Abstract
American Indians have a disproportionately high rate of kidney disease likely due to a combination of increased environmental and genetic risk factors. In an attempt to localize genes influencing kidney disease risk factors, we performed a genome wide scan of estimated glomerular filtration rate on participants of the Strong Heart Family Study. Over 3 600 men and women from 13 American Indian tribes were recruited from 3 centers (Arizona, North and South Dakota, Oklahoma). Using SOLAR 2.1.2, multipoint variance component linkage analysis was performed in each center as well as the entire cohort after controlling for center effects. Two modeling strategies were utilized: model 1 incorporated age, sex and interaction terms and model 2 additionally controlled for diabetic status, systolic and diastolic blood pressure, body mass index, low density lipoproteins, high density lipoproteins, triglycerides and smoking status. Significant evidence for linkage in Arizona lay on 12p12.2 at 39cM nearest marker D12S310 (LOD=3.5). Additional loci with suggestive evidence for linkage were detected at 1p36.31 (LOD=2.0–2.3), 2q33.3 (LOD=1.8) and 9q34.2 (LOD=2.4). No significant evidence for additive interaction with diabetes, hypertension or obesity was noted. In conclusion, we found evidence for linkage of a quantitative trait locus influencing estimated glomerular filtration rate to a region of chromosome 12p in a large cohort of American Indians.
Introduction
Chronic kidney disease (CKD) is an important public health problem affecting 8–11% of the U.S. population.(1, 2) In addition to the rising burden of end stage renal disease (ESRD), CKD is also a strong risk factor for cardiovascular disease and death. Worsening glomerular filtration rate (GFR) can result in up to a 3-fold risk of cardiovascular events and a 6-fold risk of death.(3) In the U.S., diabetes and hypertension are the most common risk factors for CKD and end-stage renal disease.(4) Even in the absence of diabetes, metabolic syndrome is strongly associated with reduced GFR.(5, 6) Other identified risk factors include glucose control(7, 8), blood pressure control(9, 10), body mass index(11), cholesterol levels(12, 13) and smoking(14, 15).
American Indians are known to have higher rates of ESRD than the general population, approximately twice that of Caucasians.(4, 16, 17) The vast majority of kidney disease in American Indians is due to diabetes, with rates of diabetic ESRD approximately four times higher than in Caucasians, and continuing to climb at a rapid pace in younger age groups.(4) The prevalence of earlier stages of CKD is also high. A cohort study of Navajo Indians found 3–6% of nondiabetics and 10–11% of diabetics had a creatinine clearance of less than 65ml/min.(18) Environmental risk factors for kidney disease are highly prevalent in this population. The prevalence of diabetes, hypertension, hypercholesterolemia and obesity in American Indians over 40 years of age has been reported to range from 40–60%, 25–35%, 30–40% and 20–40%, respectively.(19–21) Smoking is highly prevalent with 26–38% of American Indians being active smokers.(19)
Genetic predisposition to kidney disease is well accepted.(17, 22–24) Heritable factors have long been considered a component of diabetic kidney disease,(25, 26) with multiple genomic loci indicated in the development and progression of diabetic nephropathy.(23, 24, 27–31) Indeed, studies have implicated the same genes or genomic regions in the predisposition or progression of any kidney disease, irrespective of the initial insult.(32–36) Genetic factors may also play a role in the variability of creatinine and GFR in populations without kidney disease.(34, 37–39) The aim of the present study was to identify genetic loci that are linked to the gene(s) that influence phenotypic variation of GFR in a large population of American Indian families.
Results
A total of 3 665 individuals were available for analysis from the Strong Heart Family Study (SHFS) (Arizona = 1 235; Dakotas = 1 220; Oklahoma = 1 210). Descriptive characteristics of all SHFS participants are summarized in table 1. The average age ± standard deviation of participants in each center was approximately 39 years ± 15; 41 years ± 17 and 44 years ± 17 in Arizona, North and South Dakota and Oklahoma, respectively. Diabetes, hypertension and obesity were highly prevalent, especially in the Arizona center. Kidney disease was common, with albuminuria (urine albumin:creatinine ratio[ACR] >30 μg/mg) present in approximately 19% and depressed GFR (<60ml/min/1.73m2) present in approximately 7% of individuals in all centers (center-specific data not shown). There was wide variation in albuminuria and estimated GFR with means of 106μg/mg ± 598 and 99ml/min/1.73m2 ± 27 in all centers, respectively. The distribution of CKD as defined by the Kidney Disease Quality of Outcomes Initiative (KDOQI), amongst all centers was: 11% stage 1, 28% stage 2, 4% stage 3, 0.5% stage 4 and 1% stage 5 CKD. Twenty percent (n=722) of all observations had an eGFR > 120ml/min/1.73m2. Five percent (n=182) of all observations had an eGFR > 120ml/min/1.73m2 and no proteinuria. For the analyses of those not on antihypertensive medications, there were 738 participants deleted from analysis and 486 of these had either diabetes and/or proteinuria.
Table 1.
