Abstract
Angiogenesis, or neovascularization, is known to play an important role in the neoplastic progression leading to metastasis. CD31 or Factor VIII-related antigen (F VIII RAg) immunohistochemistry (IHC), is widely used in experimental studies quantifing tumor neovascularization in immunocompromised animal models implanted with transformed human cell lines. Quantification, however, can be affected extensively by variations in the methodology used to measure vascularization including antibody selection, pretreatment with antigen retrieval and evaluation techniques. To examine this further, we examined the microvessel density and the intensity of microvascular staining among five different human tumor xenografts and a mouse syngeneic tumor using anti-CD31 and F VIII RAg IHC staining. Different antigen retrieval methods also were evaluated. Maximal retrieval of CD31 was achieved using 0.5 M Tris (pH 10) buffer, while maximum retrieval of F VIII RAg was achieved using 0.05% pepsin treatment of tissue sections. For each optimized retrieval condition, compared to F VIII RAg, anti-CD31 highlighted small vessels better. Furthermore, the microvessel density of CD31 was significantly greater than that of F VIII RAg decorated vessels (p < 0.001). The choice of antibody and antigen retrieval method has a significant affect on immunohistochemical findings when studying angiogenesis. One also must use caution when comparing studies in the literature that use different techniques and reagents.
Key words and abbreviations: angiogenesis, antigen retrieval, CD31/PECAM-1, endothelial cells, factor VIII/vWf, immunohistochemistry, microvessel density, xenografts
Angiogenesis, or neovascularization, is the formation of new blood vessels originating from the endothelium of existing vasculature. New capillaries are the consequence of the growth of columns of aligned endothelial cells (ECs). Adjacent endothelial cell columns contact each other to form three-dimensional cords and loops that subsequently develop tubes with lumens. Angiogenesis is critical to tumor growth, neoplastic progression and metastasis (Meert, et al. 2002). Immunohistochemical staining of microvessels to assess microvessel density (MVD) per unit area is associated with the degree of intratumor neovascularization, tumor metastatic capability and the prognosis for patients with many types of human solid cancers (Hlatky et al. 2002). There are several immunohistochemical markers that can identify endothelial cells including antibodies that recognize epitopes on CD31 and Factor VIII-related antigen.
CD31, or platelet endothelial cell adhesion molecule-1 (PECAM-1), is found in large quantities on the surface of ECs and is less abundant on platelets and leukocytes. It plays a major role in a number of cellular interactions, particularly in adhesion between ECs and polymorphonuclear leukocytes, monocytes, and lymphocytes during inflammation, and between adjacent ECs during angiogenesis (Muller 2002). Factor VIII-related antigen, also known as von Willebrand factor (vWf), is synthesized in ECs and megakaryocytes and it mediates platelet adhesion to the walls of injured vessels. Immunohistochemical detection of CD31 and F VIII RAg has been used extensively to quantify angiogenesis of xenograft tumors in immunodeficient animal models carrying various human tumor cell loads (Vanzulli et al. 1997, Fulzele et al. 2006, Muruganandham et al. 2006, Ragel et al. 2007).
Like other immunohistochemistry-based studies, quantitative evaluation of vascularity in tissue sections may be affected significantly by variations in methodologies including antibody selection, methods of antigen retrieval (AR), and methods of vessel density assessment (Vermeulen et al. 1996, Meert et al. 2002). We compared evaluation of neovasculature staining using anti-CD31 or anti-F VIII RAg antibodies in five different human cell lines grown as tumor xenografts and one mouse syngeneic breast cancer by using a panel of AR methods including high temperature AR with different buffered and enzymatic solutions. The comparison among antibodies was based on the individually-optimized (maximal) retrieval for these two antigens.
Materials and methods
Cell lines
Five transformed human cell lines were grown as xenografts in athymic (nude) mice. Xenografts were derived from the following cell lines: MDA-MB-231 and MDA-MB-435 human breast cancer, UM-SCC-1 human head and neck squamous carcinoma, SKOV3.ip1 human ovarian carcinoma and LS174 human colon adenocarcinoma. An allograft from the syngeneic breast cancer cell line (TS/A) derived from a mammary adenocarcinoma that arose spontaneously in a BALB/c female mouse was also used. These latter cells (TS/A) were implanted in a BXD mouse, a genetically well-characterized animal model for studying the host immune response to neoplasia (Grizzle et al. 2002). Normal lung tissues from corresponding athymic mice and BXD RI mice also were processed as control samples. All tissues were fixed in 10% neutral buffered formalin for 24 h, processed, and embedded in paraffin blocks.
