Abstract
Larval competition is common in container-breeding mosquitoes. The impact of competition on larval growth has been thoroughly examined and findings that larval competition can lead to density-dependent effects on adult body size have been documented. The effects of larval competition on adult longevity have been less well explored. The effects of intraspecific larval densities on the longevity of adults maintained under relatively harsh environmental conditions were tested in the laboratory by measuring the longevity of adult Aedes aegypti (L.) and Aedes albopictus (Skuse) (Diptera: Culicidae) that had been reared under a range of larval densities and subsequently maintained in high- or low-humidity regimes (85% or 35% relative humidity [RH], respectively) as adults. We found significant negative effects of competition on adult longevity in Ae. aegypti, but not in Ae. albopictus. Multivariate analysis of variance suggested that the negative effect of the larval environment on the longevity of Ae. aegypti adults was most strongly associated with increased development time and decreased wing length as adults. Understanding how larval competition affects adult longevity under a range of environmental conditions is important in establishing the relationship between models of mosquito population regulation and epidemiological models of vector-borne disease transmission.
Keywords: Aedes aegypti, Aedes albopictus, adult environment, dengue, desiccation, larval environment, longevity, mosquito life history
Introduction
The effects of competition on mosquitoes that breed in containers and tree-holes are well documented, with findings showing negative density-dependent effects on population growth, individual growth, individual fecundity, survival to adulthood and developmental time (Ho et al., 1989; Walker et al., 1991; Juliano, 1998; Lounibos et al., 2001). In addition, intense larval competition may ultimately affect vector - parasite interactions in adult mosquitoes (Alto et al., 2005, 2008; Tseng, 2006). Despite the importance of adult longevity as a parameter in mathematical models of disease transmission (Dye, 1986; Luz et al., 2003), the impact of larval competition on adult longevity has not been well studied.
Field studies have noted a positive correlation between female body size, which is presumably influenced by larval nutrition and competition, and parity status. This finding is interpreted to mean that larger mosquitoes have greater blood-feeding success, possibly as a result of increased longevity (Haramis, 1983; Hawley, 1985; Nasci, 1986). In previous studies, reduced larval food resulted in decreased longevity of Aedes triseriatus (Say) exposed to harsh conditions (Haramis, 1985) and decreased longevity and fecundity of Aedes aegypti (L.) under benign conditions (Steinwascher, 1982). However, mark-release-recapture studies with Ae. triseriatus and Aedes hendersoni Cockerell have suggested no difference in the estimated longevity of individuals derived from low vs. high larval food regimes, although smaller individuals were less likely to be recaptured (Walker et al., 1987). Similar mark-release-recapture studies in Brazil with Ae. aegypti documented higher survival rates with high vs. low larval food regimes in adult males, but not females (Maciel de Freitas et al., 2007). This same study found no differences in longevity between mosquitoes with high vs. low food regimes in the laboratory under benign conditions of high humidity (80-85% relative humidity [RH]) and sucrose ad libitum.
Several laboratory studies have noted species-specific differences in adult longevity under low-humidity conditions. Mogi et al. (1996) documented differences in resistance to desiccation in sympatric Aedes species of the subgenus Stegomyia, with Ae. aegypti having the highest desiccation resistance, followed by Aedes albopictus and then Aedes paullusi Stone and Farner, a forest species (1996). Mogi et al. (1996) also demonstrated significant intraspecific variation in desiccation resistance in Ae. albopictus (Skuse). As Ae. aegypti and Ae. albopictus are day-biting mosquitoes, resistance to desiccation may be an important determinant of population performance, blood-feeding success and vectorial capacity (Christophers, 1960; Hawley, 1988). Others have found decreased longevity for Ae. aegypti compared with Ae. albopictus under starvation conditions (no blood or sugar), but with high humidity and water ad libitum (Klowden & Chambers, 1992). Recent research has noted differences in longevity between two species of Anopheles under low-humidity conditions, primarily attributable to the water content of adults at emergence, although larval environment was not considered in this study (Gray & Bradley, 2005). Gimnig et al. (2002) showed that density-dependent larval interactions in Anopheles gambiae Giles s.l. can lead to significant differences in size and larval survivorship, which may influence adult longevity.
To examine the interaction between larval competition and adult longevity, we tested the hypothesis that competition negatively affects adult longevity. We predicted that individuals from more competitive environments (as determined by higher initial larval densities) would have decreased longevity as adults, and that this difference would be more pronounced for adults maintained under conditions of lower humidity. To examine this prediction, we conducted a laboratory experiment with two species of medically important mosquitoes from wild populations of Ae. aegypti and Ae. albopictus collected in Florida, U.S.A.
