Abstract
An unmet need for expansion of primary cells and progenitor cells in three dimensions (3-D) is a synthetic mimic of the extracellular matrix (ECM) with user-controllable composition that would permit rapid recovery of viable cells under mild, non-enzymatic conditions. Three block copolymers based on disulfide-containing polyethylene glycol diacrylate crosslinkers were synthesized, and were used to crosslink thiol-modified hyaluronan and gelatin macromonomers in the presence of cells. The triblock PEGSSDA contained a single disulfide-containing block, the pentablock PEG(SS)2DA contained two disulfide blocks, and the heptablock PEG(SS)3DA contained three disulfide blocks. For each hydrogel composition, four cell types were encapsulated in 3-D, and growth and proliferation were evaluated. Murine NIH 3T3 fibroblasts, human HepG2 C3A hepatocytes, human bone-marrow derived mesenchymal stem cells (MSCs), and human umbilical vein endothelial cells (HUVECs) all showed excellent viability and growth during expansion in 3-D in the three disulfide block copolymer crosslinkers. After cell expansion, the hydrogels were dissociated using the thiol-disulfide exchange reaction in the presence of N-acetyl-cysteine or glutathione, which dissolved the hydrogel network. After dissolution, cells were recovered in high yield and with high viability by gentle centrifugation.
Keywords: disulfide-containing crosslinkers, thiol-disulfide exchange reaction, synthetic extracellular matrix, in situ crosslinking, primary cells, hyaluronic acid, PEGDA
1. Introduction
An ever-increasing number of hydrogels and fibrous scaffolds based on both natural and synthetic polymers are being developed for the expansion of primary cells and progenitor cells in three dimensions (3-D) [1–4]. Among the naturally-derived biomaterials is an in situ-crosslinkable synthetic mimic of the extracellular matrix (sECM) that is based on chemically-modified hyaluronan (HA) [5]. HA is a ubiquitous non-sulfated glycosaminoglycan with critical roles in morphogenesis [6], and chemically-modified HA derivatives have generated considerable interest as biomaterials for tissue engineering [7, 8]. This versatile sECM offers user-controllable composition and compliance, to which a variety of growth factors or matricellular proteins may be added [9, 10]. The elastic moduli of hydrogels can be tailored to match the cell type to achieve optimal regeneration of a neo tissue [11, 12]. The sECM as well as PEG-based hydrogels with protease-labile sequences [13–15] currently require enzymatic digestion to release expanded cells. For cells cultured in 2-D on hydrogel surfaces or by encapsulation in 3-D, hydrolytic enzymes such as trypsin, gelatinase (collagenase), and hyaluronidase are typically employed to both disassemble the matrix and to disrupt cell-matrix interactions. With undifferentiated or sensitive cells, this can lead to low cell recovery or modification of phenotype as a result of the non-physiological treatment, although very high levels hyaluronidase (2000 U) were well tolerated by human embryonic stem cells recovered from a photocrosslinked HA derivative [16]. Moreover, many enzymes employed in these processes are derived from animal sources, further complicating the use of the enzymatic reagent for cell expansion and recovery for human cell therapy. Thus, an important unmet need is the availability of an ECM mimetic that would allow expansion of primary or stem cells in 3-D, and then facilitate rapid recovery of viable cells under mild, non-enzymatic conditions.
Herein we describe the synthesis of three disulfide-containing polyethylene glycol diacrylate block copolymers. These new reagents were used to crosslink a thiol-modified hyaluronan derivative and thiol-modified gelatin macromonomers to give cytocompatible hydrogels for surface culture and for in situ cell encapsulation. The disulfide linkages can be cleaved reductively, and thus the gelation process is reversible and offers a convenient non-enzymatic pathway for partially dissolving the hydrogel and releasing the cells. Four cell types -- murine NIH 3T3 fibroblasts, human HepG2 C3A hepatocytes; human bone-marrow derived mesenchymal stem cells (MSCs), and human umbilical vein endothelial cells (HUVECs) -- were encapsulated in the sECMs, and growth and proliferation in 3-D were evaluated. After cell expansion, the hydrogels could be dissociated under physiologically compatible conditions using the thiol-disulfide exchange reaction [17] in which treatment with an excess of cysteine, N-acetyl-cysteine, or glutathione leads to breakdown of the hydrogel network. After dissolution, cells were recovered in high yield and with high viability by gentle centrifugation.
2. Materials and Methods
2.1 Materials
Fermentation-derived hyaluronan (HA, sodium salt, Mw= 1.5 MDa) was obtained from Novozymes Biopolymer A/S (Denmark). N-hydroxysuccinimide (NHS), triethylamine (TEA), acryloyl chloride, 1-ethyl-3-[3-(dimethylamino)propyl]carbodiimide (EDCI), 3,3’-dithiobis(propanoic acid) (DTP), N,N-dimethylaminopyridine (DMAP), t-butyl methyl ether, succinic acid, hydrazine hydrate, L-cysteine, N-acetyl-L-cysteine, glutathione (reduced), ethylene glycol, and polyethylene glycol (2000 Da and 3400 Da) were from Aldrich Chemical Co. (Milwaukee, WI). Phosphate-buffered saline (PBS) was from Fisher (Houston, TX). Dulbecco's Modified Eagle's Medium (DMEM), Minimum Essential Medium Eagle (MEME), and Ham's F12K medium were obtained from ATCC (Manassas, Va). Poietics™. Mesenchymal Stem Cell Basal Medium (MSCBM) was obtained from Lonza (Walkersville, MD).
2. 2. Analytical instrumentation
Proton NMR spectral data were obtained using a Varian INOVA 400 at 400MHz and are reported in ppm (δ) relative to δ (tetramethylsilane) = 0. UV-visible spectra were recorded on a Model 8453 UV-Vis spectrophotometer (Hewlett-Packard, Palo Alto, CA). Fluorescence images of viable cells were recorded using a Nikon Eclipse TE300 with epifluorescence capabilities. Cell proliferation was determined using a biochemical assay (Cell-Titer 96 Proliferation Kit, Promega, Madison, WI) at 550 nm, which was recorded on an OPTI Max microplate reader (Molecular Devices, Sunnyvale, CA).
2.3 Synthesis of disulfide-containing crosslinkers
2.3.1. Synthesis of PEGSSDA
All reactions in organic solvents were conducted under anhydrous conditions under a blanket of nitrogen. To a stirred solution of 3,3’-dithiodipropionic acid (DTP, 308 mg, 1.47 mmol) in THF/CH2Cl2 (30 ml, 2:1), was added EDCI in one portion. After 5 min, NHS (244 mg, 2.94 mmol) was added, and the solution was stirred for 10 min. The solution of the activated DTP NHS ester was added slowly via syringe to a flask containing a stirred solution of PEG (10.0 g, 2.94 mmol, MW 3400) in CH2Cl2 (80 ml). After addition, the final solution was stirred overnight. The resulting mixture was concentrated under reduced pressure and dried in vacuo. The crude solid product was dissolved in water and particulate materials were removed by filtration. The aqueous solution was dialyzed against water for 2 days, and then lyophilized to obtain the PEG-DTP-PEG intermediate in 80% yield. This material was employed without further purification. 1H-NMR (CDCl3): δ 2.75 (4H, t, CH2COO), 2.95 (4H, t, S-CH2), 3.40–3.80 (m, 672H, -CH2-CH2-O-), 3.82 (4H, t, COOCH2), 4.25 (4H, t, -CH2- CH2-OH). MALDI/ToF (m/z): 6918.
To a stirred solution of the above PEG-DTP-PEG intermediate (5 g, 0.572 mmol) in CH2Cl2 (100 ml) was added TEA (5 eq. 2.86 mmol, 0.4ml). After 5 min, acryloyl chloride (2.86 mmol, 0.28 ml) was added slowly via syringe. After addition, the mixture was stirred overnight at ambient temperature. The mixture was concentrated under reduced pressure to a volume of 20 mL and then filtered to remove precipitated triethylamine hydrochloride. The solution was poured into 300 mL of t-butyl methyl ether to precipitate the diacrylate product, which was collected by filtration. The solid was then dissolved in 50 ml of water, dialyzed against water for two days, and lyophilized to obtain 3.5 g of the triblock crosslinker PEGSSDA (70%). This preparation showed 71% bis-acryloylation as determined by NMR integration of the acrylate ester δ 4.22 (-CH2O-) and primary alcohol δ 4.32 (-CH2OH) resonances; % bisacryloylation = ester methylene area/(ester methylene area + alcohol methylene area). 1H-NMR (CDCl3): δ 2.75 (4H, t, CH2COO), 2.95 (4H, t, S-CH2), 3.4–3.8 (668H, m, -CH2-CH2-O-), 3.82 (4H, t, COOCH2), 4.35 (4H, t, CH2OOCCH=CH2), 5.82 (2H, trans, CH2=CH), 6.15 (2H, RCOOCCH=CH2), 6.43 (2H, cis, CH2=CH). MALDI/ToF (m/z): 7064.
2.3.2. Synthesis of PEG(SS)2DA
To a stirred solution of DTP (1.05 g) in THF/ CH2Cl2 (30 ml, 2:1), EDCI (1.92 g) was added in one portion, and the solution was stirred for 10 min. The resulting solution was via syringe to a stirred solution of PEG (20 g, MW 2000) and DMAP (976 mg) in CH2Cl2 (80 ml) and stirred overnight. The resulting solution was concentrated under reduced pressure and dried in vacuo. The crude solid PEG-DTP-PEG intermediate was dissolved in water, solids were removed by filtration, and the aqueous solution was dialyzed against water for 2 days, and then lyophilized to obtain the intermediate (14.4 g, 72%) as a white powder. 1H-NMR (CDCl3): δ 2.75 (4H, t, CH2COO), 2.95 (4H, t, S-CH2), 3.40–3.82 (m, 182H, -CH2-CH2-O- ), 4.25 (4H, t, -CH2-CH2-OH). MALDI/ToF (m/z): 4309.
