–mutant, or wild-type tumors), imatinib dose (400 mg [IM400] v 800 mg [IM800]), and time to progression and overall survival for patients with CD117-positive gastrointestinal stromal tumors.
Similarly, the assigned imatinib dose did not affect OS for these three subgroups of patients with GISTs (P = .99 for exon 11, P = .91 for exon 9, and P = .78 for WT, respectively). The median OS for patients with GISTs who had KIT exon 11 mutations was 60 months and has not yet been reached for the 800-mg imatinib dose. The median OS for patients with GISTs who had KIT exon 9 mutations was 38.6 and 38.4 months for doses of 400 mg and 800 mg, respectively. The median OS for patients who had WT GISTs was 49.0 and 39.5 months for doses of 400 gm and 800 mg, respectively.
Among the 382 patients who had KIT exon 11 or exon 9 mutations or WT genotype, the following cofactors were identified in univariate analyses as statistically significant with respect to TTP: KIT/PDGFRA WT genotype, KIT exon 9 mutation, Zubrod performance status, absolute neutrophil count, and hemoglobin. In multivariate analysis, patients who had KIT exon 9 –mutant or WT genotypes had inferior TTP; hazard ratios were 2.07 (P = .0008) and 1.85 (P = .0002), respectively (Appendix Table A3, online only). Patients who were men and who had a performance status of 2 to 3 also had shorter TTP.
In univariate analyses, KIT exon 9 mutation, KIT/PDGFRA WT genotype, age, sex, performance status, baseline hemoglobin, baseline absolute neutrophil count, and primary tumor site were significantly associated with worse OS (Appendix Table A4, online only). In multivariate analysis, KIT exon 9 genotype, KIT/PDGFRA WT genotype, male sex, increased age, performance status of 2 to 3, increased absolute neutrophil count, and lower hemoglobin were significantly associated with worse OS. Notably, the European Organisation for Research and Treatment of Cancer (EORTC) study did not find an association of male sex and worsened survival outcomes.15,28
CD117-Negative GISTs
To date, all phase I through III studies of imatinib for the treatment of advanced GIST have required that patients have CD117-positive tumors. At the time that these clinical studies were designed (2000 to 2001), it was widely believed that all GISTs were positive for this marker.5,6,13,14 As a consequence, regulatory approval around the world for the use of imatinib in GIST treatment has been limited to CD117-positive tumors. It is now established that 2% to 5% of all GISTs are CD117-negative; many of these harbor a PDGFRA mutation.17,18,19,21 To date, only anecdotal case reports have described clinical outcomes of patients with CD117-negative GISTs who are treated with imatinib.27 Thirteen patient cases in our trial were confirmed on central review to be CD117-negative GISTs. Genotyping was performed on 10 of these patient cases, which represents the largest group of such tumors for which imatinib treatment outcomes are available.
Consistent with previous studies, kinase mutations were identified in eight of the 10 CD117-negative patient cases,17,18 and KIT exon 11 mutations were present in six of these patient cases. The apparent absence of CD117 staining in these patient cases could reflect false-negative immunohistochemistry as a consequence of poor tumor fixation. Alternatively, the levels of KIT protein in these tumors may have been sufficient for oncogenic signal transduction but may have been less than the limit of detection by standard immunohistochemistry. Two of the CD117-negative patient cases contained PDGFRA exon 18 mutations (D842V and deletion IMHD842-846). Four of the six patients with KIT exon 11–mutant GISTs had CR (n = 1) or PR (n = 3) as the best objective response to therapy. The remaining two patients had SD or were nonassessable for response, respectively. None of the four patients with PDGFRA-mutant or WT CD117-negative GISTs had objective responses (PD, n = 3; NA, n = 1).
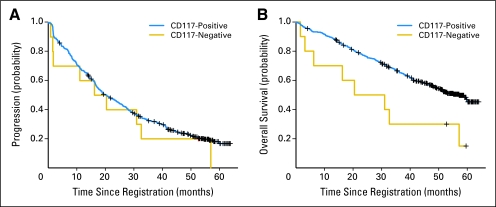
The TTP and OS of patients with CD117-negative GIST were compared with our main study population of CD117-positive tumors (Fig 4). The median TTP for CD117-negative GISTs was 18.3 months versus 20.5 months for CD-117 positive GISTs (P = .46). The median OS for the genotyped CD117-negative GISTs was 25.8 months versus 57.1 months for CD117-positive GISTs (P = .01). The median TTP and OS for the exon 11–mutant, CD117-negative GISTs were 31.9 and 44.9 months, respectively. These results are comparable to those seen for KIT exon 11–mutant, CD117-positive GISTs: 24.7 and 60.0 months, respectively (P = .81 and .42 for TTP and OS, respectively).