Descriptive statistics* of Phase IV Strong Heart Family Study participants (2001–2003).
| Characteristics | All Centers N=3665 | Arizona N=1235 | Dakotas N=1220 | Oklahoma N= 1210 | |
|---|---|---|---|---|---|
| Age, years | 40 (17) | 37 (16) | 39 (17) | 44 (17) | |
| Female gender, N (%) | 2 197 (60) | 769 (62) | 717 (59) | 711 (59) | |
| Urine ACR, μg/mg | 97 (574) | 152 (728) | 76 (508) | 64 (439) | |
| EstimatedGFR,† ml/min/1.73m2 | 100 (29) | 112 (33) | 96 (25) | 93 (24) | |
| Body mass index, kg/m2 | 32 (8) | 35 (9) | 30 (7) | 31 (7) | |
| Systolic BP, mmHg | 123 (17) | 121 (17) | 120 (16) | 127 (17) | |
| Diastolic BP, mmHg | 76 (11) | 77 (12) | 75 (11) | 77 (11) | |
| LDL-C, mmol/L | 2.5 (0.8) | 2.4 (0.7) | 2.6 (0.8) | 2.6 (0.8) | |
| HDL-C, mmol/L | 1.3 (0.4) | 1.3 (0.4) | 1.3 (0.4) | 1.4 (0.4) | |
| Triglycerides, mmol/L | 4.3 (4.4) | 4.4 (3.5) | 4.2 (5.2) | 4.5 (4.4) | |
| Hemoglobin A1C, % | 8.4 (2.1) | 8.7 (2.2) | 7.8 (1.9) | 8.2 (2.0) | |
| Hypertension, N (%) | 1 153 (31) | 420 (34) | 285 (23) | 448 (37) | |
| Antihypertensive treatment, N (%) | 738 (20) | 276 (22) | 186 (15) | 276 (23) | |
| Diabetes, N (%) | 830 (23) | 410 (33) | 172 (14) | 248 (21) | |
| Current | 1 230 (34) | 311 (25) | 518 (42) | 401 (33) | |
| Smoking, N (%) | Former | 885 (24) | 303 (25) | 284 (23) | 298 (25) |
| Never | 1 532 (42) | 606 (49) | 416 (34) | 510 (42) |
Means (± standard deviations) unless otherwise specified.
Calculated using simplified MDRD.
ACR= Albumin to Creatinine Ratio; BP=blood pressure; LDL-C=Low Density Lipoprotein Cholesterol; HDL-C= High Density Lipoprotein Cholesterol
Genetic data were available for > 60 000 relative pairs in the full dataset and > 18 000, 22 000 and 18 000 relative pairs in Arizona, the Dakotas and Oklahoma, respectively. The number of participants with available genetic and covariate data for the model 1 and model 2 analyses were: 3 605 and 3 536 in all centers; 1 215 and 1 177 in Arizona; 1 186 and 1 174 in the Dakotas; and 1 204 and 1 185 in Oklahoma. Using the fully adjusted models, the heritability estimates (standard errors [SE]) of eGFR were consistent across centers: approximately 0.33 (SE=0.03), 0.33 (SE=0.06), 0.34 (SE=0.05), 0.33 (SE=0.06) in the full sample, Arizona, the Dakotas and Oklahoma, respectively.
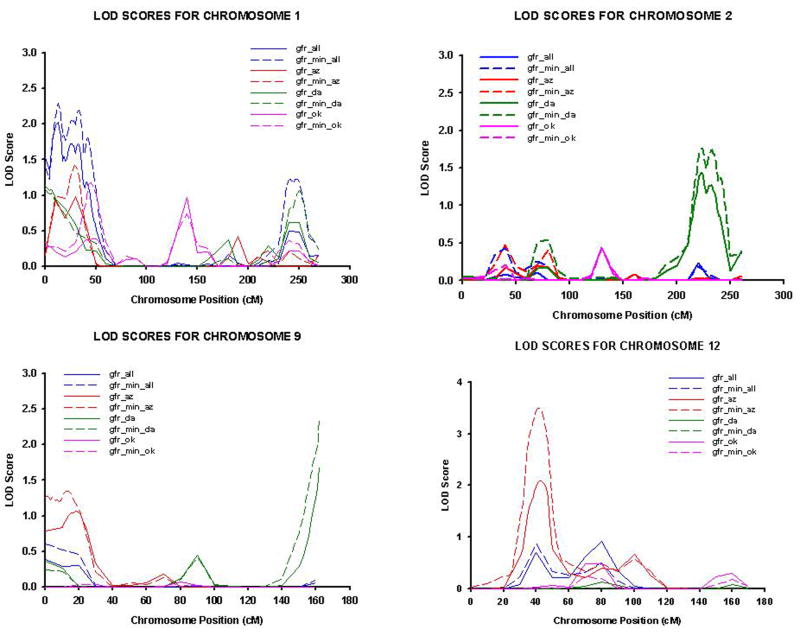
Analyses yielding logarithm of odds (LOD) scores ≥ 1.8 are displayed in table 2. There was evidence for a quantitative trait locus (QTL) for GFR in the Arizona center using model 1 (LOD = 3.5) on chromosome 12 at 39cM (nearest marker D12S31), with a 16 cM 1-LOD unit support interval spanning the regions 12p11.23 – 12p13.1.(40) Loci with suggestive evidence for linkage included 1p36.31 in all centers using both models (LOD=2.3, 2.0, respectively), 2q33.3 in the Dakotas using model 1 (LOD=1.8) and 9q34.2 using model 1 in the Dakotas (LOD=2.4).(40) Data were very similar with the use of ranked inverse serum creatinine as the outcome measure (results not shown).
Table 2.