Immunohistochemistry
Serial sections 5μm thick were cut from the formalin fixed, paraffin embedded tissue blocks and floated onto charged glass slides (Super-Frost Plus, Fisher Scientific, Pittsburgh, PA) and dried overnight at 60° C. A hemotoxylin and eosin stained section was obtained from each tissue block. All sections for immunohistochemistry were deparaffinized and hydrated using graded concentrations of ethanol to deionized water.
AR Pretreatment
The tissue sections were subjected to one of the following pretreatment protocols: no pretreatment, incubation with 0.1% trypsin in PBS (Sigma, St. Louis, MO), 0.05% pepsin (Sigma) in 0.01 M HCl (pH 2) at 37° C for 15 min, or heat treated with one of nine different buffered solutions using a pressure cooker (CEPC 800, Cook’s Essentials®, People’s Republic of China). These nine solutions (Tables 1 & 2) included 0.01 M glycine-HCl buffer (pH 3), 0.01 M sodium citrate buffer (pH 6), 0.05 M borate buffer (pH 8), 0.01 M Tris-1mM EDTA buffer (pH 9), 0.01 M Tris-1mM EDTA buffer (pH 10), 1 mM Tris-1mM EDTA buffer with 0.05% Tween 20 (pH 10), AR10 solution (pH 10, Biogenex, San Ramon, CA), 0.01 M Tris with 0.05% Tween 20 (pH 10) and 0.5 M Tris buffer (pH 10). These retrieval solutions were chosen based on their frequent use in our laboratory and in other studies (Stirling 2000, Kim et al. 2004a). The solutions were preheated in the pressure cooker for 10 min. After preheating, all slides were immersed in the respective solutions Coplin jars, then heated for another 5 min at maximum pressure (15 lb/in2). After the pressure was reduced, the slides were kept in the Coplin jars until the retrieval solution reached room temperature.
Table 1.
Evaluation of CD31 stained sections: microvessel density (MVD) and staining intensity (Int)
| Squamous cell carcinoma |
MDA-MB-231 |
MDA-MB-435 |
Colon cancer |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | I | II | III | IV | V | I | II | III | I | II | III | Mean | S.D. | ||
| Glycine | MVD | 5 | 4 | 5 | 0 | 0 | 1 | 4 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1.38 | 0.49 |
| Int | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Citric | MVD | 46 | 28 | 37 | 44 | 38 | 46 | 61 | 69 | 47 | 69 | 3 | 1 | 0 | 23 | 27 | 21 | 35.00 | 5.52 |
| Int | 1 | 2 | 1 | 1 | 2 | 2 | 1 | 2 | 2 | 1 | 1 | 1 | 0 | 2 | 2 | 2 | |||
|
|
|||||||||||||||||||
| Borate | MVD | 68 | 32 | 43 | 43 | 42 | 59 | 79 | 73 | 40 | 81 | 5 | 1 | 1 | 66 | 26 | 37 | 43.50 | 6.61 |
| Int | 2 | 3 | 3 | 2 | 3 | 3 | 3 | 2 | 2 | 2 | 1 | 1 | 1 | 3 | 3 | 3 | |||
|
|
|||||||||||||||||||
| 0.01 M Tris/EDTA | MVD | 72 | 34 | 55 | 45 | 45 | 64 | 77 | 79 | 42 | 99 | 13 | 11 | 14 | 50 | 22 | 39 | 47.56 | 6.47 |
| Int | 3 | 3 | 3 | 3 | 3 | 3 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 3 | 3 | 3 | |||
|
|
|||||||||||||||||||
| AR10 | MVD | 60 | 42 | 46 | 39 | 36 | 44 | 52 | 34 | 2 | 75 | 4 | 5 | 2 | 9 | 2 | 9 | 28.81 | 6.00 |
| Int | 2 | 2 | 1 | 1 | 2 | 2 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 2 | |||
|
|
|||||||||||||||||||
| 0.01 M Tris/Tween 20 | MVD | 53 | 45 | 43 | 20 | 21 | 13 | 44 | 19 | 0 | 26 | 1 | 1 | 1 | 16 | 7 | 15 | 20.31 | 4.38 |
| Int | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 1 | 1 | 1 | 1 | 2 | 1 | 2 | |||
|
|
|||||||||||||||||||
| 0.5 M Tris | MVD | 77 | 37 | 55 | 47 | 43 | 70 | 89 | 81 | 46 | 131 | 85 | 59 | 17 | 86 | 41 | 59 | 63.94 | 6.83 |
| Int | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 2 | 3 | 3 | 3 | 3 | |||
I–V, site of designated areas for MVD measurement; Int 0, no staining; 1, weak staining; 2, medium staining; 3, strong staining.