Materials and methods
Mosquitoes
Mosquitoes used in this experiment were F1 progeny of individuals collected as eggs, larvae and pupae in Palm Beach County, Florida. The original collection consisted of ~ 500 Ae. aegypti and ~ 1500 Ae. albopictus males and females.
Larval treatments
Three intraspecific larval densities of each species were established based on previous work in our laboratory (Alto et al., 2005). These comprised densities of 25 (low), 50 (medium) and 100 (high) first instar larvae in 0.75 L of water in enamelled larval rearing pans (18 × 30 × 4.5 cm). To provide nutrients for larvae, larval rearing water was infused with oak leaves Quercus viriginiana [Fagaceae]) at a proportion of 4 g/L for 3 days (leaves removed) and 0.025 g/L of 1: 1 yeast: albumin. Larvae were allowed to grow under a constant temperature (28 °C) and day length (light: dark [LD] 14 : 10 h) until all individuals pupated, died or 30 days was reached. Each density treatment was replicated six times for each species.
Adults
Pupae were sexed and each individual female pupa was put into a 37-mL plastic tube with approximately 5 mL of tap water and allowed to emerge. Males were discarded. Emerged adult females were individually maintained for 3 days after emergence with water and 20% sucrose ad libitum, in 37-mL plastic tubes (height × diameter: 8 × 3 cm) covered with a screen, at a mean of 86% (standard deviation [SD] ±6.4% RH, 28 °C and LD 14 : 10 h. After 3 days, water and sugar were removed and each tube was assigned to either a `low' humidity adult treatment (27 : 22 °C, LD 14 : 10 h, 35 ± 1.1% RH) or a `high' humidity adult treatment (27 : 22 °C, LD 14 : 10 h, 85 ± 1.7% RH) in separate incubators with factory-installed humidity controls (Percival Scientific Corp., Perry, IA, U.S.A.). Humidity and temperature were monitored with HOBO© data loggers (Onset Computer Corp., Pocasset, MA, U.S.A.). Assignments to high- or low-humidity treatments were made alternately so that there were approximately equal numbers of adults from each density group in each humidity treatment (Table 1). Once placed in the incubator, mosquitoes were deprived of water and sucrose, and longevity was recorded. Differences in the survival rate of pupae to emergence for each of the six replicates led to variable numbers of females available from each replicate (Table 1). No females emerged from two replicates of the high-density Ae. albopictus treatment, which were removed from the analysis of adult longevity, but retained for determining lambda (λ) (see below). In all other cases, at least two females from each replicate survived to be assigned to each of the humidity treatments.
Table 1.
Average number of adult females per replicate for each longevity treatment.
| High humidity, adult females |
Low humidity, adult females |
|||||
|---|---|---|---|---|---|---|
| Species | Larvalde nsity | Mean | Total | Mean | Total | No. of replicates tested |
| Aedes albopictus | 25 | 4.2 | 25 | 4.0 | 24 | 6 |
| 50 | 5.0 | 30 | 5.8 | 35 | 6 | |
| 100 | 2.0 | 8 | 1.8 | 7 | 4 | |
| Aedes aegypti | 25 | 3.7 | 22 | 4.3 | 26 | 6 |
| 50 | 6.0 | 36 | 5.5 | 33 | 6 | |
| 100 | 5.0 | 30 | 5.0 | 30 | 6 | |
Recording of outcome variables
Several variables were measured for each individual mosquito: longevity in days as adults; time in days from hatch to adult emergence, and wing length in mm measured under a dissecting scope using visualization software (iSolution lite; IMT i-Solution, Inc., Vancouver, BC, Canada). After the initial 3-day holding period (with sugar and water ad libitum), during which any dead adults were removed from the analysis of longevity, adults were checked every 8 h for the following 10 days of the experiment, and then every 12 h until all mosquitoes had died. To keep our estimates conservative, longevity was defined by the most recent previous time-point at which the individual had been observed alive. Longevity is given in days after the initial 3-day holding period (number of hours out of 24 alive). As we were interested in the effects of intraspecific density on longevity, each species × density replicate is the unit of analysis. Therefore, for each density × species replicate, the variables measured were: average longevity under either high or low humidity, percent larval survival to adulthood, average wing length, average time to emergence and an estimate of finite rate of population growth (λ), calculated using the formula and parameters presented in Braks et al. (2004) for these two species. In some cases only a few females (or, in one case, a single female) were used to estimate average longevity for high and low humidities for a given replicate. Although the low number of individuals may increase the amount of variation between replicates, it does not violate the assumptions of our statistical test. Therefore, the increase in variation is unbiased and conservative. The two replicates without any females were considered to have a λ of 0. Wing lengths were not measured in up to five females from each replicate (average = 0.67) (when wings were destroyed, or specimens were lost or emerged too late to be used in the experiment). Instead of removing these individuals from the dataset, the average wing length for the species across all treatments was used as the most conservative estimate of wing length to calculate λ (this estimate would bias towards seeing no treatment effect).