Logarithm of Odds (LOD) scores suggestive of linkage* (LOD ≥ 1.8) using two modeling strategies for multipoint quantitative trait linkage analyses of ranked estimated glomerular filtration rate using the simplified Modification of Diet in Renal Disease (MDRD) equation in phase IV participants of the Strong Heart Family Study (2001–2003).
| Center | Chromosome | Location (cM) | Chromosomal Region | Nearest Marker | Empirical LOD score (model 1, model 2)† |
|---|---|---|---|---|---|
| All Centers | 1 | 12 | 1p36.31 | D1S214 | 2.3, 2.0 |
| Dakotas | 2 | 205 | 2q33.3 | D2S325 | 1.8, 0.5 |
| Dakotas | 9 | 150 | 9q34.2 | D9S164 | 2.4, 1.7 |
| Arizona | 12 | 39 | 12p12.2 | D12S310 | 3.5, 2.1 |
Linkage significance criteria were as suggested by Rao and Gu (2001).
Model 1 was adjusted for age, sex, age squared and age-sex interactions.
Model 2 was additionally adjusted for diabetic status, body mass index, systolic blood pressure, diastolic blood pressure, high density lipoprotein cholesterol, low density lipoprotein cholesterol, triglycerides and smoking status.
When we excluded individuals on antihypertensive medications from analysis the LOD score on chromosome 12 increased to 4.6 in model 1 and 2.8 in model 2. LOD scores on chromosome 1 remained similar in all centers, however analyses within Arizona now also yielded suggestive LOD scores using both models (LOD= 2.5, 2.2, for models 1 and 2, respectively). Additionally, loci on chromosomes 2 and 9 were no longer significant and new loci were evident on 3q21.2 in Arizona using model 2 (LOD=1.8) and 7p21.3 in all centers and the Dakotas, using both models (LODs =1.9–2.3). Tests of additive interaction for gene-by-diabetes, -hypertension and -obesity were nonsignificant in the full sample and every center at the 0.006 p-value threshold level which accounts for multiple testing.
Discussion
This study is only the third genome-wide scan of eGFR in a general population not targeted for diabetes, hypertension or ESRD (34, 41) and is one of the first to examine gene-by-environment interactions in a linkage study of estimated GFR. We found evidence of linkage to eGFR on 12p12.2 and suggestive evidence for linkage on 1p36.31, 2q33.3 and 9q34.2 (Figure 1). The locus on 12p12.2 replicates findings from a previous genome scan of diabetic nephropathy in African Americans with a LOD score as high as 2.9 in a subset of patients with late onset diabetes.(27) Additionally, our finding of suggestive evidence for linkage to 2q33.3 replicates a study of GFR using cystatin-C in diabetics (LOD=4.1) and another of creatinine clearance in a general population of Mexican Americans.(41)
Figure 1.
Cumulative multipoint Logarithm of Odds (LOD) scores for ranked estimated glomerular filtration rate using the simplified Modification of Diet in Renal Disease (MDRD) equation on chromosomes 1, 2, 9 and 12 in phase IV participants of the Strong Heart Family Study (2001–2003). gfr_all: model 2 in all centers; gfr_min_all: model 1 in all centers; gfr_az: model 2 in Arizona; gfr_min_az: model 1 in Arizona; gfr_da: model 2 in Dakotas; gfr_min_da: model 1 in Dakotas; gfr_ok: model 2 in Oklahoma; gfr_min_ok: model 1 in Oklahoma
The lack of gene-by-diabetes, -hypertension and -obesity interaction on GFR in our study population was somewhat surprising. It is logical that there are genetic polymorphisms that predispose to kidney disease only in the setting of permissive environmental factors. Given that diabetes is the most common cause of kidney disease in American Indians, this seems a likely candidate to influence genetic susceptibility. Interaction-specific linkage analysis is underpowered and may alone explain the difference in findings for diabetes interactions. Placha et al. (2006) found strong evidence of gene-by-diabetes interaction on GFR using cystatin C in multiple extended families enriched for diabetes.(41) Cystatin C was used to calculate GFR which may be more accurate than creatinine-based methods for detecting mild CKD specifically in American Indians and/or diabetics.(42, 43) The population used in the study by Placha et al. was roughly ten years older than the SHFS and also had a lower mean BMI. It is possible that misclassification and the presence of glucose intolerance/metabolic syndrome without overt diabetes may have contributed to our finding of no interaction.
Our analysis isolated a QTL for GFR with a maximum LOD score of 3.5 at 12p12.2. In our analysis, restricting the dataset to those not on antihypertensive medications resulted in an increase of the LOD score to 4.6. Placha et al. (2006) also isolated significantly higher LOD scores after inclusion of antihypertensive treatment as a covariate.(41) The purpose of this subgroup analysis was two-fold. First, it is plausible that treatment with renin-angiotensin system (RAS) antagonists diminish the genetic propensity towards reduced kidney function. We would have liked to have stratified by treatment with RAS antagonists, however, this specific information was not available. Hence, we used treatment with antihypertensives as a surrogate for RAS antagonism. Second, since severe hypertension may also act as either a confounder or effect modifier on the propensity towards reduced kidney function, we used treatment with antihypertensives as a marker of the more severe hypertensive phenotype. The higher LOD score on 12p obtained in the subanalysis of those not on antihypertensive medications provides support for these hypotheses.