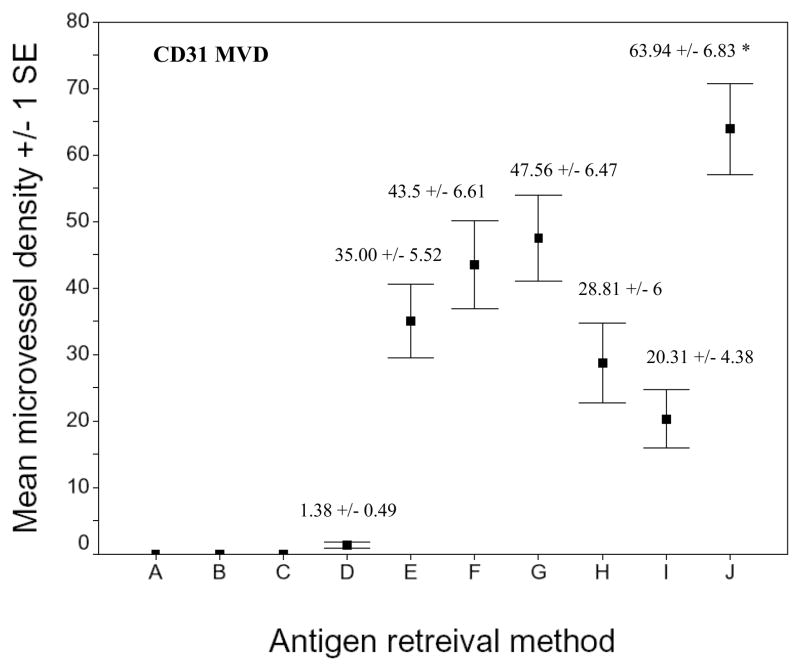
The CD31 MVD obtained with 0.5 M Tris was significantly greater than that obtained using all the other AR methods (p < 0.01). No intratumor microvessel was observed in the sections without treatment with pepsin or trypsin AR (not listed in the table). The SKOV3.ip1 human ovarian cancer cell line revealed only weak and focal microvessel staining (data not shown).
Table 2.
Evaluation of F VIII RAg stained sections: microvessel density (MVD) and staining intensity (Int)
| Squamous cell carcinoma |
MDA-MB-231 |
MDA-MB-435 |
Colon cancer |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | I | II | III | IV | V | I | II | III | I | II | III | Mean | S.D. | ||
| Trypsin | MVD | 5 | 20 | 14 | 9 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 30 | 21 | 6 | 6.81 | 2.37 |
| Int | 1 | 2 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 3 | 2 | 2 | |||
|
|
|||||||||||||||||||
| Pepsin | MVD | 22 | 20 | 19 | 19 | 31 | 1 | 4 | 3 | 3 | 3 | 4 | 13 | 7 | 41 | 15 | 15 | 13.75 | 2.85 |
| Int | 2 | 2 | 2 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 3 | 3 | 3 | |||
|
|
|||||||||||||||||||
| Glycine | MVD | 17 | 18 | 11 | 10 | 24 | 0 | 2 | 1 | 0 | 1 | 1 | 1 | 0 | 14 | 6 | 4 | 6.88 | 1.95 |
| Int | 2 | 2 | 1 | 1 | 2 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 2 | 2 | 2 | |||
|
|
|||||||||||||||||||
| Citric | MVD | 8 | 12 | 6 | 9 | 16 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 11 | 0 | 1 | 4.19 | 1.33 |
| Int | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 2 | 0 | 1 | |||
|
|
|||||||||||||||||||
| Borate | MVD | 6 | 12 | 7 | 10 | 14 | 0 | 1 | 0 | 0 | 0 | 1 | 3 | 1 | 14 | 7 | 9 | 5.31 | 1.31 |
| Int | 1 | 1 | 1 | 2 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 3 | 2 | 2 | |||
|
|
|||||||||||||||||||
| 0.01 M Tris/EDTA | MVD | 18 | 12 | 15 | 11 | 28 | 1 | 1 | 0 | 0 | 0 | 7 | 6 | 3 | 17 | 4 | 3 | 7.88 | 2.06 |
| Int | 2 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 3 | 2 | 2 | |||
|
|
|||||||||||||||||||
| AR10 | MVD | 10 | 8 | 7 | 9 | 12 | 0 | 1 | 0 | 0 | 0 | 2 | 2 | 0 | 12 | 2 | 13 | 4.88 | 1.26 |