Fig 4.
Comparison of time to progression and overall survival for patients with CD117-positive and CD117-negative gastrointestinal stromal tumors.
DISCUSSION
This prospective biomarker study of 397 patients who had genotyped CD117-positive tumors represents the largest genotyped collection of patients with GIST enrolled on a clinical study. The North American intergroup phase III trial was written in conjunction with another international phase III study (EORTC 62005) to determine the optimal imatinib dose for treating patients who have advanced GIST. Results from the EORTC trial have been published and will be compared with our current results.13,15 The frequency and spectrum of KIT and PDGFRA mutations that were identified match well with the EORTC phase III trial and with other series. Similar to previous studies, these results confirm the favorable impact of the KIT exon 11 genotype on the response to imatinib therapy compared with GISTs that have KIT exon 9–mutant or WT genotypes. This is evidenced by the following: superior objective CR/PR rates (71.7%, 44.4%, and 44.6% for KIT exon 11, KIT exon 9, and WT, respectively); superior TTP (median 24.7, 16.7, and 12.8 months for KIT exon 11, KIT exon 9, and WT, respectively); and superior OS (median 60.0, 38.4, and 49.0 months for KIT exon 11, KIT exon 9, and WT, respectively). There was no significant difference in OS between patients whose tumors had a KIT exon 9–mutant or a WT genotype.
This study also addressed the relationship between GIST genotype, imatinib dose, and treatment outcome. Patients with KIT exon 9–mutant GISTs who were treated with imatinib 800 mg had a higher objective response rate compared with patients who were treated with imatinib 400 mg. In contrast, there was no difference in objective response rates for patient with KIT exon 11–mutant or WT GISTs who were treated with either dose of imatinib. However, there was no significant difference in TTP or OS between the two dose groups for any of the three largest genotype groups (ie, KIT exon 11–mutant, KIT exon 9–mutant, or WT). Multivariate analyses showed that GIST genotype significantly impacted TTP and OS. Other variables with significant impact included sex, patient age, and Zubrod performance status. Potentially, male sex might influence response to imatinib through pharmacokinetic (eg, body mass) and/or hormonal mechanisms.28
The EORTC study reported a significant improvement in TTP, but not OS, for patients with KIT exon 9–mutant GISTs who were treated with high-dose imatinib. We did not confirm this finding. However, there were 58 patients with KIT exon 9–mutant GISTs in the EORTC study15 and only 32 in this study; this study is likely underpowered for detection of an impact of dose on TTP for this subgroup of patients with GISTs. In support of this hypothesis, the median TTP for patients with KIT exon 9–mutant GISTs who were treated with standard-dose imatinib in this study was 9.4 months compared with 18.0 months for patients who were treated with high-dose imatinib. Nine patients with KIT exon 9–mutant GISTs crossed over from the 400-mg to the 800-mg treatment arm at the time of progression, which potentially obscured any effect of dose on OS of patients with KIT exon 9–mutant GISTs. For patients with KIT exon 11–mutant or WT GISTs, our results agree with those of the EORTC report, which thus confirms that there is no effect of dose on objective response, TTP, and OS in these tumors.
The relatively large size of our treatment cohort allowed us to study clinical outcomes for genotyped CD117-negative patient cases. The TTP for this group was similar to that of CD117-positive patient cases, but OS was significantly shorter. Despite this, comparison of these results with historical chemotherapy outcome data13 suggests that patients with CD117-negative GISTs, especially those with a KIT exon 11 mutation, may benefit from imatinib treatment.