To a stirred solution of succinic acid (69 mg) in THF/ CH2Cl2 (30 ml, 2:1), EDCI (192 mg) was added as one portion, stirring was continued for 10 min. The resulting solution was added slowly via syringe to a stirred solution of the above PEG-DTP-PEG intermediate (4.2 g, MW 4200) and DMAP (61 mg) in CH2Cl2 (80 ml). After addition, the final solution was stirred overnight, concentrated, and dried as above. The crude PEG-DTP-PEG-Succ-PEG-DTP-PEG intermediate was dissolved in water, solids were removed by filtration, and the aqueous solution was dialyzed against water for 2 days, and then lyophilized to obtain the diol intermediate (3 g, 71%). 1H-NMR (CDCl3): δ 2.75 (6H, t, CH2COO), 2.90 (6H, t, S-CH2), 3.40–3.82 (m, 360H, - CH2-CH2-O-), 4.20 (4H, t, -CH2-CH2-OH). MALDI/ToF (m/z): 8328
To a stirred solution of the above diol intermediate (1.94 g) in CH2Cl2 (100 ml) was added TEA (5eq. 0.15 ml). After 5 min, acryloyl chloride (5 eq, 0.11 ml) was added slowly via syringe. After addition, the mixture was stirred overnight at ambient temperature. The resulting solution was concentrated and dried; the solid crude diacrylate was purified as described for triblock PEGSSDA, and lyophilized to obtain the pentablock crosslinker PEG(SS)2DA (1.1 g, 57%). This preparation showed 60% bis-acryloylation, calculated as described above. 1H-NMR (CDCl3): δ 2.75 (6H, t, CH2COO), 2.90 (6H, t, S-CH2), 3.40–3.82 (m, 360H, -CH2-CH2-O-), 4.25 (4H, t, -CH2-CH2-OH). 5.82 (2H, trans, CH2=CH), 6.15 (2H, RCOOCCH=CH2), 6.43 (2H, cis, CH2=CH). MALDI/ToF (m/z): 8530.
2.3.3. Synthesis of PEG(SS)3DA
To a stirred solution of DTP (1.05 g) in THF/ CH2Cl2 (30 mL, 2:1), EDCI (1.92 g) was added in one portion, and the solution was stirred for 10 min. The resulting solution was added slowly via syringe to a stirred solution of PEG (20 g, MW 2000) and DMAP (976 mg) in CH2Cl2 (80 ml). After addition, the solution was stirred overnight. The resulting solution was concentrated under reduced pressure and dried in vacuo. The solid diol was purified as above to give intermediate PEG-DTP-PEG MW 4200 (14.4 g, 72%). 1H-NMR (CDCl3): δ 2.75 (4H, t, CH2COO), 2.95 (4H, t, S-CH2), 3.40–3.82 (m, 182H, -CH2-CH2-O- ), 4.25 (4H, t, -CH2-CH2-OH). MALDI/ToF (m/z): 4309.
To a stirred solution of DTP (210 mg) in THF/ CH2Cl2 (30 ml, 2:1), EDCI (616 mg) was added in one portion and the solution was stirred for 10 min. The resulting solution was added slowly via syringe to a stirred solution of PEG-DTP-PEG (9.0 g, MW 4200) and DMAP (392 mg) in CH2Cl2 (80 ml) and stirred overnight. After addition, the final solution was stirred overnight, concentrated, dried, purified as above, and then lyophilized to give the intermediate heptablock diol PEG-DTP-PEG-DTP-PEG-DTP-PEG (5.6 g, 60.8%). 1H-NMR (CDCl3, δ): 2.75 (6H, t, CH2COO), 2.90 (6H, t, S-CH2), 3.40–3.82 (m, 364H, -CH2-CH2-O-), 4.20 (4H, t, -CH2-CH2-OH). MALDI/ToF (m/z): 8323.
To a stirred solution of the above tris-disulfide triol (1.94 g) in CH2Cl2 (100 ml) was added TEA (5 eq., 0.15 ml). After 5 min, acryloyl chloride (5 eq, 0.11 ml) was added slowly via syringe. After addition, the mixture was stirred overnight at ambient temperature. The mixture was concentrated to 20 ml and amine hydrochloride salts were removed by filtration. The solution was then poured into 300 ml of t-butyl methyl ether to precipitate the diacrylate product, and the solid product was isolated by filtration. The crude diacrylate was dissolved in 50 mL of water, and dialyzed against water for 2 days and lyophilized to obtain heptablock crosslinker PEG(SS)3DA (0.55 g, 28%). This preparation showed 70% bis-acryloylation, calculated as described above. 1H-NMR (CDCl3): δ 2.75 (6H, t, CH2COO), 2.90 (6H, t, S-CH2), 3.40–3.82-(m, 364H, -CH2-CH2-O-), 4.25 (4H, t, -CH2-CH2-OH). 5.82 (2H, trans, CH2=CH), 6.15-(2H, RCOOCCH=CH2), 6.43 (2H, cis, CH2=CH). MALDI/ToF (m/z): 8323.
2.4. Synthesis of CMHA-S and Gelatin-DTPH
Thiol-modified carboxymethyl hyaluronic acid (CMHA-S) and thiol-modified gelatin (gelatin-DTPH) were either synthesized in the Center for Therapeutic Biomaterials at the University of Utah as previously described [5, 18, 19] or obtained from Glycosan BioSystems (Salt Lake City, UT) as the Glycosil and Gelin-S components of Extracel™.
2.5. Preparation of Hydrogels and Sponges
2.5.1. Preparation of 2% (w/v) hydrogels and sponges with PEGSSDA
CMHA-S and gelatin-DTPH were dissolved in phosphate buffered saline (PBS) to give 1.5% and 3% (w/v) solutions, respectively, and the solution pH was adjusted to 7.4 by the addition of 1.0 N NaOH. Then, the CMHA-S and gelatin-DTPH solutions were mixed in a 2:1 (v:v) ratio, and then one volume of the crosslinker (PEGSSDA) solution was added to four volumes of the thiolated macromonomer solution. The solution was thoroughly mixed and then 0.5 mL was aliquoted without delay to individual vials. Gels formed in 3 minutes. The sponges were obtained by lyophilization of the corresponding hydrogels [20].
2.5.2. Preparation of 2% (w/v) hydrogels and sponges with PEG(SS)2DA and PEG(SS)3DA
The hydrogels and sponges with PEG(SS)3DA or PEG(SS)2DA at pH 7.4 were prepared as the same protocol as the above.
2.6 Swelling Determination
First, a 0.5 ml-aliquot of each hydrogel formulation was gelled in the bottom of replicate glass vials. Then, 3 ml of PBS was added on top of each gel, and the vials were placed into an incubator at 37 °C and agitated at 150 rpm. In this case, the hydrogel swelling occurred uniaxially toward the upper direction of the vials. The buffer was changed hourly, and the hydrogels plus the vials were weighed, after carefully removing the surface buffer at each time point. The mass of the swollen hydrogels was calculated by subtracting the mass of the vial from the total mass. The mass of the dry hydrogel components was calculated by the subtracting of the mass of the vial from the total mass, which was obtained after the hydrogels were thoroughly washed with distilled water and then lyophilized. The swelling ratio was defined as a ratio of the mass of swollen hydrogel to the mass of dry hydrogel. The same protocol was used to measure swelling of the sponges.
2.7 Dissolution of Hydrogels
A solution of either L-cysteine (Cys) or L-glutathione (GSH) (1.0 ml) was added to the hydrogels (0.5 mL) in order to dissociate the hydrogel network. Concentrations of Cys and GSH were 10, 25, 50, and 100 mM, and the dissolution time was defined as time from addition to the time at which no hydrogel could be visually detected. Note: In subsequent experiments, N-acetyl L-cysteine (NAcCys) was used in place of Cys to minimize precipitation near the isoelectric point during dissolution.
2.8. In Vitro Cytocompatibility – Surface Culture (“3-D on top”)
2.8.1 sECM preparation
CMHA-S and gelatin-DTPH were dissolved in PBS to give 1.0% (w/v) solutions. The crosslinkers were dissolved in PBS to give 2.0% (w/v) solutions. The pH value of each solution was adjusted to 7.4 using 1.0 N NaOH, and then sterile filtered. To form gels, the CMHA-S and gelatin-DTPH solutions were mixed in a 1:1 volume ratio. This solution was then mixed with the appropriate crosslinker in a 4:1 volume ratio; we refer to these modified HA-gelatin hydrogels as CMHA-GSX. The final solution was pipetted into wells in 24-well plates and allowed to gel. Gels were typically ready to use within 20 min. One extra set of each hydrogel type was frozen with liquid N2 and lyophilized to form porous sponges. At the end of the sECM preparation, there were eight sECM types to be evaluated: four CMHA-GSX hydrogel analogs with differing crosslinkers (PEGDA, triblock PEGSSDA, pentablock PEG(SS)2DA, and heptablock PEG(SS)3DA) and four CMHA-GSX sponges using with the same four crosslinkers.
2.8.2. Surface seeding
NIH 3T3 fibroblasts, HEPG2 C3A cells, and human mesenchymal stem cells (hMSCs) were obtained from ATCC and expanded in 2-D on tissue culture plastic using T-75 flasks until 90% confluence with DMEM, MEME, and MSCBM, respectively. Fibroblasts and hepatocytes were cultured in the presence of 10% FBS, while the hMSCs were supplemented with mesenchymal stem cell growth supplement (MCGS). Cells were removed from the substrate using accutase and seeded with 1 ml of their respective media on the hydrogels or sponges at a density of 10,000 cells/ml. Three replicates were performed for each cell type, each time point, each hydrogel composition, and each substrate examined. The plates were transferred to the incubator (37 °C, 5% CO2).
2.8.3. Cell viability
Cell viability was determined using MTS (Promega) assays at Day 3 and Day 7 of culture. Spent media was aspirated from the wells containing cells. Then, 300 µl of 15% MTS reagent in cell culture was added to each well and plates were incubated (37°C, 5% CO2) for 15 min. Next, 100 µl of the MTS/media solution was transferred from each well to a corresponding well of a 96-well plate. Absorbance values were determined at 490 nm using an Optimax Tunable Microplate Reader (Molecular Devices). Higher absorbance values are proportional to higher cell numbers.
2.9. In Vitro Cytocompatibility – Encapsulation in 3-D
2.9.1. sECM material preparation
CMHA-S and gelatin-DTPH were dissolved in phosphate buffered saline (PBS) to give 2.0% (w/v) solutions. The crosslinkers were dissolved in PBS to give 4.0% (w/v) solutions. The pH of each solution was adjusted to 7.4 using 1.0 N NaOH and sterile filtered.