| Int | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 2 | 2 | 3 | |||
|
|
|||||||||||||||||||
| 0.01 M Tris/Tween 20 | MVD | 4 | 8 | 5 | 7 | 9 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 3 | 0 | 0 | 2.31 | 0.82 |
| Int | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | |||
|
|
|||||||||||||||||||
| 0.5 M Tris | MVD | 19 | 14 | 11 | 14 | 20 | 1 | 0 | 0 | 0 | 2 | 13 | 3 | 2 | 18 | 5 | 6 | 8.00 | 1.85 |
| Int | 1 | 2 | 2 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 2 | 2 | 2 | |||
I–V, site of designated areas for MVD measurement; Int 0, no staining; 1, weak staining; 2, medium staining; 3, strong staining.
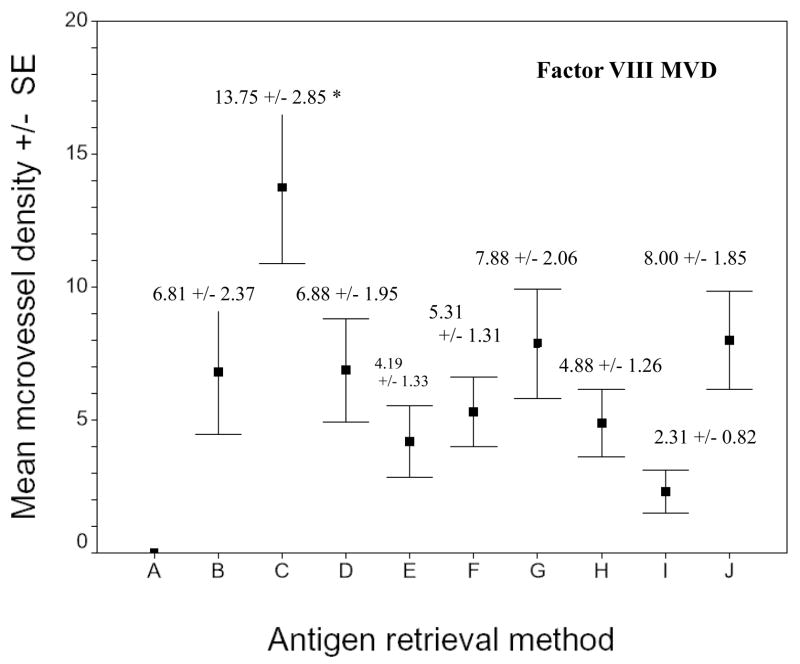
The F VIII RAg MVD obtained with pepsin was significantly greater than that obtained using all the other AR methods (p < 0.01). No intratumor microvessel was observed on the section without treatment (not listed in the table). No microvessel was observed in the SKOV3.ip1 ovarian cancer cell line (data not shown).
Immunostaining for CD31 & Factor VIII RAg
Following antigen retrieval, all sections were washed gently in deionized water, then transferred in to 0.05 M Tris-based solution in 0.15M NaCl with 0.1% v/v Triton-X-100, pH 7.6 (TBST). Endogenous peroxidase was blocked with 3% hydrogen peroxide for 10 min. To reduce further nonspecific background staining, slides were incubated with avidin and biotin blocking solutions for 15 min each (streptavidin from Jackson ImmunoResearch, West Grove, PA; biotin from Sigma), and 3% normal goat serum for 20 min (Sigma). All slides then were incubated at 4° C overnight with one of two antibodies; rabbit polyclonal antibody against CD31 (Abcam, Cambridge, MA) or rabbit polyclonal antibody against F VIII RAg (Cell Marque, Rocklin, CA). Using a lung section control, the highest titer of primary antibodies to produce optimal demonstration of microvessels with the lowest acceptable background staining was 1:200 for both anti-CD31 and anti-F VIII Rag; this dilution subsequently was used throughout the study. Negative controls were produced by eliminating the primary antibodies from the diluents.