Statistical analysis
All statistics were analysed using SAS Version 9.1 (SAS Institute, Inc., Cary, NC, U.S.A.). Following the recommendation of Scheiner (2001), two-way multivariate analysis (MANOVA) was used to examine the effects of larval density and species on all outcomes from each replicate simultaneously, except λ, as it is a composite index made up of other outcomes. Pillai's trace was used to assess significance because it is robust to departures from normality of individual response variables (e.g. average days to emergence). Standardized canonical coefficients (SCCs) were used to examine the importance of each variable on the main effects of density and species. Significant two-way MANOVAS were followed by one-way MANOVAS examining density effects for each species separately and then one-way ANOVAS on each outcome. As time to emergence was not normally distributed, a Kruskal-Wallis rank test was used in lieu of ANOVA, employing a Mann-Whitney U-test with Bonferroni's correction of alpha (α = 0.05/number of comparisons) to determine significant pairwise differences (Sokal & Rohlf, 1995). To determine how variables strictly associated with the larval environment (proportion emerged, average days to emergence and wing length) correlated with adult characteristics (longevity under high and low humidities), we conducted a canonical correspondence analysis (CCA) using the three larval variables as predictors of adult longevity for the two humidity treatments on both species and for each species separately (Quinn & Keogh, 2002; Sherry & Henson, 2005). This approach allowed us to examine which larval characteristics might be most important in determining adult longevity. Following the recommendations of Sherry & Henson (2005), we evaluated overall significance with Wilks' λ, and examined canonical coefficients and structural coefficients to determine which variables contributed to significant correlations between larval growth variables and adult longevity variables. A correlation coefficient > 0.045 (|r|> 0.45) was considered to indicate an important correlation based on the criteria in Sherry & Henson (2005).
Results
Density and species effects
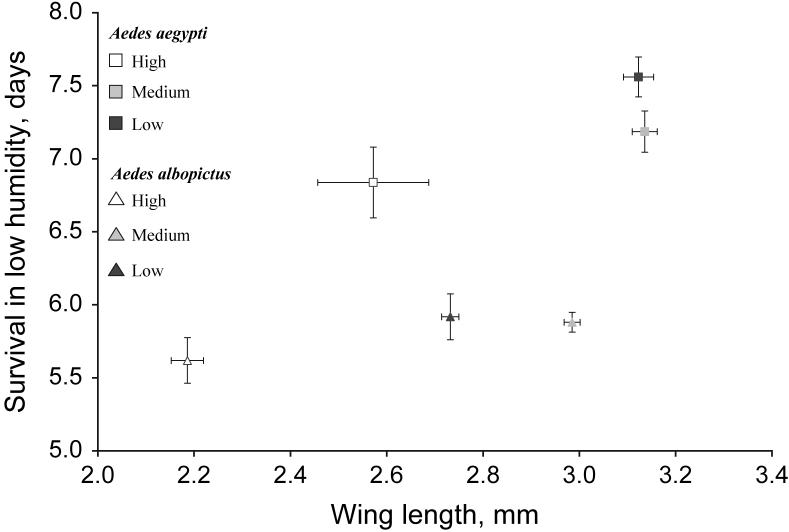
The full two-way MANOVA demonstrated significant effects of density, species and the interaction density*species when all five outcomes were considered simultaneously (density: Pillai's trace = 1.146, F10,48 = 6.45, P < 0.0001; species: Pillai's trace= 0.857, F5,23 = 27.66, P < 0.0001; density*species: Pillai's trace = 0.631, F10,48 = 2.21, P = 0.0329). Longevity under low-humidity conditions and wing length were important contributors to the effects of density and the density*species interaction, although their opposite signs suggests their relationship to be inconsistent across density levels (Table 2). This result may reflect the larger wings in Ae. albopictus reared under medium-density compared with low-density conditions and the lack of a difference in wing length in Ae. aegypti reared under medium and low densities (Fig. 1). Species differences primarily reflected differences in longevity under low-humidity conditions (large relative SCC for longevity; Table 2).