Within the one-LOD drop support interval of our peak on 12p12.2 lies the gene protein-tyrosine phosphatase receptor type-O (PTPRO). The protein, termed glomerular epithelial protein 1 (GLEPP1), is primarily located on the apical surface of glomerular podocytes and is present from early-on in development.(44, 45) Abnormalities in distribution are present in multiple human primary glomerulopathies, including minimal change disease and focal segmental glomerulosclerosis.(46) PTPRO knockout mice are viable, but podocytes have blunted, widened foot processes and decreased amounts of nephrin.(47) Knockout mice have no difference in urine albumin excretion or GFR as long as renal reserve is intact. Uninephrectomized knockout mice, however, have a 25–50% lower GFR than their wild-type counterparts, suggesting an increase in susceptibility to decreased GFR in the presence of states of hyperfiltration.(47) This is highly applicable to the Strong Heart population, given the high prevalence of obesity, diabetes and hypertension which also result in glomerular hyperfiltration.
There are several caveats to our reported findings. The MDRD equation is less accurate in those with an estimated GFR > 60ml/min/1.73m2 and the vast majority of the SHFS falls into this category. Additionally, the MDRD equation was not developed in American Indians, the estimates of GFR may have been biased. However, the MDRD equation has been validated in two studies of Pima Indians using iothalamate as the gold standard measurement.(42) Although estimates of GFR may be more accurate using cystatin C than creatinine, cystatin C measures were not available. Analyses were not repeated using the Cockroft-Gault equation since the high prevalence of obesity in our study population would have made it difficult to interpret results of creatinine clearance based on weight.
The intra-individual variability of serum creatinine tends to be high thus an average of repeated measures of serum creatinine, had they been available, would have provided a more optimal measurement of serum creatinine and estimated GFR. Other factors which may affect levels of meaured serum creatinine in populations include hydration status, concomitant medications and random testing errors.
Estimates of GFR may also have been affected by obesity, hypertension and/or diabetes as a consequence of glomerular hyperfiltration. The presence of a supranormal GFR is the first stage of kidney disease for these conditions and hence may misrepresent the true pathologic status when placed within a continuous outcome variable paradigm. According to this logic, 20% of our subjects fell into this category of having a supranormal GFR, and excluding these participants would not have been appropriate. Further complicating this issue is that persons with a GFR 90–120ml/min/1.73m2 may, in fact, possess more severe renal pathology than those with a supranormal GFR, as they may have surpassed this state and have declining renal function even though their GFR is in the ‘normal range’. This is an issue inherent to renal pathology which cannot be overcome or corrected for in the analysis. From a physiologic standpoint, it would have been interesting to have an ordinal outcome variable using the KDOQI staging system for CKD, however, such an approach would be difficult to implement, as linkage analysis requires either a two category discrete or continuous outcome variable.
There was a significant decrease in the LOD score for the locus on 12p12.2 in the fully adjusted model, however, this may be explained by a loss of power alone. Analysis of the fully adjusted model in Arizona incorporated 1177 individuals whereas the minimally adjusted model incorporated 1215 individuals. Although this difference would be miniscule in a general epidemiologic study, it is possible in a linkage study that crucial individuals were dropped from the analysis. The alternative possibility is that covariates introduced in the maximum model are confounders in the minimum model. We addressed this by running the analysis using the covariates diabetes, hypertension, hypertension medication, obesity, BMI, systolic and diastolic blood pressures as the outcome variable using the minimum model over the 20 centimorgan region on 12p. None of these analyses were remotely suggestive of linkage. To address whether albuminuria had confounded our analysis, we also ran the analysis with UACR as the outcome variable and found no evidence of linkage. In addition, there was no evidence of genetic correlation between GFR and UACR. Since we did not incorporate hypertension, hypertensive medication or UACR into the model, we also ran the analysis incorporating these variables into the model and obtained the exact same results.
The validity of our findings is strengthened by two indicators of robustness. First, the addition of hypertension, hypertensive medication and UACR did not alter the LOD score isolated on 12p12.2. Second, this locus is a replication of a previous genome scan of kidney disease and we additionally replicated effect modification by antihypertensive treatment. Lastly, within our 1-LOD drop support interval is the candidate gene for GLEPP-1 which could logically influence eGFR in a population prone to states of hyperfiltration. Since very few genome wide linkage scans of eGFR have been published, and this region of 12p has been implicated in other populations, we should direct future research into the refined mapping of the 12p12.2 region.
Materials and Methods
Study Population
The Strong Heart Study (SHS) was begun in 1988 to investigate cardiovascular disease (CVD) and its risk factors in a geographically diverse group of resident American Indian tribal members at three study centers in Arizona, Oklahoma, and North and South Dakota. The SHFS, a component of the SHS, was initiated in 1996 with the goal of localizing genes that influence CVD and its risk factors. The data utilized for the current analysis were gathered during phase IV of the SHFS, which occurred between 2001 and 2003. More than 3600 men and women aged 14 to 93 were recruited from over 90 extended families originating from 13 separate American Indian tribes. The Arizona center is primarily composed of Pima Indians, but there are also representatives of the closely related Maricopa and Tohono O’odham Indian tribes. The vast majority of participants in the Dakotas center are Dakota/Lakota/Nakota. The Oklahoma Center is represented by members of the Kiowa, Comanche, Delaware, Apache, Caddo, Fort Sill Apache, and Wichita tribes. All protocols were approved by the Indian Health Service Institutional Review Board, by the institutional review boards of the participating institutions and by the 13 American Indian tribes participating in these studies.