From their inception, results from the North American and the EORTC phase III trials were intended to be integrated into a common data set for a prospectively planned combined analysis. This analysis will include the integration of clinical and molecular data; analysis of this combined data set should additionally define the relationship between genotype, imatinib dose, and clinical outcome of patients with advanced GIST who are treated with imatinib.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Michael C. Heinrich, Novartis (C), MolecularMD (U), Pfizer Inc (C); Christopher L. Corless, Novartis Inc (U); Christopher W. Ryan, Novartis Inc (C), Pfizer Inc (C); Margaret von Mehren, Novartis Inc (C); Robert S. Benjamin, Novartis Inc (C); George D. Demetri, Novartis Inc (C), Pfizer Inc (C), Infinity Pharmaceuticals (C) Stock Ownership: Michael C. Heinrich, MolecularMD Honoraria: Michael C. Heinrich, Novartis Inc, Pfizer Inc; Christopher L. Corless, Novartis Inc; Christopher W. Ryan, Novartis Inc, Pfizer Inc; Margaret von Mehren, Novartis Inc; Robert S. Benjamin, Novartis Inc; George D. Demetri, Novartis Inc, Pfizer Inc Research Funding: Michael C. Heinrich, Novartis Inc, Pfizer Inc; Christopher L. Corless, Novartis Inc; Christopher W. Ryan, Novartis Inc, Pfizer Inc; Margaret von Mehren, Novartis Inc; Charles D. Blanke, Novartis Inc; Robert S. Benjamin, Novartis Inc; George D. Demetri, Novartis Inc, Pfizer Inc, Infinity Pharmaceuticals Expert Testimony: George D. Demetri, Novartis Inc (U), Pfizer Inc (U), Infinity Pharmaceuticals (U) Other Remuneration: Robert S. Benjamin, Novartis Inc
AUTHOR CONTRIBUTIONS
Conception and design: Michael C. Heinrich, Christopher L. Corless, Ernest C. Borden, Christopher D.M. Fletcher, Christopher W. Ryan, Margaret von Mehren, Charles D. Blanke, Cathryn Rankin, Robert S. Benjamin, Vivien H. Bramwell, George D. Demetri, Monica M. Bertagnolli, Jonathan A. Fletcher
Financial support: Michael C. Heinrich, Kouros Owzar, Christopher L. Corless, Ernest C. Borden, Cathryn Rankin, Monica M. Bertagnolli
Administrative support: Michael C. Heinrich, Donna Hollis, Ernest C. Borden, Christopher W. Ryan, Margaret von Mehren, Charles D. Blanke, Cathryn Rankin, Robert S. Benjamin, George D. Demetri, Monica M. Bertagnolli, Jonathan A. Fletcher
Provision of study materials or patients: Michael C. Heinrich, Christopher L. Corless, Ernest C. Borden, Christopher D.M. Fletcher, Christopher W. Ryan, Margaret von Mehren, Charles D. Blanke, Robert S. Benjamin, Vivien H. Bramwell, George D. Demetri, Monica M. Bertagnolli
Collection and assembly of data: Michael C. Heinrich, Kouros Owzar, Christopher L. Corless, Donna Hollis, Christopher D.M. Fletcher, Charles D. Blanke, Cathryn Rankin, Vivien H. Bramwell, George D. Demetri, Jonathan A. Fletcher
Data analysis and interpretation: Michael C. Heinrich, Kouros Owzar, Christopher L. Corless, Donna Hollis, Christopher D.M. Fletcher, Charles D. Blanke, Cathryn Rankin, George D. Demetri, Monica M. Bertagnolli, Jonathan A. Fletcher
Manuscript writing: Michael C. Heinrich, Kouros Owzar, Christopher L. Corless, Donna Hollis, Ernest C. Borden, Christopher D.M. Fletcher, Christopher W. Ryan, Margaret von Mehren, Charles D. Blanke, Robert S. Benjamin, Vivien H. Bramwell, George D. Demetri, Monica M. Bertagnolli, Jonathan A. Fletcher
Final approval of manuscript: Michael C. Heinrich, Kouros Owzar, Christopher L. Corless, Donna Hollis, Ernest C. Borden, Christopher D.M. Fletcher, Christopher W. Ryan, Margaret von Mehren, Charles D. Blanke, Cathryn Rankin, Robert S. Benjamin, Vivien H. Bramwell, George D. Demetri, Monica M. Bertagnolli, Jonathan A. Fletcher
Appendix
Genotyping methods:
Mutational analyses were performed on genomic DNA extracted from paraffin-embedded or fresh frozen tumor tissue by using a combination of polymerase chain reaction (PCR) amplification, denaturing high-performance liquid chromatography (D-HPLC) screening, and automated sequencing, as described previously (Corless CL, McGreevey L, Town A, et al: J Mol Diagn 6:366-370, 2004).8,26 Experiments that involved recombinant DNA were performed by using Biosafety Level 2 safety conditions in accordance with National Institutes of Health Guidelines for Research Involving Recombinant DNA Molecules (http://www4.od.nih.gov/oba/rac/guidelines/guidelines.html). PCR primer pairs and D-HPLC conditions are listed in Appendix Table A1. Tumors that lacked mutation in either gene (ie, no mutations in KIT exons 9, 11, 13, or 17 or in PDGFRA exons 12, 14, or 18) were classified as wild-type (WT) genotypes.9,15,26
Statistical methods.