After washing with TBST, biotinylated goat anti-rabbit IgG (1:1000; Jackson ImmunoResearch, West Grove, PA) were applied to the sections for 30 min at room temperature. Sections then were incubated with Strepavidin-HRP (Sigma) for 30 min at room temperature. Diaminobenzidine (DAB; Scy Tek Laboratories, Logan, UT) was used as the chromagen and hematoxylin (no. 7211, Richard-Allen Scientific, Kalamazoo, MI) as the counterstain.
Assessment of immunostaining
Depending on the size of the H & E section, three to five 1mm2 areas within the tumor were selected randomly at magnification X 40 for evaluation. These areas subsequently were used for all immunohistochemical comparisons. Bioquant® Image Analysis software (Rtm Biometrics, Nashville, TN) was used to “lock” on these preselected areas for each histological section of the same paraffin block regardless of retrieval method or antibody applied. The MVD measurements and intensity scoring for either CD31 or F VIII RAg staining were obtained simultaneously within each area at a X 200 magnification. The MVD was measured based on Weidner’s method (Weidner 1995). Each positive endothelial cell cluster of immunoreactivity in contact with the selected field was counted as an individual vessel in addition to the morphologically identifiable vessels with a lumen. The intensity of the staining was scored as 0, 1, 2, 3, indicating absence of staining, weak, moderate, or strong intensity, respectively.
Statistical analysis
The paired t-test was used to compare the mean MVD obtained using the method described above. The correlation between MVD and staining intensity using different methods of AR was compared using the Pearson Correlation Coefficient. Statistical analysis was carried out using the SPSS version 9.C software. A p value ≤ 0.05 was considered statistically significant.
Results
The CD31 and Factor VIII MVD counts within the xenografts using rabbit pAbs are summarized in Tables 1 and 2. The sections pretreated with either 0.01 M Tris-EDTA (pH 10) or 1 mM Tris-EDTA/0.05% Tween-20 (pH 10) in the pressure-cooker detached from the slides and thus were not available for evaluation. All control sections (antibody deleted) had no staining after all AR procedures.
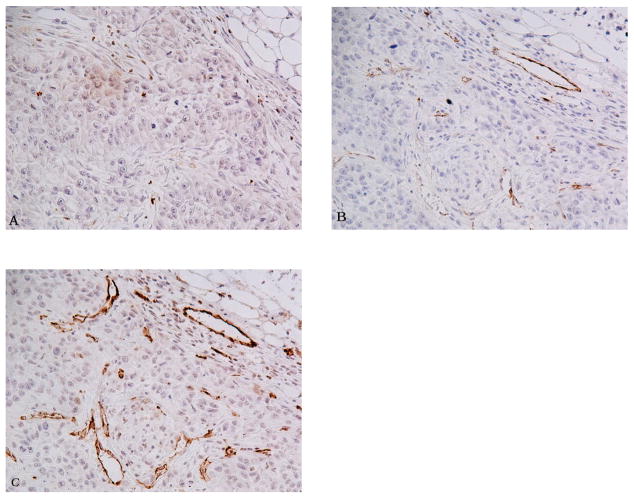
Compared to other AR pretreatments, 0.5 M Tris (pH 10) buffer produced the most intense and consistent staining of endothelial cells with anti-CD31 (Table 1; Fig. 1). The difference in MVD between 0.5 M Tris (pH 10) and the other antigen retrieval methods was statistically significant (p < 0.01) (Fig. 2). Using Tris-EDTA (pH 9) produced a staining pattern of CD31 immunoreactivity similar to that of 0.5 M Tris buffer in most cases, but this treatment resulted in a much higher background. For the remaining two high-pH Tris-based buffers, 0.01 M Tris/0.05% Tween-20 (pH 10) and the commercially available AR10 solution (pH 10), both had weaker signals than those in sections treated with 0.5 M Tris or Tris-EDTA buffers. In the study reported here, sections treated with citric acid yielded unacceptably weak and scattered CD31 staining of vascular endothelium (Fig. 1).