2.9.2. Cell encapsulation
Previous encapsulation methods [9, 10, 21] were modified to use the new crosslinkers. Thus, NIH 3T3 fibroblasts, HepG2 C3As, and human mesenchymal stem cells (hMSCs) were cultured as described above. For encapsulation, we added human umbilical vein endothelial cells (HUVECs), obtained from ATCC and expanded in 2-D on tissue culture plastic were cultured in T-75 flasks until 90% confluence Ham's F-12K medium. Cells were removed from the substrate using accutase and resuspended in the crosslinker solutions. To form gels, the CMHA-S and gelatin-DTPH solutions were mixed in a 1:1 volume ratio. This solution was then mixed with the appropriate crosslinker/cell solution in a 4:1 volume ratio. Then, 100 µl of each combination was pipetted into tissue culture inserts in 24-well plates. The final cell seeding density in each insert was 10,000 cells per 100 µl of each sECM hydrogel. Three replicates were performed for each cell type, each time point, and each hydrogel composition examined. The plates were transferred to an incubator (37 °C, 5% CO2) and cell viability was determined using MTS (Promega) assays at day 3 and day 7 of culture as described above.
2.9.3. Recovery of Encapsulated Cells from Pentablock-Crosslinked sECMs
In a separate set of experiments, NIH 3T3 fibroblasts, HepG2 C3A cells, hMSCs, and HUVECs were encapsulated in tissue culture inserts at a density of 100,000 cells in a 200-µl hydrogel (cell density = 500,000 cells/ml) formed using the pentablock PEGSS2DA crosslinker as described above. Each hydrogel was cultured for 3 days in 800 µl of the appropriate media, and then the spent media was removed by aspiration. Gels were removed from the tissue culture inserts by excising the mesh layer of the insert, and then using the large end of a sterile plastic pipette tip to pushthe gel through the opening into a 15-ml sterile plastic centrifuge tube. Next, solutions containing 50 mM N-acetyl-L-cysteine solutions were prepared in each cell type's appropriate media, and the pH values were adjusted to 7.4. Then, 3 ml of the NAcCys media solutions was added into each gel-containing tube and placed on a rocker in an incubator (37 °C, 5% CO2) for 1 h. Tubes were centrifuged (1500 rpm, 5 min) and the supernatant was removed by aspiration. The remaining contents were resuspended in the appropriate media and plated on the surface of 2% Extracel™ (Glycosan BioSystems) gels in 24-well plates following the 3-D on top protocol described above. Three replicates were performed for each cell type and each time point examined.
Three wells from each cell type were assayed at 24, 48, and 72 h after re-plating onto Extracel™ using the LIVE/DEAD® Viability/Cytotoxity Kit for mammalian cells (Molecular Probes, Eugene, OR). Concentrations of 4 µM calcein-AM and 4 µM ethidium homodimer-1 in DPBS were prepared for the assay. Media was aspirated and 400 µl of the assay solutions were added to each well. Cells were incubated for 20 min (37 °C, 5% CO2) and then imaged and photographed with fluorescence under the microscope (Olympus, Center Valley, PA). All pictures taken were representative of the entire well. LIVE and DEAD photos were overlaid and cells were counted, and the percentage of live cells was calculated.
2.10. Statistical analysis
The data are presented as the means ± standard deviation (S.D.) of the number of replicates. Values were compared using Student’s t-test (2-tailed) with two sample unequal variance, and p < 0.05 or less was considered statistically significant.
3.0 Results
3.1. Synthesis and characterization of disulfide-containing block copolymers
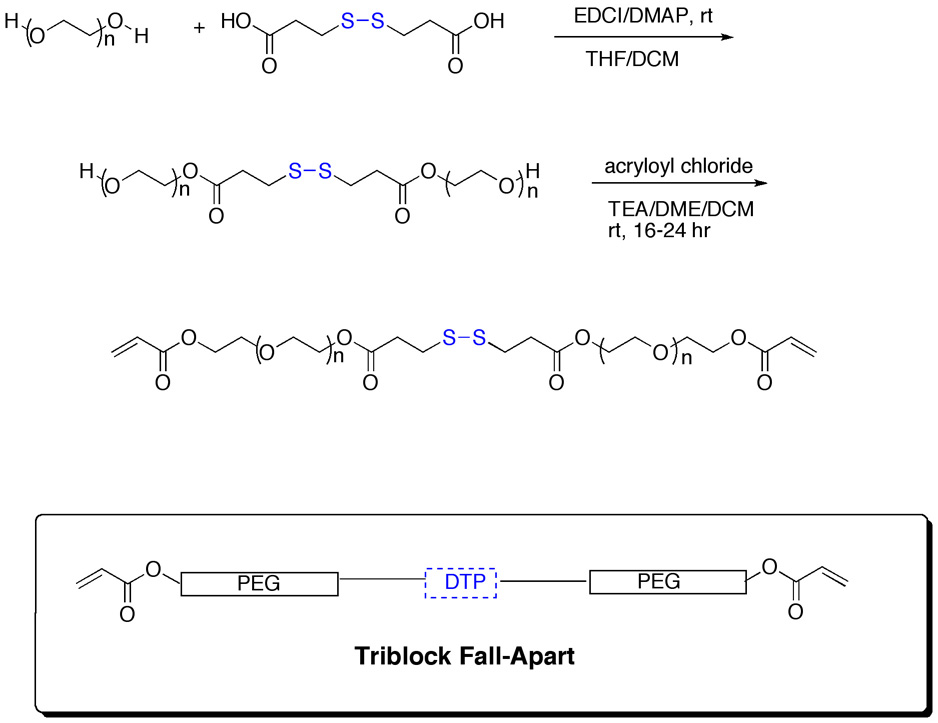
Three disulfide-containing block copolymers – the triblock PEGSSDA, pentablock PEG(SS)2DA and heptablock PEG(SS)3DA crosslinkers -- were prepared to determine the ease of dissolution for crosslinkers with single or multiple reductive cleavage sites. The triblock crosslinker was synthesized using a carbodiimide-mediated coupling of PEG (MW 3400) with the disulfide containing bis(3,3’-dithiopropionic acid, DTP); oligomerization was minimized by adding the activated ester of DTP to excess PEG in mixed tetrahydrofuran-dichloromethane solution (Scheme 1). The crude triblock PEG-DTP-PEG intermediate was purified by dialysis against water to remove small molecule byproducts. Next, the lyophilized PEG-DTP-PEG intermediate was bis-acryloylated under anhydrous conditions to provide the triblock crosslinker PEGSSDA, which was purified by dialysis and lyophilized. The final crosslinkers were characterized by 1H-NMR and Maldi/ToF mass spectrometry.
Scheme 1.
Synthesis of the triblock fall-apart crosslinker with a single disulfide bond (PEGSSDA). The crosslinker with mono-disulfide was made by coupling of PEG 3400 to DTP followed by acryloylation. See text for details.
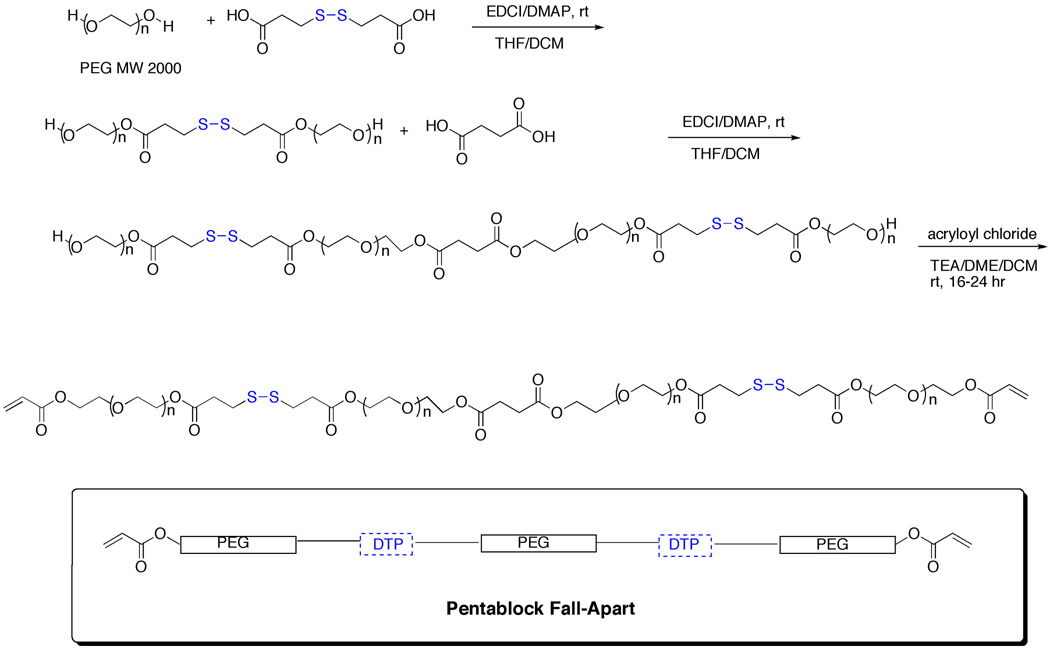
The pentablock crosslinker was synthesized by first preparing the triblock PEG-DTP-PEG intermediate using MW2000 PEG, in order to ultimately obtain a pentablock crosslinker with a size under 10 kDa (Scheme 2). Then, a solution of EDCI-activated succinic acid was added to excess PEG-DTP-PEG, and the resulting PEG-DTP-PEG-Succ-PEG-DTP-PEG intermediate was isolated, purified by dialysis, and lyophilized. (Note: the central PEG-Succ-PEG unit is considered as a single “PEG” block for comparison purposes). Bisacryloylation generated the pentablock crosslinker PEG(SS)2DA.
Scheme 2.