Phenotypes
Detailed descriptions of the SHS and SHFS study design and laboratory protocols have been published previously.( 48, 49) Blood pressure and body morphometrics were measured during the physical exam. After five minutes of rest, upper arm seated blood pressure was measured three times by a trained technician using a mercury column sphygmomanometer (WA Baum Co) and size-adjusted cuffs. The first and fifth Korotkoff sounds were recorded. The average of the last two measures was used for all analyses. Hypertension was defined by a systolic blood pressure (SBP) ≥ 140 mm Hg or diastolic blood pressure (DBP) ≥ 90 mm Hg or use of antihypertensive drugs.(10) Body mass index was calculated as weight (kg)/height (m)2, and obesity was defined as a BMI ≥ 30 kg/m2. Type II diabetes was determined according to the American Diabetes Association criteria.(50)
Fasting blood samples were assayed at MedStar Research Institute, Washington, D.C., using standard laboratory methods as previously described.(49) Triglycerides (TG), total cholesterol and high density lipoprotein cholesterol (HDL-C) were measured using enzymatic reagents and the Hitachi 717. Low density lipoprotein cholesterol (LDL-C) was derived using the Friedewald equation; it was directly measured in subjects with triglyceride values of >400 mg/dl.(51) Serum creatinine was measured by the picric acid method.(52) GFR was estimated by the abbreviated MDRD equation: GFR (ml/min/1.73m2) = 186.3 × (serum creatinine)−1.154 × (Age)−0.203 × (0.742 if female) × (1.210 if African American).(53) This prediction equation has been previously validated in an American Indian population.(42) GFR was calculated using the formula for Caucasians. Information regarding smoking status was obtained during a personal interview and was defined as having ever had at least 100 cigarettes.
Genotypes
The SHFS genotyping procedures have been previously described.(54) In brief, DNA was isolated from fasting blood samples using organic solvents, and then amplified in separate polymerase chain reactions (PCR) with primers specific for short tandem repeat markers using the ABI PRISM Linkage Mapping Set-MD10 Version 2.5 (Applied Biosystems, Foster City,CA). PCR products were loaded into an ABI PRISM 377 DNA sequencer for laser-based automated genotyping. Analyses and assignment of the marker alleles were done using computerized algorithms (Applied Biosystems).
Genetic distances were obtained using sex-averaged chromosomal maps from the Marshfield Center for Medical Genetics (http://research.marshfieldclinic.org/genetics) and are reported in Haldane centiMorgans (cM). Pedigree relationships were verified using the PREST (pedigree relationship statistical tests) package, which employs likelihood-based inference statistics for genome-wide identity-by-descent (IBD) allele sharing.(55) Mendelian inconsistencies and spurious double recombinants were detected using the SimWalk2 package.(56) The overall blanking rate for both types of errors was less than 1% of the total number of genotypes for Arizona, Dakotas and Oklahoma. The cytogenetic locations of markers were determined using the web resources of the University of California Santa Cruz (UCSC) (http://genome/ucsc.edu) and the Marshfield Linkage maps (http://research.marshfieldclinic.org/genetics/MarkerSearch/buildMap.asp).
Quantitative Genetic Analyses
GFR was rank transformed to normalize the distribution and attain a kurtosis of less than 1.0. SAS, version 9.1, was used to calculate the residual variability after covariate adjustment. To maximize the power to detect genetic effects, we considered two different models of covariate adjustment in each center and the full sample. In model 1, adjustments were made for age, sex, age2, as well as age-by-sex interactions. Model 2 incorporated additional covariates supported by current literature as potential confounders including: BMI, SBP, DBP, TG, HDL-C, LDL-C, diabetic status (yes versus no) and smoking status (current and former versus never). TG levels were log transformed and outliers from the distribution of systolic blood pressure were set to 200 mmHg (Winsorization) to maintain normal distributions. The presence of ACEI/ARB medications as well as hypertension severity could potentially confound or modify the genetic influence over GFR. Therefore, we additionally ran models 1 and 2 excluding individuals on any antihypertensive medication (specific medication use was unknown). All models were stratified by center.
SOLAR, version 2.1.2, was used to perform multipoint variance component linkage analysis of the residuals. Details of this model have been described previously.(57, 58) The use of the variance component approach requires an estimate of the IBD matrix. We used the Loki package which employs a Markov chain Monte Carlo stochastic procedure to compute the IBD allele sharing at points throughout the genome conditional on the genotype information available at neighboring points.(59)
Interaction Analyses
Genotype-by-diabetes, -hypertension and -obesity interactions on GFR were explored using a three step strategy. We initially tested for evidence of additive interaction by accounting for the genetic covariance differences according to diabetic, hypertension or obesity status in relative pairs. In these analyses, the likelihood of a model including genotype-by-diabetes, -hypertension or -obesity interaction is compared to the likelihood of restricted models in which such interactions are excluded. We tested for differential additive genetic effects among diabetic versus non-diabetic, hypertensive versus normotensive or obese versus non-obese participants (genetic correlation (ρg) ≠1); for differences in the magnitude of the genetic effects among diabetic versus non-diabetic, hypertensive versus normotensive and obese versus non-obese participants (genetic variance (σg)≠ among two groups); and for differences in residual environmental interaction with diabetes, hypertension or obesity status (environmental variance (σe)≠ among two groups).