Table 2.
Standardized canonical coefficients (SCCs) for the first canonical for density, species and the density*species interaction. For all factors only the first canonical was significant.
| Factor | Percent variation explained | Longevity, low humidity | Longevity, high humidity | Proportion emerged | Time to emergence | Wing length |
|---|---|---|---|---|---|---|
| Density | 97.81 | -1.704 | -0.685 | 0.569 | -0.032 | 3.727 |
| Species | 100.0 | 2.177 | 0.756 | -0.227 | -0.209 | -0.78 |
| Density*species | 78.59 | -1.744 | -1.172 | 0.614 | 0.124 | 3.511 |
Fig. 1.
Bivariate plot of average (± 1 standard error of the mean) wing length and longevity under low-humidity conditions for each density by species.
Larval outcome variables (proportion emerged, average time to emergence and wing length) and adult longevity outcomes (longevity under high and low humidities) were significantly correlated in a canonical correlation (squared canonical correlation: 0.5315; Wilks' λ = 0.4479, F6,56 = 4.61, P = 0.0007). Only the first canonical variable was significant for each class of the outcomes (data not shown). The standardized correlation coefficients showed that all larval outcomes (proportion emerged, time to emergence and wing length) were important (r > |0.45|) amongst the larval outcomes variables, whereas only longevity under low-humidity conditions was important amongst the adult outcomes variables (Table S1). All structural coefficients were important in the CCA except the effect of proportion emerged on the adult correlate. The CCA showed that time to emergence was negatively correlated with the adult correlate (longevity in high- and low-humidity conditions) and wing length was positively correlated with the adult correlate. Both adult longevity outcomes were positively correlated with the larval canonical variable.
Density effects within Aedes aegypti
For Ae. aegypti, manova demonstrated the importance of density with both larval (percent survival, wing length and time to emergence) and adult (longevity under high and low humidities) outcomes in the model (Pillai's trace: 1.3328, F10,22 = 4.39, P = 0.0018). Only the first canonical was significant, explaining 88% of the variation between densities. Standardized canonical coefficients demonstrated that wing length and longevity under low-humidity conditions contributed the most to differences between density treatments, with proportion emerged also a significant contributor (Fig. 1, Table 3). Opposite signs of wing length and longevity under low humidity mean these two outcomes are responding in opposite ways to density. This may be the result of slightly larger wings at the intermediate density of larvae, whereas longevity under low humidity seems to respond in a positive, linear manner to larval density. There were no differences between the low- and medium-density treatments, but both the low- and medium-density treatments differed from the high-density treatment by pairwise MANOVA.
Table 3.
Results of MANOVA on larval density treatments, including all pairwise comparisons, showing relationships to univariate measures of larval growth and survival and adult longevity. Only standardized canonical coefficients from the first canonical variate (explaining 87% of variation in the fullmode l)a res hown.