Synthesis of the pentablock fall-apart crosslinker with two disulfide bonds (PEG(SS)2DA)
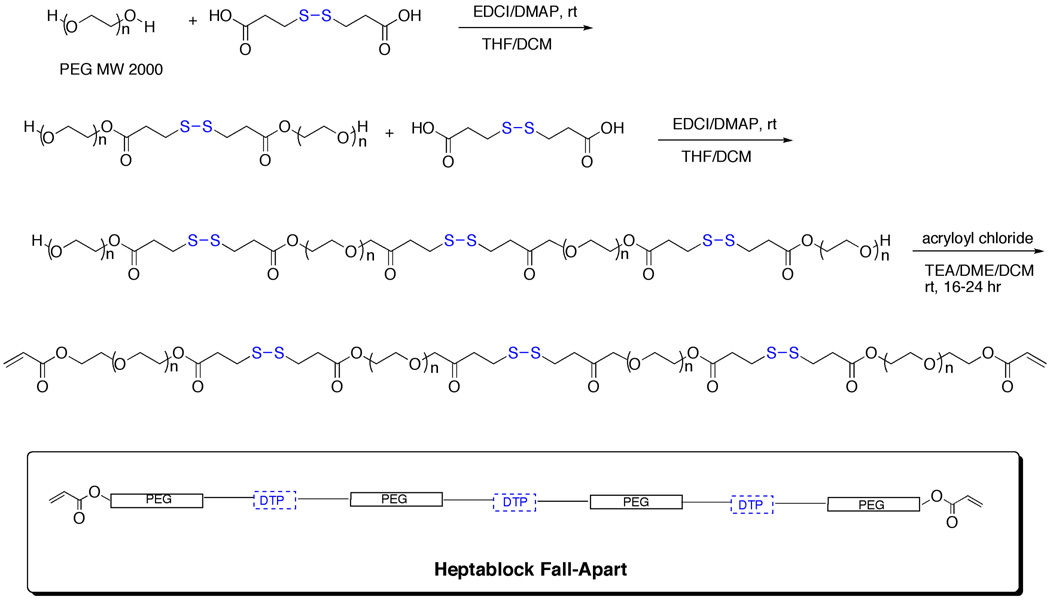
Finally, the heptablock crosslinker was prepared from the above triblock PEG-DTP-PEG intermediate with MW2000 PEG using DTP instead of succinic acid as the internal diacid to provide a third disulfide-containing block (Scheme 3). Thus, a solution of EDCI-activated DTP acid was added to excess PEG-DTP-PEG, and the resulting PEG-DTP-PEG-DTP-PEG-DTP-PEG intermediate was isolated, purified by dialysis, and lyophilized. Bisacryloylation provided the heptablock crosslinker PEG(SS)3DA, which was purified and analyzed as above.
Scheme 3.
Synthesis of the heptablock fall-apart crosslinker with three disulfide bonds (PEG(SS)3DA)
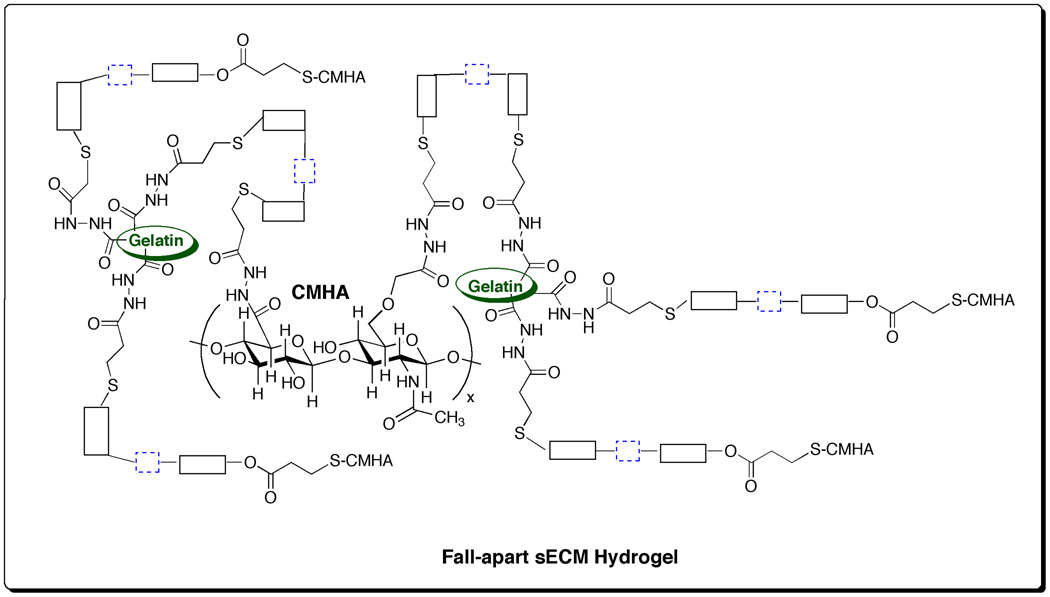
3.2. sECM hydrogels with disulfide-containing crosslinkers
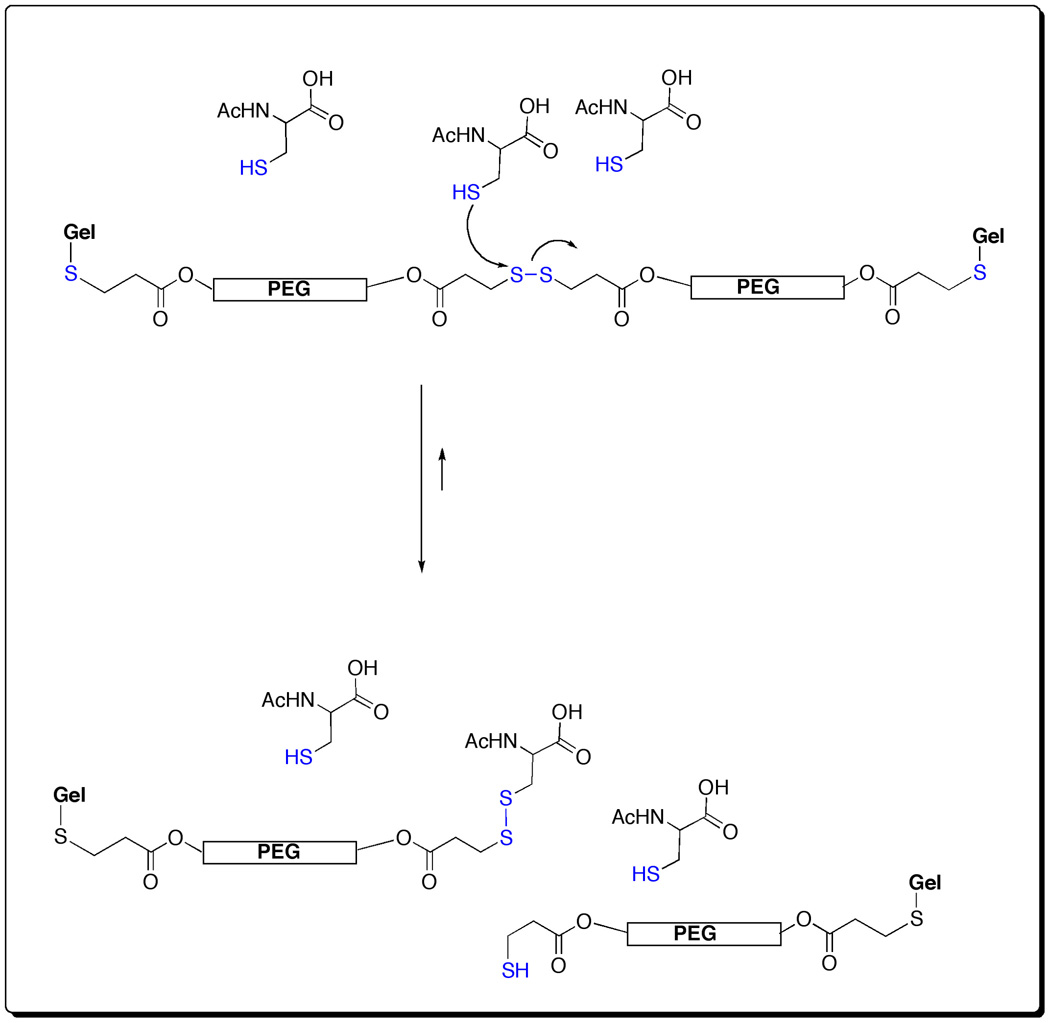
We first established that the new crosslinkers gave sECM hydrogels with suitable gelation and dissolution properties, and could be prepared from CMHA-S and gelatin-DTPH solutions (pH 7.4) using the triblock, pentablock, and heptablock disulfide-containing crosslinkers in place of PEGDA. Scheme 4 shows a representation of the molecular network generated during the crosslinking with the triblock PEGSSDA. Gelation was observed within 3 – 5 min as previously described for PEGDA [5, 21] and other newer PEG and HA-derived crosslinkers [22, 23]. Samples of the triblock, pentablock, and heptablock disulfide-containing hydrogels were also lyophilized to form porous sponges [20]. The swelling of the disulfide block copolymer hydrogels and sponges was measured in PBS at pH 7.4 over the course of 6 h (Supplementary Figure 1). Each of the disulfide block copolymer hydrogels was essentially fully swollen when prepared, with only minor (10–20%) additional swelling during this time period. In contrast, the lyophilized sponges all swelled by 13 to15–fold, with the most rapid changes occurring in the first three hours.
Scheme 4.
Network structure for sECM hydrogels formed with triblock PEGSSDA crosslinker
3.3. Redissolution of disulfide block copolymer crosslinked hydrogels
We employed the thiol-disulfide exchange reaction (Scheme 5) that occurs commonly in biochemistry to “un-crosslink” the hydrogels by cleaving many of the disulfide bonds that maintained the hydrogel structure. The kinetics of this reaction are first-order in both disulfide and thiolate anion, and are generally believed to occur by a bimolecular nucleophilic substitution reaction [17]. Given these kinetic parameters, it was necessary to employ an excess of the thiol component at a pH as high as possible, consistent with maintaining physiological compatibility. The rate of dissolution for each of the three disulfide block copolymer hydrogels (0.5 ml each) was measured at pH 7.4 during mechanical agitation in the presence of 1.0 ml of 10, 25, 50, and 100 mM of either L-cysteine (Cys) or L-glutathione (GSH) (Supplementary Figure 2). As anticipated, the re-dissolution times decreased with the increased thiol concentration. Each of the three disulfide-block containing gels was completely dissolved with 50 mM and 100 mM Cys within 90 min, the heptablock showing the most rapid dissocation. At 10 mM and 25 mM, dissolution took much longer, and at lower volumes (0.5 ml) of Cys solution, gels would re-form within a few hours. The use of Cys near its isoelectric point also led to precipitation and cloudy suspensions at 50 mM, which necessitated a switch to NAcCys in subsequent studies. With GSH as the thiol, even 10 mM and 25 mM GSH both led to dissociation in as little as 40 min for the heptablock and pentablock crosslinkers (Supplementary Figure 2).
Scheme 5.
Mechanism of re-dissolution of triblock PEGSSDA via thiol-disulfide exchange reaction
3.4. Surface culture on disulfide block copolymer crosslinked sECMs
Next, the cytocompatibility of the disulfide block copolymer crosslinked sECMs was determined. HepG2 C3A, NIH 3T3 fibroblasts, and hMSCs were seeded onto the surface of triblock PEGSSDA, pentablock PEG(SS)2DA, and heptablock PEG(SS)3DA crosslinked hydrogels and sponges (Supplementary Figure 3). As controls, cells were also seeded on tissue culture plastic and PEGDA-crosslinked sECMs (Extracel). MTS assays were conducted at Day 3 and Day 7 of surface culture on sECM gels and sponges. For all of the hydrogels, the number of cells, based on cell number proportionality to A490 measurements, was higher on day 7 than on day 3, indicating expected proliferation on a cytocompatible substratum. There were no significant differences among the disulfide block copolymers or between the disulfide block copolymers and the two controls, suggesting that each of the three disulfide-containing crosslinkers could be readily substituted for PEGDA.