Acknowledgments
We would first like to thank the Strong Heart Family Study participants. Without their participation, this project would not have been possible. In addition, the cooperation of the Indian Health Service hospitals and clinics, and the directors of the SHS clinics, and the many collaborators and staff of the Strong Heart Study have made this project possible. This research was funded by a cooperative agreement that includes grants U01 HL65520, U01 HL41642, U01 HL41652, U01 HL41654, and U01 HL65521 from the National Heart, Lung, and Blood Institute. Development of SOLAR and the methods implemented in it are supported by US Public Health Service grant MH059490 from the National Institutes of Health. The views expressed in this paper are those of the authors and do not necessarily reflect those of the Indian Health Service.
This research was funded by a cooperative agreement from the National Heart, Lung, and Blood Institute.
Footnotes
GFR in American Indians linked to 12p12.2
Disclosures
There are no competing financial interests to report.
References
- 1.Coresh J, Astor B, Greene T, et al. Prevalence of chronic kidney disease and decreased kidney function in the adult U.S. population: Third National Health and Nutrition Examination Survey. American Journal of Kidney Disease. 2003;41:1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- 2.Culleton BF, Larson MG, Evans JC, et al. Prevalence and correlates of elevated serum creatinine levels: the Framingham Heart Study. Arch Intern Med. 1999;159:1785–1790. doi: 10.1001/archinte.159.15.1785. [DOI] [PubMed] [Google Scholar]
- 3.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 4.U.S. Renal Data System. USRDS 2006 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2006. [Google Scholar]
- 5.Kurella M, Lo JC, Chertow GM. Metabolic syndrome and the risk for chronic kidney disease among nondiabetic adults. J Am Soc Nephrol. 2005;16:2134–2140. doi: 10.1681/ASN.2005010106. [DOI] [PubMed] [Google Scholar]
- 6.Lucove J, Vupputuri S, Heiss G, et al. Department of Epidemiology. Chapel Hill: University of North Carolina; 2006. Metabolic Syndrome and Chronic Kidney Disease: the Strong Heart Study. [Google Scholar]
- 7.Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA. 2003;290:2159–2167. doi: 10.1001/jama.290.16.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) The Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 9.Bakris GL, Weir MR, Shanifar S, et al. Effects of blood pressure level on progression of diabetic nephropathy: results from the RENAAL study. Arch Intern Med. 2003;163:1555–1565. doi: 10.1001/archinte.163.13.1555. [DOI] [PubMed] [Google Scholar]
- 10.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 11.Hsu CY, McCulloch CE, Iribarren C, et al. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006;144:21–28. doi: 10.7326/0003-4819-144-1-200601030-00006. [DOI] [PubMed] [Google Scholar]
- 12.Fried LF, Orchard TJ, Kasiske BL. Effect of lipid reduction on the progression of renal disease: a meta-analysis. Kidney Int. 2001;59:260–269. doi: 10.1046/j.1523-1755.2001.00487.x. [DOI] [PubMed] [Google Scholar]
- 13.Fagot-Campagna A, Nelson RG, Knowler WC, et al. Plasma lipoproteins and the incidence of abnormal excretion of albumin in diabetic American Indians: the Strong Heart Study. Diabetologia. 1998;41:1002–1009. doi: 10.1007/s001250051023. [DOI] [PubMed] [Google Scholar]
- 14.Gambaro G, Verlato F, Budakovic A, et al. Renal impairment in chronic cigarette smokers. J Am Soc Nephrol. 1998;9:562–567. doi: 10.1681/ASN.V94562. [DOI] [PubMed] [Google Scholar]
- 15.Pinto-Sietsma SJ, Mulder J, Janssen WM, et al. Smoking is related to albuminuria and abnormal renal function in nondiabetic persons. Ann Intern Med. 2000;133:585–591. doi: 10.7326/0003-4819-133-8-200010170-00008. [DOI] [PubMed] [Google Scholar]
- 16.Stidley CA, Shah VO, Narva AS, et al. A population-based, cross-sectional survey of the Zuni Pueblo: a collaborative approach to an epidemic of kidney disease. Am J Kidney Dis. 2002;39:358–368. doi: 10.1053/ajkd.2002.30557. [DOI] [PubMed] [Google Scholar]
- 17.Robbins DC, Knowler WC, Lee ET, et al. Regional differences in albuminuria among American Indians: an epidemic of renal disease. Kidney Int. 1996;49:557–563. doi: 10.1038/ki.1996.79. [DOI] [PubMed] [Google Scholar]
- 18.Hoy W, Jim S, Warrington W, et al. Urinary findings and renal function in adult Navajo Indians and associations with type 2 diabetes. Am J Kidney Dis. 1996;28:339–349. doi: 10.1016/s0272-6386(96)90490-4. [DOI] [PubMed] [Google Scholar]