| Standardized canonical coefficients (first canonical variate) |
||||||||
|---|---|---|---|---|---|---|---|---|
| d.f.( n,de nsity) | Pillai'stra ce | P | Wing length | Proportion emerged | Days to emerge | Longevity, low humidity | Longevity, high humidity | |
| Aedesae gypti | ||||||||
| Density | 10,22 | 1.33 | 0.0018 | 3.306 | 0.205 | -0.293 | -1.42 | -0.478 |
| High vs. low | 5,10 | 0.785 | 0.0040 | 2.944 | -0.186 | -0.79 | -1.03 | -0.607 |
| High vs. medium | 5,10 | 0.861 | 0.0005 | 3.333 | 0.313 | -0.143 | -1.499 | -0.429 |
| Med vs. low | 5,10 | 0.535 | 0.1230 | 2.126 | 1.023 | 1.09 | -1.478 | 0.131 |
| Aedesalbopic tus | ||||||||
| Density | 10,20 | 1.267 | 0.0088 | 8.437 | 1.282 | -0.2101 | -0.04 | -0.114 |
| High vs. low | 5,9 | 0.98 | < 0.0001 | 8.374 | 1.327 | -0.241 | -0.02 | -0.081 |
| High vs. medium | 5,9 | 0.99 | < 0.0001 | 8.443 | 1.276 | -0.206 | -0.043 | -0.118 |
| Medium vs. low | 5,9 | 0.919 | 0.0001 | 8.438 | 1.133 | -0.121 | -0.096 | -0.203 |
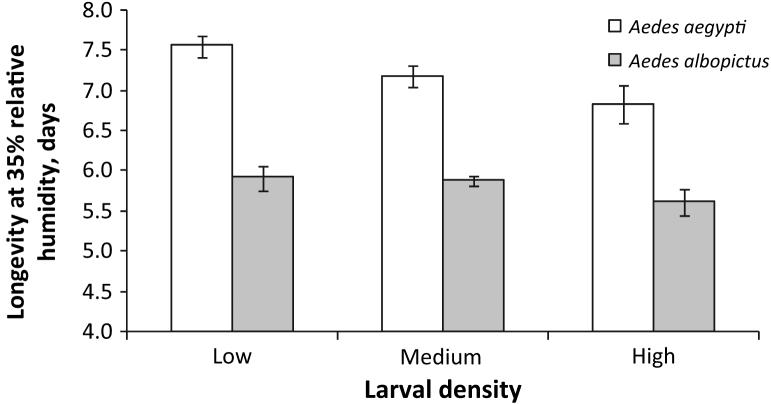
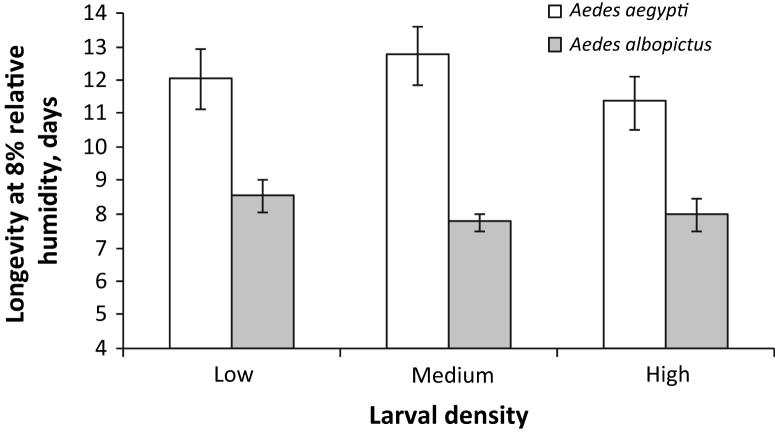
Following the significant MANOVA, individual ANOVAS demonstrated that Ae. aegypti density treatments differed significantly in proportion emerged (data not shown, one-way ANOVA, high < medium = low, F2 = 12.11, P = 0.0007), wing length (data not shown, one-way ANOVA, high < medium = low, F2 = 25.55, P < 0.0001), average days to emergence (data not shown, Kruskal-Wallis test on ranks: χ22 = 14.7485, P = 0.0006, post hoc test on ranks high < medium < low), and longevity under low-humidity conditions (Fig. 2, one-way ANOVA, high < low, high = medium, low = medium, F2 = 4.28, P = 0.0355), but not for longevity under high-humidity conditions (Fig. 3, one-way ANOVA, high = medium = low, F2 = 0.79, P = 0.4739). Lambda, analysed separately from the MANOVA, was significantly different between densities for Ae. aegypti (data not shown, one-way ANOVA, high < medium < low, F2 = 494.79, P < 0.0001).
Fig. 2.
Longevity under low humidity by larval density for Aedes albopictus and Aedes aegypti.
Fig. 3.
Longevity under high humidity by larval density for Aedes albopictus and Aedes aegypti.
Larval outcome variables (proportion emerged, average time to emergence and wing length) and adult longevity outcomes (longevity under high and low humidities) were significantly correlated in a CCA for Ae. aegypti (squared canonical correlation: 0.71; Wilks' λ = 0.2886, F6,24 = 3.45, P = 0.0134). Only the first canonical variable was significant for each class of outcomes. The standardized correlation coefficients showed that wing length and days to emergence were important (r > |0.45|) in the larval outcomes variable, whereas only longevity under low-humidity conditions was important in the adult outcome variable (Table S2) All structural coefficients were important in the CCA, and showed that time to emergence was negatively correlated with longevity in high- and low-humidity conditions and wing length was positively correlated with longevity in high- and low-humidity conditions, as was proportion emerged. Both adult longevity outcomes were positively correlated with the larval canonical variable.