There were some differences among the cell lines on both gels and sponges. The NIH 3T3 fibroblasts exhibited greater proliferation on the hydrogel sECMs than on the sponges, but both exhibited continued cell growth over 7 days. In contrast, HEPG2 C3As and hMSCs proliferated well on all hydrogel sECMs, but showed decreased proliferation on sponge sECMs relative to controls (Supplementary Figure 3). The decreased proliferation of HEPG2 C3As and hMSCs can likely be attributed the stiffer mechanical properties of the sponges, but this was not pursued further.
3.5. Encapsulation in 3-D and recovery of encapsulated cells
Having established that cells would grow on the surface of all disulfide block crosslinked sECM hydrogels, and having established initial parameters for dissolution of the hydrogels, we next examined the ability of cells to survive in 3-D culture following encapsulation using in situ crosslinked hydrogels. Thus, HepG2 C3A, NIH 3T3 fibroblasts, hMSCs, and HUVECs were encapsulated in tissue culture inserts at a cell density of 10,000 cells/100 µl into triblock PEGSSDA, pentablock PEG(SS)2DA, and heptablock PEG(SS)3DA crosslinked hydrogels. Cell viability and proliferation was determined at Day 3 and Day 7 using MTS assays. Figure 1 shows that increased proliferation was observed on Day 7 compared to Day 3 on each of the three disulfide-containing crosslinked sECMs for HepG2 C3A, NIH 3T3 fibroblasts, hMSCs. The proliferation of cells within the three disulfide-containing crosslinked sECMs were indistinguishable from the 2-D control and the PEGDA encapsulation control. For the HUVEC cells, proliferation was considerably slower, but again there were no differences among the disulfide-containing crosslinked sECMs and the controls.
Figure 1.
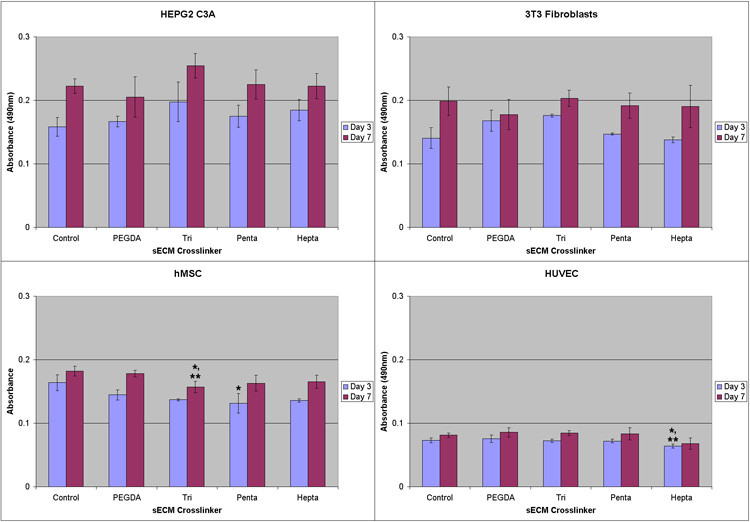
MTS absorbance readings on culture day 3 and 7. Clockwise from upper left: HEPG2 C3A hepatocytes; NIH 3T3 fibroblasts; hMSCs; HUVECs. Cells were encapsulated in sECM hydrogels using PEGDA, triblock PEGSSDA, pentablock PEG(SS)2DA and heptablock PEG(SS)3DA crosslinkers. One asterisk (*) indicates statistical difference relative to plastic control (p < 0.01) and two asterisks (**) indicate statistical difference relative to PEGDA control.
Next, we asked whether it was possible to recover viable cells from the disulfide block copolymer crosslinked sECM hydrogels. To this end, we focused on a single crosslinker, the pentablock PEG(SS)2DA, which offered two reductively cleavable modules. For these experiments, HepG2 C3A, NIH 3T3 fibroblasts, hMSCs, and HUVECs (100,000 cells) were encapsulated in 200 µl of pentablock PEG(SS)2DA crosslinked sECM hydrogels in tissue culture inserts and cultured for 3 days in 800 µl media. After removing the gel from the insert, a solution of 50 mM NAcCys in cell-specific media was prepared, adjusted to pH 7.4, and 3 ml of the NAcCys media was added to the 200 µl hydrogel in a sterile centrifuge tube and agitated by rocking at 37 °C, 5% CO2 for 1 h. By this time, the entire gel piece had completely dissolved. The tubes were then centrifuged gently to obtain a cell pellet, the dissolved sECM solution was removed by aspiration, the cells were resuspended in the appropriate medium, and then seeded onto 2% Extracel in 3-D on top cultures to determine viability and the proportion of live cells.
It should be noted that the conditions employed for cell recovery differ significantly from the simple solution-overlay method employed to assess dissolution rates. First, the gels containing encapsulated cells were completely surrounded by the dissolution solution, rather than being overlaid with solution. Second, the solution:gel ratio for the initial experiments were 2:1, while in the cell recovery studies, the ratios were increased to 15:1. Third, the agitation from the rocker platform created turbulence that further accelerated dissolution. Since the goal is to minimize the time required for cell recovery, these three simple changes had important practical implications.
A live-dead assay was first conducted for each cell type at 24, 48, and 72 h following cell recovery and re-seeding onto Extracel. Figure 2 shows representative images exhibiting the characteristic morphology and cell survival at 24 hours for HepG2 C3A hepatocytes (Figure 2a), NIH 3T3 fibroblasts (Figure 2b), hMSCs (Figure 2c), and HUVECs (Figure 2d); the HEPG2C3A cells were clustered, while the 3T3s, hMSCs, and HUVECs had adopted their normal spread and spindle-like appearance. Importantly, for each cell type, the percentage of live cells increased at each time point (Figure 3), with 80–95% viability at 3 days. This result confirmed that the treatment used to induce the dissolution of the reductively cleavable sECM hydrogels was indeed mild enough for the vast majority of the encapsulated cells to survive.
Figure 2.
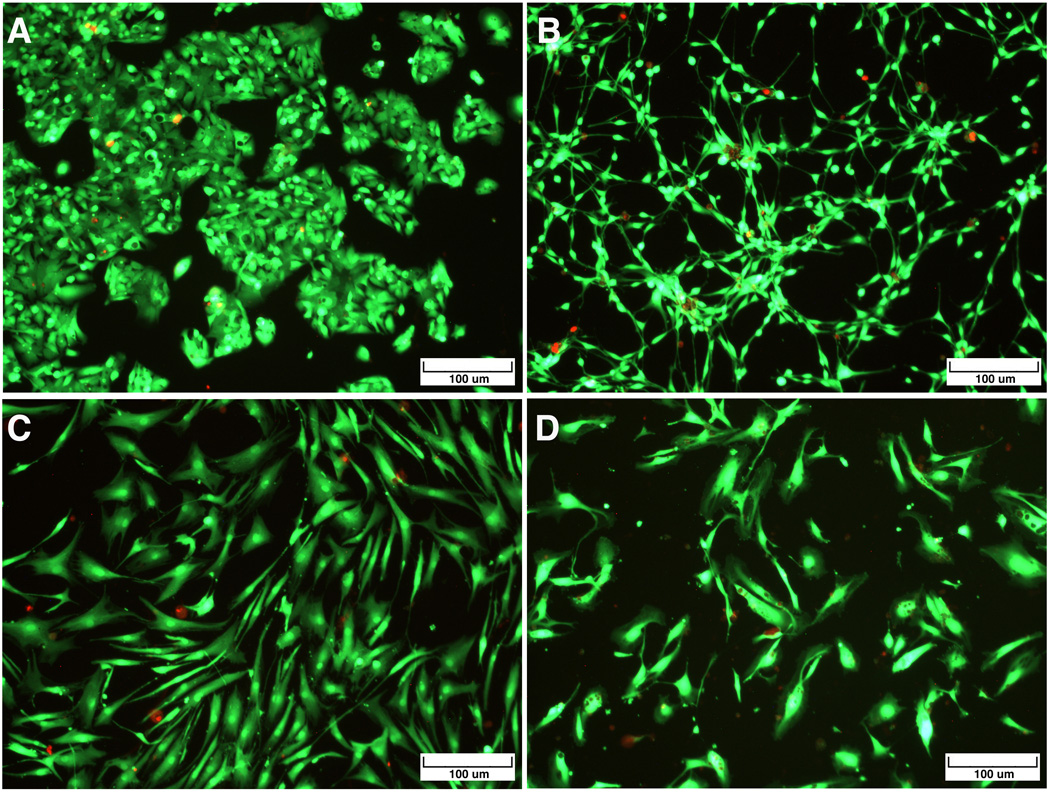
Live/Dead Images of cells recovered from pentablock PEG(SS)2DA-crosslinked sECM hydrogels by NAcCys dissolution and then re-cultured for 3 days on Extracel™. Green fluorescent calcein marks the live cells and red fluorescent EthD-1 is taken up by the dead cells. Clockwise from upper left: HEPG2 C3A hepatocytes; NIH 3T3 fibroblasts; hMSCs; HUVECs.
Figure 3.