Fig. 1.
Comparision of CD31 immunohistochemistry using various AR methods on serial sections of the squamous carcinoma xenograft. A) 0.05% pepsin treated section. B) 0.01 M Citric acid (pH 6) treated section. C) 0.5 M Tris buffer (pH 10) treated section. All panels 200 X.
Fig. 2.
Microvessel density of CD31 stained sections of xenografts using different AR methods. A) No treatment. B) 0.1% trypsin. C) 0.05% pepsin. D) 0.01 M glycine (pH 3). E) 0.01 M sodium citric buffer (pH 6). F) 0.05 M borate buffer (pH 8). G) 0.01 M Tris-EDTA buffer (pH 9). H) AR10 solution (pH 10). I) 0.01 M Tris/0.05% Tween 20 (pH 10). J) 0.5 M Tris (pH 10). Asterisk indicates that the CD31 microvellel development seen with 0.5 M Tris (pH 10) was significantly greater than that obtained using the other AR methods (p < 0.01).
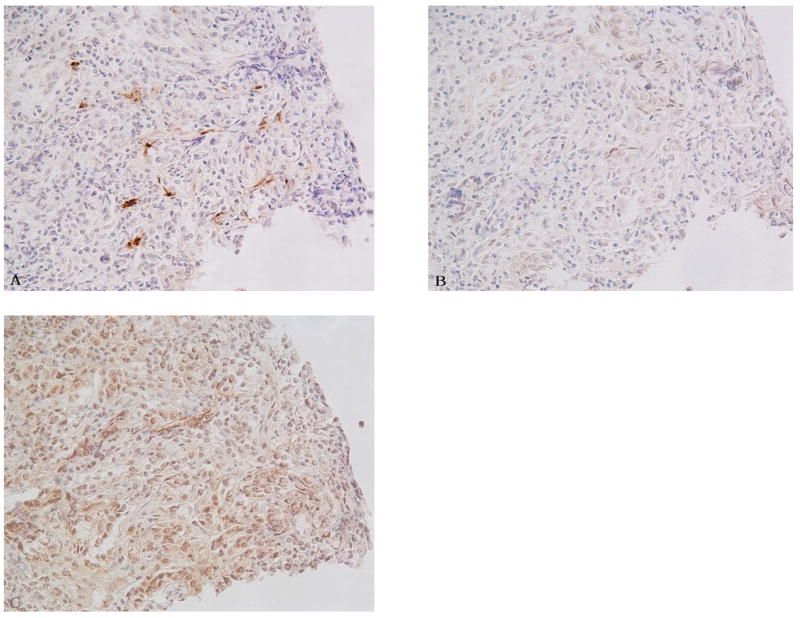
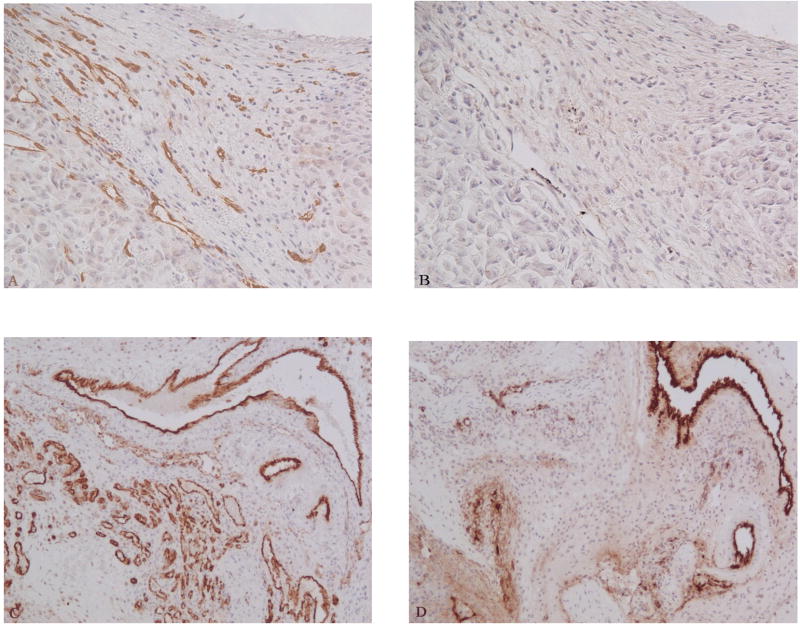
Immunohistochemical staining using antibody against anti-F VIII RAg showed an enhanced signal after pretreatment by proteolytic enzymes, specifically pepsin or trypsin (Table 2; Fig. 3). Furthermore, pepsin was superior to trypsin in all cases. Heat induced epitope retrieval (HIER) using 0.5 M Tris also was effective in most cases, but with much higher background staining (Fig. 3). The difference in MVD between pepsin (higher MVD) and the other antigen retrieval methods was statistically significant (p < 0.01) (Fig. 4).