Density effects within Aedes albopictus
The overall MANOVA for Ae. albopictus demonstrated a significant difference in density treatments (Pillai's trace: 1.2668, F10,20 = 3.46, P = 0.0088). Again, only the first canonical was significant, explaining > 99% of the variation. Standardized canonical coefficients demonstrated that wing length and proportion emerged were the most important factors in determining differences between density treatments (Table 3). Pairwise comparisons demonstrated significant differences between all densities (all P < 0.001), and canonical coefficients suggested the overwhelming importance of wing length in this relationship.
Following significant MANOVAS, individual ANOVAS demonstrated that Ae. albopictus density treatments differed significantly in proportion emerged (data not shown, one-way ANOVA, high < medium = low, F2 = 28.58, P = 0.0001) and wing length (data not shown, one-way ANOVA, high << low < medium, F2 = 397.54, P = 0.0001), but not in average days to emergence (data not shown, Kruskal-Wallis, χ22 = 4.5264 P = 0.1040), longevity under low-humidity conditions (Fig. 2; one-way ANOVA, F2 = 1.65, P = 0.2304) or longevity under high-humidity conditions (Fig.3; one-way ANOVA, F2 = 1.29, P = 0.3083). Lambda, analysed separately from the MANOVA, was significantly different between densities for Ae. albopictus (data not shown, Kruskal-Wallis test on ranks: high < medium = low, χ22 = 15.1736, P = 0.0005).
For Ae. albopictus, larval outcomes and adult outcomes were not significantly correlated (squared canonical correlation: 0.22; Wilks' λ = 0.7137, F6,22 = 0.67, P = 0.6721). None of the canonical variables was significant, and there were no structural coefficients > 0.45, the aforementioned critical value (data not shown).
Discussion
The degree of intraspecific competition had a significant effect on adult longevity under low-humidity conditions for Ae. aegypti, but not for Ae. albopictus. Larval density also had significant effects on wing length and proportion emerged for Ae. albopictus. Surprisingly, Ae. albopictus reared at medium density exhibited longer wings than adults derived from the low-density treatment. We cannot attribute this effect to any known error in methodology, and note a precedent for certain non-linearities in the relationship between density and growth in a congeneric species, Ae. triseriatus, which has a similar ecology (Livdahl, 1984). The results of the Ae. aegypti treatments demonstrate that six replicates for each density was sufficient to detect differences in the effects of intraspecific larval density on adult longevity. Furthermore, we saw highly significant differences in other outcomes of the larval environment, such as wing length and time to emergence. Therefore, we conclude that if the effects of larval environment on adult longevity were large, the densities chosen should represent a range of conditions broad enough to allow the detection of differences in adult longevity resulting from larval competition. However, the loss of two high-density Ae. albopictus replicates because no females survived to emergence limited our ability to detect differences in longevity for this species at high larval densities. Indeed, the point estimates for Ae. albopictus longevity under drier conditions show the same patterns as for Ae. aegypti (adults derived from the high-density larval treatment had the shortest survival times), albeit non-significantly.
This study suggests that larval competition has significant effects on adult longevity in Ae. aegypti. This result supports other evidence from field (Haramis, 1983; Hawley, 1985; Nasci, 1986) and laboratory studies for related species, Ae. triseriatus and Aedes sierennsis (Ludlow) (Haramis, 1985). A similar result was found in the laboratory with Ae. aegypti under high-humidity adult conditions (Steinwascher, 1982). However, mark-release-recapture studies have generally failed to detect differences in longevity between adult females derived from different larval feeding regimes (Walker et al., 1987; Maciel de Freitas et al., 2007) Our observation that competitive environments affect longevity for Ae. aegypti but not Ae. albopictus suggests this phenomenon is complicated and may be species- and condition-specific. Further experiments into the effects of larval competition on longevity in Ae. albopictus may need to include a more diverse array of adult conditions than that examined here, or larger sample sizes.
Previous studies have noted small differences between these species in longevity under conditions of water and sugar ad libitum, and daily blood availability (Braks et al., 2006), and large differences under desiccating conditions (Mogi et al., 1996). In agreement with Mogi et al. (1996), the longevity of Ae. aegypti was greater than that of Ae. albopictus under both high- and low-humidity conditions. Our finding that adult longevity under low-humidity conditions was a function of larval competition for Ae. aegypti, but not Ae. albopictus, also supports the findings of Mogi et al. (1996), who noted a correlation between wing length and longevity in Ae. aegypti, but not in Ae. albopictus. Considering the differences in overall longevity between the species, it is possible that the conditions examined were so unfavourable to Ae. albopictus adult females that differences caused by larval environment were overwhelmed by rapid death.