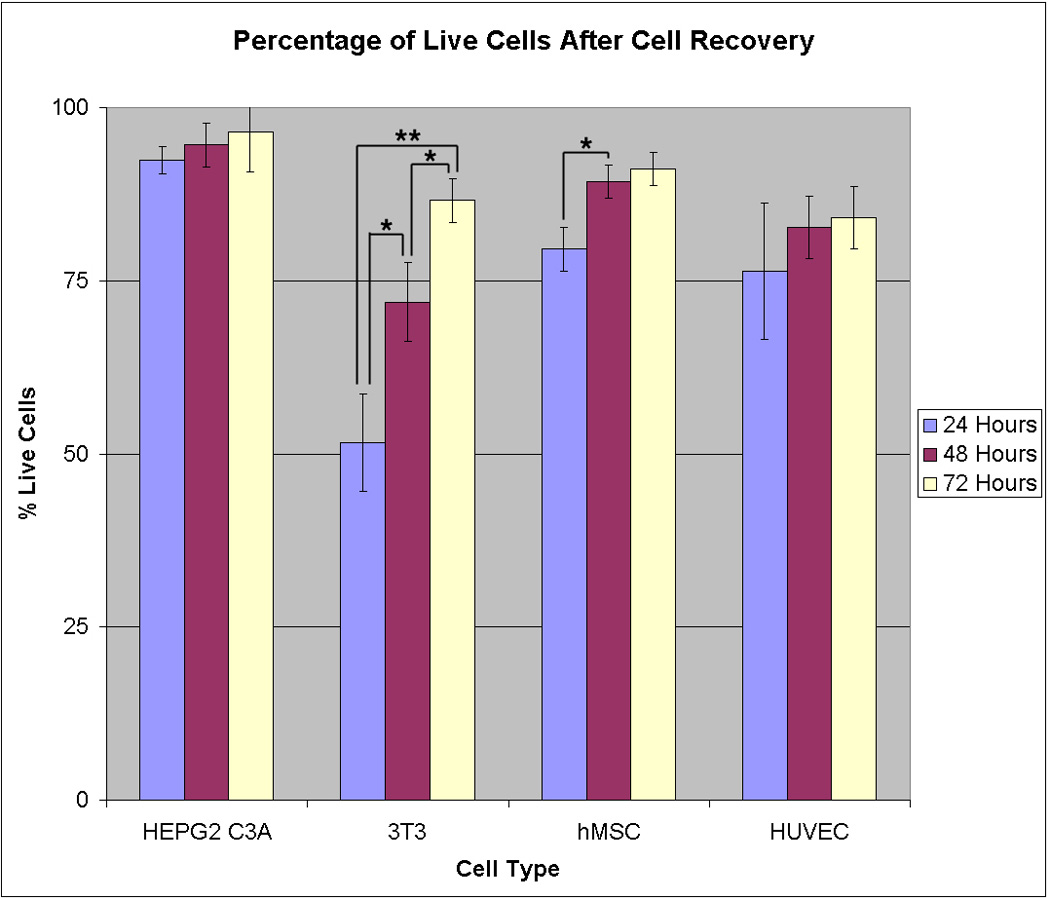
Viability of cells recovered from pentablock PEG(SS)2DA-crosslinked sECM hydrogels by NAcCys dissolution and then re-cultured for 1, 2, and 3 days on Extracel™. From left to right: HEPG2 C3A hepatocytes; NIH 3T3 fibroblasts; hMSCs; HUVECs. One asterisk (*) indicates statistical difference at p < 0.05, and two asterisks (**) indicate statistical difference at p < 0.01.
4. Discussion
To expand cells in a 3-D environment, a variety of natural, synthetic or semi-synthetic materials have been developed to recapitulate the functions of the natural extracellular matrix (ECM) in orchestrating cell proliferation, migration, differentiation, angiogenesis and invasion [24, 25]. Design principles must recreate the interwoven set of biochemical and mechanical cues in the cellular microenvironment [2, 26] and allow versatility in fabrication methods for engineered microenvironments [1, 27, 28]. Recently, the natural and synthetic materials that have been developed specifically to provide environments that mimic the stem cell niche have been reviewed [3].
A wide range of natural and synthetic hydrogels, water-swollen cross-linked polymeric structures with good biocompatibility, have been used for controlled release of bioactive macromolecules [4, 29–31] and tissue engineering [32, 33]. Hydrogels are promising cell delivery systems for the healing and regeneration of damaged tissues for several reasons. First, the soft nature of hydrogels minimizes mechanical or frictional irritation to the surrounding tissue [34]. Second, the hydrophilic surface has low interfacial tension in contact with body fluids, thus minimizing protein adsorption and unwanted cell adhesion [35]. Third, hydrogels are highly permeable, facilitating the transport of nutrients and metabolites [36]. The outstanding properties of alginate hydrogels as tools in cell delivery and tissue engineering have been recently reviewed [37], but cell recovery from these gels has been problematic. A clever enzymatic solution was recently described, in which alginate gels containing PLGA microspheres loaded with alginate lyase were employed for expansion of neural progenitor cells [38].
Many in situ-crosslinkable hydrogels based on derivatives of the polyanionic glycosaminoglycan HA derivatives have been developed in the past decade [7, 8]. Most prominent among the hydrogels useful for cell encapsulation are the chemically-crosslinked thiolated HA-PEGDA hydrogels [39] and the photocrosslinked methacrylated HA [40]. Photopolymerized HA has been manipulated to control degradation and mechanical behavior [41], used as a template for addition of peptides and to adjust modulus and swelling [42], used for chondrocyte or MSC encapsulation for repair of osteochondral defects [43, 44], and developed as a matrix for self-renewal and differentiation of human stem cells [16]. For in vitro cell recovery, a large excess of bovine-derived hyaluronidase was employed. Other applications of photocrossed glycosaminoglycan-based gels include the culturing of chondrocytes in poly(vinyl alcohol) – chondrotin sulfate photocrosslinked gels, which can be degraded with chondroitinase ABC [45].
Two alternatives to cell-recovery by non-enzymatic methods should be noted: thermoresponsive “smart” gels and physically crosslinked self-assembling peptides. Thermoresponsive polymers [31] have been used to recover cell sheets from an elastin polymer-coated inert membrane [46], from cells cultured on poly(N-isopropylacrylamide) (PIPAAm) grafted PEG surfaces [47, 48], or from cells cultured on pluronic-coated surfaces [49]. These novel surfaces allow the cultivation of cells without using enzymes by utilizing the thermoresponsive phase transition property of PNIPAAm [50]. Nonetheless, these systems have not been adapted to the 3-D encapsulation environment for cell expansion and recovery.
Polypeptide -based hydrogels can be physically crosslinked via leucine zipper domains [51], or allowed to self-assemble into a shear-thinning gel suitable for injectable cell therapy [52]. The synthetic, peptide-based ECM substitute PuraMatrix™ forms fibrous scaffolds that can be used for 3D cell embedding or surface plating, but cell recovery by “on-demand” dissociation of the matrix in vitro is not practical [53, 54].
Recently, we have emphasized the importance of using the simplest applicable material to facilitate manufacture and regulatory approval, that is, including the end-user needs as design criteria for materials to be used for cell therapy [5] and for development of drug evaluation tools [55]. Examples of end-user parameters that can now be met by pragmatic sECMs include: (i) experimentally variable composition, (ii) experimentally variable compliance, (iii) controllable biodegradability in vitro and in vivo, (iv) multiple physical forms for in situ crosslinking during cell encapsulation and fabrication, (v) batch to batch consistency, (vi) ease of use at physiological temperature and pH, (vii) transparency for ease of visualization, (viii) compatibility with high throughput screening (HTS) platforms, and (ix) translational potential. These sECMs are modular in nature, allowing the user to add soluble factors, attachment peptides, matricellular proteins, different crosslinkers, and different macromomers in any required combination [9].
Notably absent from this list is the development of hydrogels for the expansion and differentiation of cells ex vivo that permit rapid recovery of the expanded and differentiated cells. In particular, the value of being able to expand undifferentiated human postnatal or embryonic stem cells in an animal product-free environment is negated if one requires an animal-derived protease or glycosidase to release the cells from the 3-D hydrogel environment. This design criterion is not a necessity for classical forms of tissue engineering or cell therapy, in which “cells + scaffold + soluble factors” are used to create an implantable or injectable scaffold. For these constructs, a biodegradable material is sufficient, and engineers have developed a myriad of materials that degrade by bulk erosion via hydrolysis or by endogenous enzymatic degradation in vivo as the two most common mechanisms of biodegradation. However, for the ex vivo expansion and ultimate manipulation of stem cells for producing clinically-useful engineered tissues or cell therapy products, a simple and practical non-enzymatic method was required.
To this end, we prepared the disulfide block PEG(SS)nDA copolymers and created the first covalently-crosslinked 3-D sECM hydrogel that permits a non-enzymatic dissociation of the gel under mild physiologically well-tolerated conditions. Furthermore, encapsulated cells grow and proliferate in 3-D, and a high yield of viable cells can be obtained following gel dissolution by using the well-known thiol-disulfide exchange reaction that occurs in normal biochemical processes. The process of cell encapsulation and cell recovery is illustrated schematically in Figure 4. While the proof-of-concepts experiments described herein employed a chemically-modified gelatin derivative for attachment and spreading of normal adherent cells, the same principles are applicable to using the disulfide block PEG(SS)nDA copolymers to crosslink chemically modified HA hydrogels, such as the CMHA-S (a.k.a., Glycosil) employed for a metabolomic study involving self-renewal and expansion of human fetal hepatic stem cells [56], or the photocrosslinked HA methyacrylate gels [16] used to expand and differentiate human embryonic stem cells.
Figure 4.
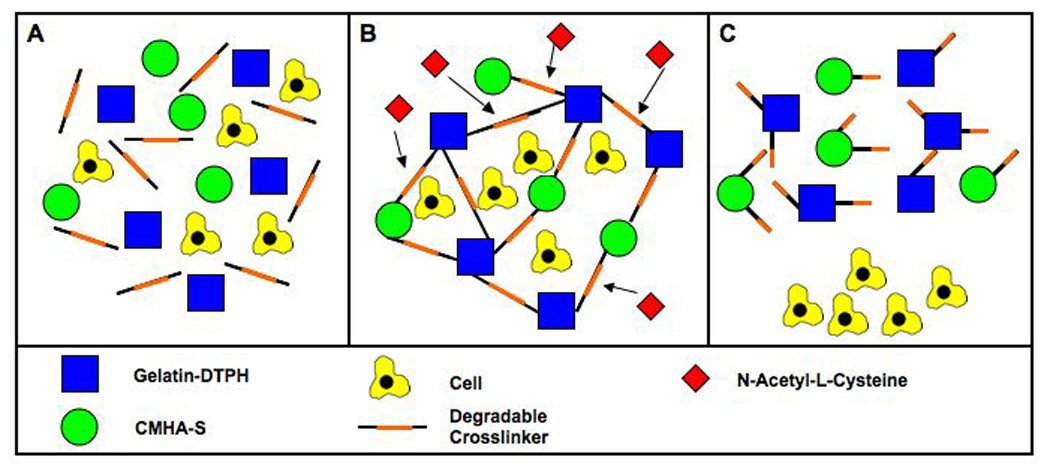
Schematic representation of encapsulation and recovery of cells from fall-apart crosslinked sECMs.