Fig. 3.
Comparision of Factor VIII IHC by various AR methods on serial sections of colon adenocacinoma xenograft. A) 0.05% pepsin treated section. B) 0.01 M vitric acid (pH 6) treated section. C) 0.5 M Tris buffer (pH 10) treated section. All figures 200 X.
Fig. 4.
Microvessel density of F VIII RAg stained sections of xenografts using different AR methods. A) No treatment. B) 0.1% trypsin. C = 0.05% pepsin. D) 0.01M glycine (pH 3). E) 0.01 M sodium citric buffer (pH 6). F) 0.05 M borate buffer (pH 8). G) 0.01 M Tris-EDTA buffer (pH 9). H) AR10 solution (pH 10). I) 0.01 M Tris/0.05% Tween 20 (pH 10). J) 0.5 M Tris (pH 10). Asterisk indicates that the F VIII RAg microvessel development seen after pepsin digestion was significantly greater than that obtained by using the other AR methods (p < 0.01).
As shown in Fig. 5, we compared the staining of two endothelium markers after pretreatment with the optimized AR (0.5 M Tris, pH 10, for anti-CD31 stain and 0.05% pepsin for anti-F VIII RAg). The higher value for CD31 MVD was statistically significant (p < 0.001). The major targets of anti-F VIII RAg staining were the large to medium size vessels within and around the peripheral edge of the tumor. Microvessels within tumors were scarcely detected using F VII Rag in all cases, and often there was cross-reaction with tumor cells and RBCs. By contrast, immunohistochemistry with anti-CD31 antibody usually displayed homogeneously strong staining of all vessels. The one exception was the xenograft using the ovarian cancer cell line, SKOV3.ip1. Anti-CD31 staining revealed only weak and focal signaling of microvessels, even using its optimized AR method, 0.5 M Tris buffer. Anti-F VIII RAg staining did not improve the signal in the xenograft model of ovarian cancer. The CD31 and F VIII RAg microvessel staining of the syngeneic breast cancer using the same panel of AR methods showed results similar to those for human cell line xenografts (Fig. 5C,D).
Fig. 5.
Comparision of anti-CD31 and anti-F VIII RAg stains using the optimized AR method. Serial sections from MDA-MB-231 breast cancer xenograft (A and B) and syngeneic breast cancer (C and D). Sections were treated with 0.5 M Tris buffer (pH 10) followed by the CD31 immunohistochemistry (A and C). Sections were treated with 0.05% pepsin followed by F VIII RAg immunohistochemistry (B and D). Anti-CD31 produced better microvessel staining compared to anti-F VIII RAg staining. A and B: 200 X; C and D 100 X.
The ECs lining normal lung vessels from both athymic nude mice and BXD mice were stained with anti-CD31 and anti-F VIII RAg Abs. The staining pattern was similar to that observed in the tumors. Anti-CD31 staining produced very strong signals in a wide range of blood vessels of various sizes, while the anti-F VIII RAg antibody stained only large to medium-size vessels.
There was a significant correlation between the MVD and intensity of staining using both antibodies (r = 0.682, p < 0.01 for CD31 and r = 0.729, p <0.01 for F VIII Rag).