Competition has large effects on growth and survival for larval mosquitoes, and some effects of competition may continue into adulthood. Alto et al. (2005, 2008) have shown that larval competition can affect interactions of adult mosquitoes with viral pathogens, and that the effects of larval competition are species-specific. Likewise, other researchers have suggested that the larval environment of mosquitoes may have an effect on the evolution of virulence in an obligate parasite (Tseng, 2006). The results of our study suggest that longevity of adults in desiccating conditions is affected by the degree of larval competition in Ae. aegypti. Identification of the relationship between an ecological process, such as intraspecific larval competition, and adult longevity can help make explicit the connection between density-dependent population models of larval mosquitoes and epidemiological models of mosquito-borne disease transmission. Models of mosquito-borne disease transmission are sensitive to changes in adult longevity parameters, which suggests that even small changes in adult longevity caused by larval competition may result in large changes in disease transmission (Dye, 1986; Luz et al., 2003).
Acknowledgements
We thank Naoya Nishimura and Krystle Greene for assistance in measuring wing lengths and maintaining mosquito colonies. This work was supported by a National Institutes of Health grant (5R01AI-044793) to LPL. We are grateful to two anonymous referees and to Dr Gabriella Gibson, whose comments greatly improved this article.
Footnotes
Supporting Information Additional supporting information may be found in the online version of this article.
Table S1. Canonical correlation analysis between larval outcomes (proportion emerged, average days to emergence and wing length) and adult outcomes (longevity under high- and low-humidity conditions) for both species. Important correlations (r > |0.45|, by convention (Sherry & Keogh, 2005), are in bold.
Table S2. Canonical correlation analysis between larval outcomes (proportion emerged, average days to emergence and wing length) and adult longevity outcomes (longevity under high- and low-humidity conditions) for Aedes aegypti. Important correlations (r > |0.45|, by convention) are in bold.
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than for missing material) should be directed to the corresponding author for the article.
References
- Alto BW, Lounibos LP, Higgs S, Juliano SA. Larval competition differentially affects arbovirus infection in Aedes mosquitoes. Ecology. 2005;86:3279–3288. doi: 10.1890/05-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alto BW, Lounibos LP, Mores C, Reiskind M. Larval competition alters the susceptibility of adult Aedes mosquitoes to dengue infection. Proceedings of the Royal Society of London Series B-Biological Sciences. 2008;275:463–471. doi: 10.1098/rspb.2007.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braks M, Honorio N, Lounibos LP, Lourenco-de-Oliveira R, Juliano SA. Interspecific competition between two invasive species of container mosquitoes, Aedes aegypti and Aedes albopictus (Diptera: Culicidae), in Brazil. Annals of the Entomological Society of America. 2004;97:130–139. [Google Scholar]
- Braks M, Juliano SA, Lounibos LP. Superior reproductive success on human blood without sugar is not limited to highly anthropophilic mosquito species. Medical and Veterinary Entomology. 2006;20:53–59. doi: 10.1111/j.1365-2915.2006.00612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophers S. Aedes aegypti (L.): The Yellow Fever Mosquito. Cambridge University Press; Cambridge: 1960. pp. xxx–xxx. [Google Scholar]
- Dye C. Vectorial capacity: must we measure all its components? Parasitology Today. 1986;2:203–209. doi: 10.1016/0169-4758(86)90082-7. [DOI] [PubMed] [Google Scholar]
- Gimnig JE, Ombok M, Otieno S, Kaufman MG, Vulule JM, Walker ED. Density-dependent development of Anopheles gambiae (Diptera: Culicidae) larvae in artificial habitats. Journal of Medical Entomology. 2002;39:162–172. doi: 10.1603/0022-2585-39.1.162. [DOI] [PubMed] [Google Scholar]
- Gray E, Bradley T. Physiology of desiccation resistance in Anopheles gambiae and Anopheles arabiensis. American Journal of Tropical Medicine and Hygiene. 2005;73:553–559. [PubMed] [Google Scholar]