Two time-to-event end points were considered: overall survival (OS) and time to tumor progression (TTP). The reference time for both was the time of random assignment. For TTP, death as a result of any cause was considered an event. Patients who were lost to follow-up were censored with respect to TTP if there was no evidence of progression on or before the dropout time. Objective response was defined as complete response (CR; confirmed or unconfirmed) plus partial response (PR; confirmed or unconfirmed) compared with all assessed patients (ie, excluded nonassessable ([NA] patients). Discrepancies among time-to-event distributions with respect to the exon itself (9, 11, or WT) were investigated by using the log-rank test, whereas discrepancies among response probabilities with respect to the exon mutation status were investigated by using Fisher's test. The interaction of tumor genotype and treatment arm (standard- v high-dose imatinib) with respect to the clinical outcomes was investigated in a framework of two-way, multiplicative, log-linear Cox and log-linear logistic models. Multivariate analyses were carried out more generally in the framework of additive log-linear Cox and logistic models. Odds ratios were estimated by using conditional maximum likelihood. Survival functions were estimated by using a Kaplan-Meier estimator (Cox DR, Snell EJ: Analysis of Binary Data. Capman & Hall/CRC, 1989; Klabfleisch JD, Prentice RL: The Statistical Analysis of Failure Time Data. John Wiley & Sons, 2002).
Statistical analysis was performed by statisticians at the Cancer and Leukemia Group B (CALGB) Statistical Center by using the R statistical environment (version 2.6.1; R Foundation for Statistical Computing, University of Auckland, Auckland, New Zealand; R Development Core Team: A language and environment for statistical computing, 2007. http://www.r-project.org/). Patients were registered and were randomly assigned to the treatment trial, and clinical data were collected and managed by the Southwest Oncology Group Statistical Center. Tumor samples were received and managed by the CALGB Pathology Coordinating Office. All data were frozen on September 14, 2006.
Table A1.
Primer and D-HPLC Conditions Used for KIT and PDGFRA Genotyping Studies
| Exon | Forward Primer | Reverse Primer | D-HPLC Temperatures (°C) |
|---|---|---|---|
| K8 | GCTGAGGTTTTCCAGCACTC | AATTGCAGTCCTTCCCCTCT | 50.0 |
| K9 | ATGCTCTGCTTCTGTACTGCC | CAGAGCCTAAACATCCCCTTA | 50.0 |
| K11 | CCAGAGTGCTCTAATGACTG | ACCCAAAAAGGTGACATGGA | 50.0/56.2 |
| K13 | CATCAGTTTGCCAGTTGTGC | ACACGGCTTTACCTCCAATG | 59.5 |
| K17 | TGTATTCACAGAGACTTGGC | GGATTTACATTATGAAAGTCACAGG | 58.0 |
| P12 | TCCAGTCACTGTGCTGCTTC | GCAAGGGAAAAGGGAGTCTT | 50.0/59.7 |
| P14 | TGGTAGCTCAGCTGGACTGAT | GGGATGGAGAGTGGAGGATT | 59.1 |
| P18 | ACCATGGATCAGCCAGTCTT | TGAAGGAGGATGAGCCTGAC | 50/61.6 |
Abbreviation: D-HPLC, denaturing high-performance liquid chromatography.
Table A2.
Correlation of Imatinib Dose, GIST Genotype, and Objective Response Rate
| Genotype | Imatinib Dose (%)
|
Analysis
|
|||
|---|---|---|---|---|---|
| 400 mg | 800 mg | OR | 95% CI | P | |
| KIT exon 9 | 17 | 67 | 9.05 | 1.24 to 116.74 | .02 |
| KIT exon 11 | 71 | 72 | 1.05 | 0.59 to 1.90 | .89 |
| WT | 42 | 50 | 1.39 | 0.40 to 4.83 | .59 |
NOTE. Objective response rate includes complete response and partial response rates.
Abbreviations: GIST, gastrointestinal stromal tumor; OR, odds ratio; WT, wild type.
Table A3.