The incorporation of degradable crosslinkers makes 3-D expansion and differentiation of progenitor cells more practical and scalable. The principal drawback of the majority of hydrogels is in recovering cells from the 3-D environment. Simple enzymatic treatment does not always allow facile recovery of cell encapsulated in 3-D. In contrast, the disulfide containing crosslinkers were readily dissociated using the thiol-disulfide exchange reaction with Cys, NAcCys, or GSH as the biocompatible thiol component. Preliminary studies with encapsulated cells suggested that for maximum viability, it was necessary to limit the thiol component concentration to 25 mM and to employ agitation and diffusion conditions to limit the exposure time to one or two hours. At 25 mM GSH or NAcCys, even after 12 hours of incubation, enough healthy cells survived to proliferate into a confluent monolayer 3 days after replating, but viability was clearly reduced. In each of the triblock, pentablock, and heptablock crosslinked gels cells consistently at Day 3 and Day 7 relative to PEGDA and plastic controls, indicating complete biocompatibility of the new crosslinkers. Thus, the “fall-apart” disulfide-containing crosslinkers can be used in place for PEGDA.
There was a noticeable difference between the proliferation of cells on and in gels and on sponges. All cell types performed well when surface or encapsulation cultured using our hydrogels. However, when cultured on sponges, 3T3 fibroblasts were the only cell type to proliferate after day 3. HEPG2 C3As and hMSCs decreased in number from day 3 to day 7. Based on the encouraging data from all of our gels and that of the 3T3 fibroblasts on sponges, we can conclude that this decrease in cell number is not due to toxicity. It is likely that the mechanical properties, or simply the stiffness, of the sponges affected the proliferation rates of the different cell types.
Photopolymerized poly(ethylene glycol) diacrylate (PEGDA) has become a staple as a research tool for preparing “bio-neutral”, synthetic, degradable, and readily customized matrices for tissue engineering and drug delivery [57]. Multiarmed PEG derivatives with matrix metalloprotease-cleavable peptide linkages provide specific microenvironments engineered to direct morphogenesis [25] and cell-invasion characteristics [13]. Photopolymerized poly(ethylene glycol) (PEG) hydrogels have also used for manipulating cell matrix interactions of hMSCs in response to peptides and pendant phosphates [58]. Recently, photopolymerized pluronic hydrogels were developed for injectable cell delivery [59]. We considered the possibility that the disulfide block PEG(SS)nDA copolymers could be used for photochemical crosslinking, analogous to the use of PEGDA as a “blank slate” [1, 33, 60] onto which additional peptides may be grafted or other factors embedded. We hypothesized that we could create a blank slate environment that could be designed to “fall apart” on demand under non-enzymatic conditions. To this end, we attempted to crosslink a 10% w/v solution of the triblock PEGSSDA in the presence of a catalytic (1%) amount of acetophenone in N-vinylpyrrolidone added to the solution, followed by irradiation at 365 nm for 1 min (data not shown). Robust gels were formed using this protocol with PEGDA, but none of the PEG(SS)nDA crosslinkers formed a hydrogel over a wide range of conditions. Addition of cysteamine, a soluble disulfide at 100 mM failed to “protect” the crosslinker disulfide against radical-induced scission of the PEGSSDA; indeed, 100 mM cysteamine was an effective inhibitor of gelation of PEGDA by virtue of its radical scavenging ability.
5. Conclusion
Three block disulfide-block containing polyethylene glycol diacrylate crosslinkers were synthesized, characterized, and used to prepare sECMs that could be disassembled under mild reductive conditions. Murine NIH 3T3 fibroblasts, human HepG2 C3A hepatocytes, human bone-marrow derived mesenchymal stem cells (MSCs), and human umbilical vein endothelial cells (HUVECs) all showed excellent viability and growth during expansion in 3-D in each of the triblock, pentablock, and heptablock copolymer crosslinked sECMs. After cell expansion, the reductively-cleavable hydrogels could be dissociated by the thiol-disulfide exchange using N-acetyl-cysteine or glutathione. After dissolution of the hydrogels, cells were recovered in high yield and with high viability by gentle centrifugation. The reductively-cleavable crosslinkers offer a platform that can be employed for a wide variety of hydrogel sECM compositions. Moreover, the readily dissolvable hydrogels allow for non-enzymatic cell recovery to facilitate comparative proteomic and genomic profiling of cells grown in 2-D vs. 3-D, with specific growth factors, with different gel compliance, or with different added matricellular proteins.
Supplementary Material
Acknowledgement
This research was supported by an NSF FIBR Grant (EF-0526854), by the State of Utah Centers of Excellence Program, and by NIH Grant 2 R01 DC04336 (to S. L. Thibeault and G.D. Prestwich). We thank Dr. J. A. Scott, Glycosan BioSystems, Inc. for providing the Extracel™ kits and selected crosslinkable sECM components.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jianxing Zhang, Center for Therapeutic Biomaterials, Department of Medicinal Chemistry, The University of Utah, 419 Wakara Way Suite 205, Salt Lake City, UT 84108-1257.
Aleksander Skardal, Center for Therapeutic Biomaterials, Department of Bioengineering, The University of Utah, 419 Wakara Way Suite 205, Salt Lake City, UT 84108-1257.
Glenn D. Prestwich, Center for Therapeutic Biomaterials, Department of Medicinal Chemistry, Department of Bioengineering, The University of Utah, 419 Wakara Way Suite 205, Salt Lake City, UT 84108-1257
References
- 1.Cushing MC, Anseth KS. Materials science. Hydrogel cell cultures. Science. 2007;316:1133–1134. doi: 10.1126/science.1140171. [DOI] [PubMed] [Google Scholar]
- 2.Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24:4337–4351. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 3.Dawson E, Mapili G, Erickson K, Taqvi S, Roy K. Biomaterials for stem cell differentiation. Adv Drug Deliv Rev. 2008;60:215–228. doi: 10.1016/j.addr.2007.08.037. [DOI] [PubMed] [Google Scholar]
- 4.Hoffman AS. Hydrogels for biomedical applications. Adv Drug Deliv Rev. 2002;54:3–12. doi: 10.1016/s0169-409x(01)00239-3. [DOI] [PubMed] [Google Scholar]
- 5.Prestwich GD. Simplifying the extracellular matrix for 3-D cell culture and tissue engineering: A pragmatic approach. J. Cell. Biochem. 2007;101:1370–1383. doi: 10.1002/jcb.21386. [DOI] [PubMed] [Google Scholar]
- 6.Toole BP. Hyaluronan in morphogenesis. Semin. Cell Dev. 2001;12:79–87. doi: 10.1006/scdb.2000.0244. [DOI] [PubMed] [Google Scholar]
- 7.Allison D, Grande-Allen K. Hyaluronan: a powerful tissue engineering tool. Biomaterials. 2006;12:2131–2140. doi: 10.1089/ten.2006.12.2131. [DOI] [PubMed] [Google Scholar]
- 8.Shu XZ, Prestwich GD. Therapeutic biomaterials from chemically modified hyaluronan. In: Garg HG, Hales CA, editors. Chemistry and Biology of Hyaluronan. Amsterdam: Elsevier Press; 2004. pp. 475–504. [Google Scholar]
- 9.Serban MA. Making modular extracellular matrices: solutions for the puzzle. Methods. 2008;45:93–98. doi: 10.1016/j.ymeth.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serban MA, Liu Y, Prestwich GD. Effects of synthetic extracellular matrices on primary human fibroblast behavior. Acta Biomaterialia. 2008;4:67–75. doi: 10.1016/j.actbio.2007.09.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Discher D, Janmey P, Wang Y-l. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 12.Ghosh K, Pan Z, Guan E, Ge S, Liu Y, Nakamura T, et al. Cell adaptation to a physiologically relevant ECM mimic with different viscoelastic properties. Biomaterials. 2007;28:671–679. doi: 10.1016/j.biomaterials.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lutolf MP, Lauer-Fields JL, Schmoekel HG, Metters AT, Weber FE, Fields GB, et al. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: engineering cell-invasion characteristics. Proc Natl Acad Sci U S A. 2003;100:5413–5418. doi: 10.1073/pnas.0737381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moss JA, Stokols S, Hixon MS, Ashley FT, Chang JY, Janda KD. Solid-phase synthesis and kinetic characterization of fluorogenic enzyme-degradable hydrogel cross-linkers. Biomacromolecules. 2006;7:1011–1016. doi: 10.1021/bm051001s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mann BK, Gobin AS, Tsai AT, Schmedlen RH, West JL. Smooth muscle cell growth in photopolymerized hydrogels with cell adhesive and proteolytically degradable domains: synthetic ECM analogs for tissue engineering. Biomaterials. 2001;22:3045–3051. doi: 10.1016/s0142-9612(01)00051-5. [DOI] [PubMed] [Google Scholar]
- 16.Gerecht S, Burdick J, Ferreira L, Townsend S, Langer R, Vunjak-Novakovic G. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proc Natl. Acad. Sci USA. 2007;104:11298–11303. doi: 10.1073/pnas.0703723104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bach RD, Dmitrenko O, Thorpe C. Mechanism of thiolate-disulfide interchange reactions in biochemistry. J. Org. Chem. 2008;73:12–21. doi: 10.1021/jo702051f. [DOI] [PubMed] [Google Scholar]
- 18.Shu XZ, Liu Y, Luo Y, Roberts MC, Prestwich GD. Disulfide cross-linked hyaluronan hydrogels. Biomacromolecules. 2002;3:1304–1311. doi: 10.1021/bm025603c. [DOI] [PubMed] [Google Scholar]