- 19.Welty TK, Rhoades DA, Yeh F, et al. Changes in cardiovascular disease risk factors among American Indians. The Strong Heart Study. Ann Epidemiol. 2002;12:97–106. doi: 10.1016/s1047-2797(01)00270-8. [DOI] [PubMed] [Google Scholar]
- 20.Stidley CA, Shah VO, Scavini M, et al. The Zuni kidney project: a collaborative approach to an epidemic of kidney disease. J Am Soc Nephrol. 2003;14:S139–143. doi: 10.1097/01.asn.0000070151.95421.87. [DOI] [PubMed] [Google Scholar]
- 21.Diabetes prevalence among American Indians and Alaska Natives and the overall population--United States, 1994–2002. MMWR Morb Mortal Wkly Rep. 2003;52:702–704. [PubMed] [Google Scholar]
- 22.Imperatore G, Knowler WC, Pettitt DJ, et al. Segregation analysis of diabetic nephropathy in Pima Indians. Diabetes. 2000;49:1049–1056. doi: 10.2337/diabetes.49.6.1049. [DOI] [PubMed] [Google Scholar]
- 23.Imperatore G, Hanson RL, Pettitt DJ, et al. Sib-pair linkage analysis for susceptibility genes for microvascular complications among Pima Indians with type 2 diabetes. Pima Diabetes Genes Group. Diabetes. 1998;47:821–830. doi: 10.2337/diabetes.47.5.821. [DOI] [PubMed] [Google Scholar]
- 24.Vardarli I, Baier LJ, Hanson RL, et al. Gene for susceptibility to diabetic nephropathy in type 2 diabetes maps to 18q22.3–23. Kidney Int. 2002;62:2176–2183. doi: 10.1046/j.1523-1755.2002.00663.x. [DOI] [PubMed] [Google Scholar]
- 25.Fogarty DG, Rich SS, Hanna L, et al. Urinary albumin excretion in families with type 2 diabetes is heritable and genetically correlated to blood pressure. Kidney Int. 2000;57:250–257. doi: 10.1046/j.1523-1755.2000.00833.x. [DOI] [PubMed] [Google Scholar]
- 26.Langefeld CD, Beck SR, Bowden DW, et al. Heritability of GFR and albuminuria in Caucasians with type 2 diabetes mellitus. Am J Kidney Dis. 2004;43:796–800. doi: 10.1053/j.ajkd.2003.12.043. [DOI] [PubMed] [Google Scholar]
- 27.Bowden DW, Colicigno CJ, Langefeld CD, et al. A genome scan for diabetic nephropathy in African Americans. Kidney Int. 2004;66:1517–1526. doi: 10.1111/j.1523-1755.2004.00915.x. [DOI] [PubMed] [Google Scholar]
- 28.Moczulski DK, Rogus JJ, Antonellis A, et al. Major susceptibility locus for nephropathy in type 1 diabetes on chromosome 3q: results of novel discordant sib-pair analysis. Diabetes. 1998;47:1164–1169. doi: 10.2337/diabetes.47.7.1164. [DOI] [PubMed] [Google Scholar]
- 29.Krolewski AS, Poznik GD, Placha G, et al. A genome-wide linkage scan for genes controlling variation in urinary albumin excretion in type II diabetes. Kidney Int. 2006;69:129–136. doi: 10.1038/sj.ki.5000023. [DOI] [PubMed] [Google Scholar]
- 30.Iyengar SK, Fox KA, Schachere M, et al. Linkage analysis of candidate loci for end-stage renal disease due to diabetic nephropathy. J Am Soc Nephrol. 2003;14:S195–201. doi: 10.1097/01.asn.0000070078.66465.55. [DOI] [PubMed] [Google Scholar]
- 31.Sale MM, Freedman BI, Langefeld CD, et al. A genome-wide scan for type 2 diabetes in African-American families reveals evidence for a locus on chromosome 6q. Diabetes. 2004;53:830–837. doi: 10.2337/diabetes.53.3.830. [DOI] [PubMed] [Google Scholar]
- 32.Fox CS, Yang Q, Guo CY, et al. Genome-wide linkage analysis to urinary microalbuminuria in a community-based sample: the Framingham Heart Study. Kidney Int. 2005;67:70–74. doi: 10.1111/j.1523-1755.2005.00056.x. [DOI] [PubMed] [Google Scholar]
- 33.Freedman BI, Bowden DW, Rich SS, et al. A genome scan for all-cause end-stage renal disease in African Americans. Nephrol Dial Transplant. 2005;20:712–718. doi: 10.1093/ndt/gfh704. [DOI] [PubMed] [Google Scholar]
- 34.Fox CS, Yang Q, Cupples LA, et al. Genomewide linkage analysis to serum creatinine, GFR, and creatinine clearance in a community-based population: the Framingham Heart Study. J Am Soc Nephrol. 2004;15:2457–2461. doi: 10.1097/01.ASN.0000135972.13396.6F. [DOI] [PubMed] [Google Scholar]
- 35.Freedman BI, Yu H, Spray BJ, et al. Genetic linkage analysis of growth factor loci and end-stage renal disease in African Americans. Kidney Int. 1997;51:819–825. doi: 10.1038/ki.1997.115. [DOI] [PubMed] [Google Scholar]
- 36.Freedman BI, Rich SS, Yu H, et al. Linkage heterogeneity of end-stage renal disease on human chromosome 10. Kidney Int. 2002;62:770–774. doi: 10.1046/j.1523-1755.2002.00534.x. [DOI] [PubMed] [Google Scholar]
- 37.Whitfield JB, Martin NG. The effects of inheritance on constituents of plasma: a twin study on some biochemical variables. Ann Clin Biochem. 1981;21(Pt 3):176–183. doi: 10.1177/000456328402100303. [DOI] [PubMed] [Google Scholar]