Univariate and Multivariate Analysis of Cofactors Associated With TTP
| Cofactor | Analysis of Progression-Free Survival
|
|||||
|---|---|---|---|---|---|---|
| Univariate
|
Multivariate
|
|||||
| P | HR | 95% CI | P | HR | 95% CI | |
| KIT mutation | ||||||
| Exon 9 | .0022 | 1.82 | 1.24 to 2.68 | .0008 | 2.07 | 1.35 to 3.16 |
| WT | .0051 | 1.53 | 1.14 to 2.06 | .0002 | 1.85 | 1.34 to 2.56 |
| Treatment* | .69 | 1.05 | 0.83 to 1.32 | .18 | 1.18 | 0.93 to 1.50 |
| Sex, male | .06 | 1.25 | 0.99 to 1.57 | .007 | 1.41 | 1.10 to 1.82 |
| Age, by decades | .78 | 0.99 | 0.90 to 1.08 | .74 | 1.02 | 0.93 to 1.11 |
| Zubrod performance† | 1.6 × 10−6 | 2.14 | 1.57 to 2.92 | 8.1 × 10−5 | 2.02 | 1.42 to 2.87 |
| ANC | .0061 | 1.07 | 1.02 to 1.12 | .10 | 1.04 | 0.99 to 1.10 |
| HGB | .0061 | 0.92 | 0.86 to 0.98 | .21 | 0.96 | 0.89 to 1.03 |
| Primary tumor type‡ | .61 | 1.06 | 0.84 to 1.35 | .19 | 1.19 | 0.92 to 1.54 |
Abbreviations: TTP, time to progression; HR, hazard ratio; WT, wild type; ANC, absolute neutrophil count; HGB, hemoglobin.
Treatment of imatinib 400 mg/d v 800 mg/d.
Performance score of 0 to 1 v 2 to 3.
Gastric v nongastric tumor type.
Table A4.
Univariate and Multivariate Analysis of Cofactors Associated With OS
| Cofactor | Analysis of OS
|
|||||
|---|---|---|---|---|---|---|
| Univariate
|
Multivariate
|
|||||
| P | HR | 95% CI | P | HR | 95% CI | |
| KIT mutation | ||||||
| Exon 9 | .014 | 1.79 | 1.13 to 2.85 | .002 | 2.28 | 1.36 to 3.84 |
| WT | .049 | 1.46 | 1.00 to 2.13 | .0003 | 2.08 | 1.40 to 3.10 |
| Treatment* | .96 | 1.01 | 0.75 to 1.35 | .65 | 1.07 | 0.79 to 1.46 |
| Sex, male | .035 | 1.39 | 1.02 to 1.87 | .003 | 1.65 | 1.18 to 2.25 |
| Age, by decades | .00038 | 1.23 | 1.09 to 1.39 | .00006 | 1.28 | 1.14 to 1.45 |
| Zubrod performance† | 9 × 10−13 | 3.63 | 2.55 to 5.17 | 8.6 × 10−9 | 3.40 | 2.24 to 5.15 |
| ANC | 7.5 × 10−9 | 1.16 | 1.11 to 1.23 | .044 | 1.07 | 1.00 to 1.14 |
| HGB | 7.6 × 10−5 | 0.84 | 0.77 to 0.92 | .023 | 0.90 | 0.82 to 0.99 |
| Primary tumor type‡ | .65 | 1.07 | 0.79 to 1.46 | .11 | 1.31 | 0.94 to 1.82 |
Abbreviations: OS, overall survival; HR, hazard ratio; WT, wild type; ANC, absolute neutrophil count; HGB, hemoglobin
Treatment of imatinib 400 mg/d v 800 mg/d.
Performance score of 0 to 1 v 2 to 3.
Gastric v nongastric tumor type.
published online ahead of print at www.jco.org on October 27, 2008
Supported in part by Grants No. CA33601, CA04919, CA32291, CA27525, CA31946, CA32102, and CA77202 from the National Cancer Institute (NCI); by Grants No. U01-CA70172-01 and N01-CM-17003 from the NCI; by a Veterans Affairs Merit Review Grant (M.H.); and by research funding from Novartis Pharmaceuticals.
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Authors‘ disclosures of potential conflicts of interest are found at the end of this article.