- 19.Shu XZ, Liu Y, Palumbo F, Prestwich GD. Disulfide-crosslinked hyaluronan-gelatin hydrogel films: a covalent mimic of the extracellular matrix for in vitro cell growth. Biomaterials. 2003;24:3825–3834. doi: 10.1016/s0142-9612(03)00267-9. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Ahmad S, Shu XZ, Sanders RK, Kopesec SA, Prestwich GD. Accelerated repair of cortical bone defects using a synthetic extracellular matrix to deliver human demineralized bone matrix. J. Orthoped. Res. 2006;24:1454–1462. doi: 10.1002/jor.20148. [DOI] [PubMed] [Google Scholar]
- 21.Shu XZ, Ahmad S, Liu Y, Prestwich GD. Synthesis and evaluation of injectable, in situ crosslinkable synthetic extracellular matrices (sECMs) for tissue engineering. J. Biomed. Mater. Res. A. 2006;79A:902–912. doi: 10.1002/jbm.a.30831. [DOI] [PubMed] [Google Scholar]
- 22.Serban MA, Prestwich GD. Synthesis of hyaluronan haloacetates and biology of novel crosslinker-free synthetic extracellular matrix hydrogels. Biomacromolecules. 2007;8:2821–2828. doi: 10.1021/bm700595s. [DOI] [PubMed] [Google Scholar]
- 23.Vanderhooft JL, Mann BK, Prestwich GD. Synthesis and characterization of novel thiol-reactive poly(ethylene glycol) crosslinkers for biomaterials. Biomacromolecules. 2007;8:2883–2889. doi: 10.1021/bm0703564. [DOI] [PubMed] [Google Scholar]
- 24.Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix-cytoskeleton crosstalk. Nat. Rev. Mol. Cell Biol. 2001;2:793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- 25.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 26.Griffith L, Swartz M. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Molec. Cell Biol. 2006;7:211–224. doi: 10.1038/nrm1858. [DOI] [PubMed] [Google Scholar]
- 27.Yeh J, Ling Y, Karp JM, Gantz J, Chandawarkar A, et al. Micromolding of shape-controlled, harvestable cell-laden hydrogels. Biomaterials. 2006;27:5391–5398. doi: 10.1016/j.biomaterials.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Bhadriraju K, Chen CS. Engineering cellular microenvironments to improve cell-based drug testing. Drug Discov Today. 2002;7:612–620. doi: 10.1016/s1359-6446(02)02273-0. [DOI] [PubMed] [Google Scholar]
- 29.Recum H, Okano T, Kim SW. Growth factor release from thermally reversible tissue culture substrate. J. Controlled Release. 1998;55:121–130. doi: 10.1016/s0168-3659(98)00042-x. [DOI] [PubMed] [Google Scholar]
- 30.Elbert DL, Pratt AB, Lutolf MP, Halstenberg S, Hubbell JA. Protein delivery from materials formed by self-selective conjugate addition reactions. J Control Release. 2001;76:11–25. doi: 10.1016/s0168-3659(01)00398-4. [DOI] [PubMed] [Google Scholar]
- 31.Jeong B, Kim SW, Bae YH. Thermosensitive sol-gel reversible hydrogels. Adv Drug Deliv Rev. 2002;54:37–51. doi: 10.1016/s0169-409x(01)00242-3. [DOI] [PubMed] [Google Scholar]
- 32.Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev. 2001;101:1869–1879. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 33.Bryant SJ, Anseth KS. Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly(ethylene glycol) hydrogels. J Biomed Mater Res. 2002;59:63–72. doi: 10.1002/jbm.1217. [DOI] [PubMed] [Google Scholar]
- 34.Uludag H, De Vos P, Tresco PA. Technology of mammalian cell encapsulation. Adv Drug Deliv Rev. 2000;42:29–64. doi: 10.1016/s0169-409x(00)00053-3. [DOI] [PubMed] [Google Scholar]
- 35.Abraham S, Brahim S, Ishihara K, Guiseppi-Elie A. Molecularly engineered p(HEMA)-based hydrogels for implant biochip biocompatibility. Biomaterials. 2005;26:4767–4778. doi: 10.1016/j.biomaterials.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 36.Tse MU H, Sefton MV, Chang PL. Secretion of recombinant proteins from hydroxyethyl methacrylate-methyl methacrylate capsules. Biotechnol. bioeng. 1996;51:271–280. doi: 10.1002/(SICI)1097-0290(19960805)51:3<271::AID-BIT3>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 37.Augst AD, Kong HJ, Mooney DJ. Alginate hydrogels as biomaterials. Macromol Biosci. 2006;6:623–633. doi: 10.1002/mabi.200600069. [DOI] [PubMed] [Google Scholar]
- 38.Ashton RS, Banerjee A, Punyani S, Schaffer DV, Kane RS. Scaffolds based on degradable alginate hydrogels and poly(lactide-co-glycolide) microspheres for stem cell culture. Biomaterials. 2007;28:5518–5525. doi: 10.1016/j.biomaterials.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 39.Shu XZ, Liu Y, Palumbo FS, Luo Y, Prestwich CD. In situ crosslinkable hyaluronan hydrogels for tissue engineering. Biomaterials. 2004;25:1339–1348. doi: 10.1016/j.biomaterials.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 40.Baier Leach J, Bivens KA, Patrick CW, Jr, Schmidt CE. Photocrosslinked hyaluronic acid hydrogels: natural, biodegradable tissue engineering scaffolds. Biotechnol Bioeng. 2003;82:578–589. doi: 10.1002/bit.10605. [DOI] [PubMed] [Google Scholar]
- 41.Burdick JA, Chung C, Jia XQ, Randolph MA, Langer R. Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules. 2005;6:386–391. doi: 10.1021/bm049508a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park YD, Tirelli N, Hubbell JA. Photopolymerized hyaluronic acid-based hydrogels and interpenetrating networks. Biomaterials. 2003;24:893–900. doi: 10.1016/s0142-9612(02)00420-9. [DOI] [PubMed] [Google Scholar]
- 43.Nettles DL, Vail TP, Morgan MT, Grinstaff MW, Setton LA. Photocrosslinkable hyaluronan as a scaffold for articular cartilage repair. Ann Biomed Eng. 2004;32:391–397. doi: 10.1023/b:abme.0000017552.65260.94. [DOI] [PubMed] [Google Scholar]
- 44.Liu Y, Shu XZ, Prestwich GD. Osteochondral defect repair with autologous bone marrow-derived mesenchymal stem cells in an injectable, in situ crosslinked synthetic extracellular matrix. Tissue Eng. 2006:3405–3416. doi: 10.1089/ten.2006.12.3405. [DOI] [PubMed] [Google Scholar]
- 45.Bryant SJ, Davis-Arehart KA, Luo N, Shoemaker RK, Arthur JA, Anseth KS. Synthesis and characterization of photopolymerized multifunctional hydrogels: water-soluble poly(vinyl alcohol) and chondroitin sulfate macromers for chondrocyte encapsulation. Macromolecules. 2004;37:6726–6733. [Google Scholar]
- 46.Zhang H, Iwama M, Akaike T, Urry DW, Pattanaik A, Parker TM, et al. Human amniotic cell sheet harvest using a novel temperature-responsive culture surface coated with protein-based polymer. Tissue Eng. 2006;12:391–401. doi: 10.1089/ten.2006.12.391. [DOI] [PubMed] [Google Scholar]
- 47.Kwon HO, Kikuchi A, Yamato M, Okano T. Accelerated cell sheet recovery by co-grafting of PEG with PIPAAm onto porous cell culture membranes. Biomaterials. 2003;24:1223–1232. doi: 10.1016/s0142-9612(02)00469-6. [DOI] [PubMed] [Google Scholar]
- 48.Kwon OH, Kikuchi A, Yamato M, Sakurai Y, Okano T. Rapid cell sheet detachment from poly(N-isopropylacrylamide)-grafted porous cell culture membranes. J Biomed Mater Res. 2000;50:82–89. doi: 10.1002/(sici)1097-4636(200004)50:1<82::aid-jbm12>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 49.Higuchi A, Yamamoto T, Sugiyama K, Hayashi S, Tak TM, Nakagawa T. Temperature-dependent cell detachment on Pluronic gels. Biomacromolecules. 2005;6:691–696. doi: 10.1021/bm0494850. [DOI] [PubMed] [Google Scholar]
- 50.Moran MT, Carroll WM, Selezneva I, Gorelov A, Rochev Y. Cell growth and detachment from protein-coated PNIPAAm-based copolymers. J Biomed Mater Res A. 2007;81:870–876. doi: 10.1002/jbm.a.31089. [DOI] [PubMed] [Google Scholar]
- 51.Shen W, Zhang K, Kornfield JA, Tirrell DA. Tuning the erosion rate of artificial protein hydrogels through control of network topology. Nat Mater. 2006;5:153–158. doi: 10.1038/nmat1573. [DOI] [PubMed] [Google Scholar]
- 52.Haines-Butterick L, Rajagopal K, Branco M, Salick D, Rughani R, Pilarz M, et al. Controlling hydrogelation kinetics by peptide design for three-dimensional encapsulation and injectable delivery of cells. Proc Natl Acad Sci U S A. 2007;104:7791–7796. doi: 10.1073/pnas.0701980104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang S, Holmes TC, DiPersio CM, Hynes RO, Su X, Rich A. Self-complementary oligopeptide matrices support mammalian cell attachment. Biomaterials. 1995;16:1385–1393. doi: 10.1016/0142-9612(95)96874-y. [DOI] [PubMed] [Google Scholar]
- 54.Semino CE, Merok JR, Crane GG, Panagiotakos G, Zhang S. Functional differentiation of hepatocyte-like spheroid structures from putative liver progenitor cells in three-dimensional peptide scaffolds. Differentiation. 2003;71:262–270. doi: 10.1046/j.1432-0436.2003.7104503.x. [DOI] [PubMed] [Google Scholar]
- 55.Prestwich GD. Evaluating drug toxicity and efficacy in three dimensions: using synthetic extracellular matrices in drug discovery. Acc. Chem. Res. 2008;41:139–148. doi: 10.1021/ar7000827. [DOI] [PubMed] [Google Scholar]
- 56.Turner WS, Seagle C, Galanko J, Favorov O, Prestwich GD, Macdonald JM, et al. Nuclear magnetic resonance metabolomic footprinting of human hepatic stem cells and hepatoblasts cultured in hyaluronan hydrogels. Stem Cells. 2008 doi: 10.1634/stemcells.2007-0863. in press. [DOI] [PubMed] [Google Scholar]
- 57.Anseth KS, Metters AT, Bryant SJ, Martens PJ, Eliseeff JH, Bowman CN. In situ forming degradable networks and their application in tissue engineering and drug delivery. J. Control. Release. 2002;78:199–209. doi: 10.1016/s0168-3659(01)00500-4. [DOI] [PubMed] [Google Scholar]
- 58.Nuttelman CR, Tripodi MC, Anseth KS. Synthetic hydrogel niches that promote hMSC viability. Matrix Biol. 2005;24:208–218. doi: 10.1016/j.matbio.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 59.Lee SY, Tae G. Formulation and in vitro characterization of an in situ gelable, photo-polymerizable Pluronic hydrogel suitable for injection. J Control Release. 2007;119:313–319. doi: 10.1016/j.jconrel.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 60.Mahoney MJ, Anseth KS. Three-dimensional growth and function of neural tissue in degradable polyethylene glycol hydrogels. Biomaterials. 2006;27:2265–2274. doi: 10.1016/j.biomaterials.2005.11.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.