- 38.Bathum L, Fagnani C, Christiansen L, Christensen K. Heritability of biochemical kidney markers and relation to survival in the elderly--results from a Danish population-based twin study. Clin Chim Acta. 2004;349:143–150. doi: 10.1016/j.cccn.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 39.Hunter DJ, Lange M, Snieder H, et al. Genetic contribution to renal function and electrolyte balance: a twin study. Clin Sci (Lond) 2002;103:259–265. doi: 10.1042/cs1030259. [DOI] [PubMed] [Google Scholar]
- 40.Rao DCCG. False positives and false negatives in genome scans. Advances in Genetics. 2001;42:487–498. doi: 10.1016/s0065-2660(01)42038-4. [DOI] [PubMed] [Google Scholar]
- 41.Placha G, Poznik GD, Dunn J, et al. A genome-wide linkage scan for genes controlling variation in renal function estimated by serum cystatin C levels in extended families with type 2 diabetes. Diabetes. 2006;55:3358–3365. doi: 10.2337/db06-0781. [DOI] [PubMed] [Google Scholar]
- 42.Perkins BA, Nelson RG, Ostrander BE, et al. Detection of renal function decline in patients with diabetes and normal or elevated GFR by serial measurements of serum cystatin C concentration: results of a 4-year follow-up study. J Am Soc Nephrol. 2005;16:1404–1412. doi: 10.1681/ASN.2004100854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Macisaac RJ, Tsalamandris C, Thomas MC, et al. Estimating glomerular filtration rate in diabetes: a comparison of cystatin-C- and creatinine-based methods. Diabetologia. 2006;49:1686–1689. doi: 10.1007/s00125-006-0275-7. [DOI] [PubMed] [Google Scholar]
- 44.Wiggins RC, Wiggins JE, Goyal M, et al. Molecular cloning of cDNAs encoding human GLEPP1, a membrane protein tyrosine phosphatase: characterization of the GLEPP1 protein distribution in human kidney and assignment of the GLEPP1 gene to human chromosome 12p12-p13. Genomics. 1995;27:174–181. doi: 10.1006/geno.1995.1021. [DOI] [PubMed] [Google Scholar]
- 45.Wang R, St John PL, Kretzler M, et al. Molecular cloning, expression, and distribution of glomerular epithelial protein 1 in developing mouse kidney. Kidney Int. 2000;57:1847–1859. doi: 10.1046/j.1523-1755.2000.00034.x. [DOI] [PubMed] [Google Scholar]
- 46.Sharif K, Goyal M, Kershaw D, et al. Podocyte phenotypes as defined by expression and distribution of GLEPP1 in the developing glomerulus and in nephrotic glomeruli from MCD, CNF, and FSGS. A dedifferentiation hypothesis for the nephrotic syndrome. Exp Nephrol. 1998;6:234–244. doi: 10.1159/000020528. [DOI] [PubMed] [Google Scholar]
- 47.Wharram BL, Goyal M, Gillespie PJ, et al. Altered podocyte structure in GLEPP1 (Ptpro)-deficient mice associated with hypertension and low glomerular filtration rate. J Clin Invest. 2000;106:1281–1290. doi: 10.1172/JCI7236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.North KE, Howard BV, Welty TK, et al. Genetic and environmental contributions to cardiovascular disease risk in American Indians: the strong heart family study. Am J Epidemiol. 2003;157:303–314. doi: 10.1093/aje/kwf208. [DOI] [PubMed] [Google Scholar]
- 49.Lee ET, Welty TK, Fabsitz R, et al. The Strong Heart Study. A study of cardiovascular disease in American Indians: design and methods. Am J Epidemiol. 1990;132:1141–1155. doi: 10.1093/oxfordjournals.aje.a115757. [DOI] [PubMed] [Google Scholar]
- 50.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 51.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 52.Chasson A, Grady H, Stanley M. Determination of creatinine by means of automatic chemical analysis. Tech Bull Regist Med Tech. 1957;30:207–212. [PubMed] [Google Scholar]
- 53.Levey A, Bosch J, Lewis J, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Annales of Internal Medicine. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 54.North KE, Goring HH, Cole SA, et al. Linkage analysis of LDL cholesterol in American Indian populations: the Strong Heart Family Study. J Lipid Res. 2006;47:59–66. doi: 10.1194/jlr.M500395-JLR200. [DOI] [PubMed] [Google Scholar]
- 55.Sun L, Wilder K, McPeek MS. Enhanced pedigree error detection. Hum Hered. 2002;54:99–110. doi: 10.1159/000067666. [DOI] [PubMed] [Google Scholar]
- 56.Sobel E, Papp JC, Lange K. Detection and integration of genotyping errors in statistical genetics. Am J Hum Genet. 2002;70:496–508. doi: 10.1086/338920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62:1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blangero J, Almasy L. Multipoint oligogenic linkage analysis of quantitative traits. Genet Epidemiol. 1997;14:959–964. doi: 10.1002/(SICI)1098-2272(1997)14:6<959::AID-GEPI66>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 59.Heath SC. Markov chain Monte Carlo segregation and linkage analysis for oligogenic models. Am J Hum Genet. 1997;61:748–760. doi: 10.1086/515506. [DOI] [PMC free article] [PubMed] [Google Scholar]