REFERENCES
- 1.Hirota S, Isozaki K, Moriyama Y, et al: Gain-of-function mutations of c-KIT in human gastrointestinal stromal tumors. Science 279:577-580, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Heinrich MC, Griffith DJ, Druker BJ, et al: Inhibition of c-kit receptor tyrosine kinase activity by STI 571, a selective tyrosine kinase inhibitor. Blood 96:925-932, 2000 [PubMed] [Google Scholar]
- 3.Tuveson DA, Willis NA, Jacks T, et al: STI571 inactivation of the gastrointestinal stromal tumor c-KIT oncoprotein: Biological and clinical implications. Oncogene 20:5054-5058, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Joensuu H, Roberts PJ, Sarlomo-Rikala M, et al: Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med 344:1052-1056, 2001 [DOI] [PubMed] [Google Scholar]
- 5.van Oosterom AT, Judson I, Verweij J, et al: Safety and efficacy of imatinib (STI571) in metastatic gastrointestinal stromal tumours: A phase I study. Lancet 358:1421-1423, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Demetri GD, von Mehren M, Blanke CD, et al: Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 347:472-480, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Blanke CD, Demetri GD, von Mehren M, et al: Long-term results from a randomized phase II trial of standard versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol 26:620-625, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Heinrich MC, Corless CL, Demetri GD, et al: Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol 21:4342-4349, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Corless CL, Fletcher JA, Heinrich MC: Biology of gastrointestinal stromal tumors. J Clin Oncol 22:3813-3825, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Heinrich MC, Corless CL, Duensing A, et al: PDGFRA activating mutations in gastrointestinal stromal tumors. Science 299:708-710, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Hirota S, Ohashi A, Nishida T, et al: Gain-of-function mutations of platelet-derived growth factor receptor α gene in gastrointestinal stromal tumors. Gastroenterology 125:660-667, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Debiec-Rychter M, Dumez H, Judson I, et al: Use of c-KIT/PDGFRA mutational analysis to predict the clinical response to imatinib in patients with advanced gastrointestinal stromal tumours entered on phase I and II studies of the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer 40:689-695, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Verweij J, Casali PG, Zalcberg J, et al: Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: Randomised trial. Lancet 364:1127-1134, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Blanke CD, Rankin C, Demetri GD, et al: Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol 26:626-632, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Debiec-Rychter M, Sciot R, Le CA, et al: KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur J Cancer 42:1093-1103, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Sihto H, Sarlomo-Rikala M, Tynninen O, et al: KIT and platelet-derived growth factor receptor alpha tyrosine kinase gene mutations and KIT amplifications in human solid tumors. J Clin Oncol 23:49-57, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Medeiros F, Corless CL, Duensing A, et al: KIT-negative gastrointestinal stromal tumors: Proof of concept and therapeutic implications. Am J Surg Pathol 28:889-894, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Debiec-Rychter M, Wasag B, Stul M, et al: Gastrointestinal stromal tumours (GISTs) negative for KIT (CD117 antigen) immunoreactivity. J Pathol 202:430-438, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Miettinen M, Sobin LH, Lasota J: Gastrointestinal stromal tumors of the stomach: A clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol 29:52-68, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Miettinen M, Lasota J: Gastrointestinal stromal tumors: Review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med 130:1466-1478, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Kontogianni-Katsarou K, Lariou C, Tsompanaki E, et al: KIT-negative gastrointestinal stromal tumors with a long term follow-up: A new subgroup does exist. World J Gastroenterol 13:1098-1102, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lasota J, Kopczynski J, Sarlomo-Rikala M, et al: KIT 1530ins6 mutation defines a subset of predominantly malignant gastrointestinal stromal tumors of intestinal origin. Hum Pathol 34:1306-1312, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Lux ML, Rubin BP, Biase TL, et al: KIT extracellular and kinase domain mutations in gastrointestinal stromal tumors. Am J Pathol 156:791-795, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakurai S, Oguni S, Hironaka M, et al: Mutations in c-KIT gene exons 9 and 13 in gastrointestinal stromal tumors among Japanese. Jpn J Cancer Res 92:494-498, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hartmann K, Wardelmann E, Ma Y, et al: Novel germline mutation of KIT associated with familial gastrointestinal stromal tumors and mastocytosis. Gastroenterology 129:1042-1046, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Corless CL, Schroeder A, Griffith D, et al: PDGFRA mutations in gastrointestinal stromal tumors: Frequency, spectrum and in vitro sensitivity to imatinib. J Clin Oncol 23:5357-5364, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Bauer S, Corless CL, Heinrich MC, et al: Response to imatinib mesylate of a gastrointestinal stromal tumor with very low expression of KIT. Cancer Chemother Pharmacol 51:261-265, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Van Glabbeke M, Verweij J, Casali PG, et al: Initial and late resistance to imatinib in advanced gastrointestinal stromal tumors are predicted by different prognostic factors: A European Organisation for Research and Treatment of Cancer-Italian Sarcoma Group-Australasian Gastrointestinal Trials Group study. J Clin Oncol 23:5795-5804, 2005 [DOI] [PubMed] [Google Scholar]