- Haramis L. Increased adult size correlated with parity in Aedes triseriatus. Mosquito News. 1983;43:77–79. [Google Scholar]
- Haramis L. Larval nutrition and adult ecology. In: Lounibos LP, Rey J, editors. Mosquito Ecology: Proceedings of a Symposium. University of Florida Press; Gainesville, FL: 1985. pp. xxx–xxx. [Google Scholar]
- Hawley W. The effect of larval density on adult longevity of a mosquito Aedes sierrensis - epidemiological consequences. Journal of Animal Ecology. 1985;54:955–964. [Google Scholar]
- Hawley WA. Biology of Aedes albopictus. Journal of the American Mosquito Control Association. 1988;4:1–39. [PubMed] [Google Scholar]
- Ho B, Ewert A, Chew L. Interspecific competition among Aedes aegypti, Ae. albopictus, and Ae. triseriatus (Diptera: Culicidae): larval development in mixed cultures. Journal of Medical Entomology. 1989;26:615–623. doi: 10.1093/jmedent/26.6.615. [DOI] [PubMed] [Google Scholar]
- Juliano SA. Species introduction and replacement among mosquitoes: interspecific resource competition or apparent competition? Ecology. 1998;79:255–268. [Google Scholar]
- Klowden M, Chambers G. Reproductive and metabolic differences between Aedes aegypti and Ae. albopictus (Diptera: Culicidae) Journal of Medical Entomology. 1992;29:467–471. doi: 10.1093/jmedent/29.3.467. [DOI] [PubMed] [Google Scholar]
- Livdahl T. Interspecific interactions and the r-K continuum: laboratory comparisons of geographic strains of Aedes triseriatus. Oikos. 1984;42:193–202. [Google Scholar]
- Lounibos LP, Omeara GF, Escher RL, et al. Testing predictions of displacement of native Aedes by the invasive Asian Tiger Mosquito Aedes albopictus in Florida, U.S.A. Biological Invasions. 2001;3:151–166. [Google Scholar]
- Luz P, Codeco C, Massad E, Struchiner C. Uncertainties regarding dengue modelling in Rio de Janeiro, Brazil. Memorias do Instituto do Oswaldo Cruz. 2003;98:871–878. [PubMed] [Google Scholar]
- Maciel de Freitas R, Codeco C, Lourenco de Oliveira R. Body size-associated survival and dispersal rates of Aedes aeygpti in Rio de Janeiro. Medical and Veterinary Entomology. 2007;21:284–292. doi: 10.1111/j.1365-2915.2007.00694.x. [DOI] [PubMed] [Google Scholar]
- Mogi M, Miyagi I, Abadi K, Syafruddin Inter- and intraspecific variation in resistance to desiccation by adult Aedes (Stegomyia) spp. (Diptera: Culicidae) from Indonesia. Journal of Medical Entomology. 1996;33:53–57. doi: 10.1093/jmedent/33.1.53. [DOI] [PubMed] [Google Scholar]
- Nasci R. Relationship between adult mosquito (Diptera: Culicidae) body size and parity in field populations. Environmental Entomology. 1986;15:874–876. [Google Scholar]
- Quinn G, Keogh M. Experimental Design and Data Analysis for Biologists. Cambridge University Press; Cambridge: 2002. pp. xxx–xxx. [Google Scholar]
- Scheiner S. MANOVA. In: Scheiner S, Gurevitch, editors. Design and Analysis of Ecological Experiments. J Princeton University Press; Princeton, N J: 2001. pp. xxx–xxx. [Google Scholar]
- Sherry A, Henson R. Conducting and interpreting canonical correlation analysis in personality research: a user-friendly primer. Journal of Personality Assessment. 2005;84:37–48. doi: 10.1207/s15327752jpa8401_09. [DOI] [PubMed] [Google Scholar]
- Sokal R, Rohlf F. Biometry. WH Freeman & Co.; New York, N Y: 1995. pp. xxx–xxx. [Google Scholar]
- Steinwascher K. Relationship between pupal mass and adult survivorship and fecundity for Aedes aegypti. Environmental Entomology. 1982;11:150–153. [Google Scholar]
- Tseng M. Interactions between the parasite's previous and current environment mediate the outcome of parasite infection. American Naturalist. 2006;168:1–9. doi: 10.1086/507997. [DOI] [PubMed] [Google Scholar]
- Walker ED, Copeland RS, Paulson SL, Munstermann LE. Adult survivorship, population density, and body size in sympatric populations of Aedes triseriatus and Aedes hendersoni (Diptera, Culicidae) Journal of Medical Entomology. 1987;24:485–493. doi: 10.1093/jmedent/24.4.485. [DOI] [PubMed] [Google Scholar]
- Walker ED, Lawson D, Merritt R, Morgan W, Klug M. Nutrient dynamics, bacterial populations, and mosquito productivity in tree hole ecosystems and microcosms. Ecology. 1991;72:1529–1546. [Google Scholar]