Discussion
The aim of the study reported here was to identify an optimal method for evaluating the neovasculature of xenograft and syngenic tumors in mice. Our approach was to compare the immunohistochemical staining of neovascular endothelium detected either by immunohistochemical staining with either CD31 or Factor VIII-related antigen, each stained under optimized AR conditions. The two polyclonal antibodies used, anti-CD31 and anti-F VIII RAg, cross-reacted with mouse endothelium. Choosing antibodies that bind directly to or cross-react with a murine endothelial marker is essential for specific detection of angiogenesis in mouse xenografts. It has been reported by Lehr et al. 1997) that the newly formed intratumor microvessels in human xenografts were lined by ECs of the host mouse.
CD31 (PECAM-1) is a transmembrane glycoprotein that is highly expressed in endothelium. Its localization at the endothelial cell junctions suggests an important role in transendothelial cellular migration (Zocchi et al. 1996). CD31 and Factor VIII-related antigen are both commonly used endothelial markers for quantifying angiogenesis by calculation of Microvessel Density (MVD) (Weidner 1995, Fox 1997, Ushijima et al. 2001, Norrby and Ridell 2003). The quantification of vascularity in tissue sections can be influenced greatly by variations in methodology and one of the most crucial factors is the use of AR in the immunohistochemical staining process.
In our study, AR with 0.5 M Tris (pH 10) buffer achieved the most intense and consistent staining of CD31 in the endothelial cells from the xenografts compared other pretreatments. On the other hand, anti-F VIII RAg antibody produced enhanced staining after pretreating the sections with 0.05% pepsin. Factor VIII Rag MVD of pepsin treated sections was significantly higher than other methods (p < 0.01). Knowing that the degree of staining positivity is altered by differences in AR methods suggests the need for standardization of AR for each antibody.
One goal of AR standardization is to maximize recovery of certain epitopes previously “masked” by formalin fixation, the so-called “maximal retrieval” (Shi et al. 1996). Different retrieval solutions may provide reaction environments that favor the uncovering of certain groups of antigens. Findings from the study of anti-CD31 staining indicate that pH, chemical composition and molarity of the buffers are important factors in addition to temperature in HIER; this agrees with previously published reports (Shi et al. 1995, Kim et al. 2004b ). Citrate buffer, one of the most commonly used ARs, yielded only weak to moderate staining of the neovasculature in the xenografts. This reduction in immunohistochemical staining in formalin fixed, paraffin embedded sections has been suggested by other investigators also (Kim et al. 2004, Yamashita 2007). Our findings are supported further by the study by Cattoretti et al. (1993), in which they sought to optimize the antigen unmasking method on various formalin fixed, paraffin embedded tissue sections using a panel of antibodies including anti-PECAM-1/CD31 and anti-vWf/Factor VIII. Enzymatic treatment and non-enzymatic heat induced treatment were the most suitable AR techniques for anti-F VIII RAg and anti-CD31 staining, respectively (Cattoretti et al. 1993).
Our study suggests the superiority of CD31 over F VIII RAg as a marker for angiogenesis in the various xenografts under each optimized retrieval condition. Staining of capillary-size intratumor vessels was significantly dependent on the antibody. In all the xenografts tested, the small vessels were more numerous and stained more intensely with anti-CD31 compared to anti-F VIII RAg (Fig. 5). We postulate that the anti-CD31 antibody stained the small vessels with immature endothelium, indicating active neoangiogenesis within the tumor. Anti-F VIII RAg antibody was shown to stain mainly the large to medium-size vessels in most cases. The lack of differentiation of tumor vasculature endothelial cells is believed to be one contributor to the inconsistent and unreliable application of markers for normal endothelium (Takahashi et al. 1998, Tsuji et al. 2002). In addition, F VIII RAg/vWf is localized selectively in Weibel-Palade bodies, a unique type of endothelial cell-specific inclusion, which is expressed least in microvessels and greatest in blood vessels close to the heart (Thorin and Shreeve 1998). The lack of vWf staining in certain tissue endothelium could be explained by insufficient vWf, resulting in fewer Weibel-Palade bodies to be detected by immunohistochemistry. Some investigators suggest that CD31 is the most sensitive marker for endothelial cell, and therefore consistently stains more vessels than F VIII RAg (Giatromanolaki et al. 1997, Leong 2004). An international consensus on methodology and criteria of evaluation of MVD also proposed that anti-CD31 immunostaining be the standard for microvessel assessment (Vermeulen et al. 1996).
Considering the intrinsic diversity of endothelial cells (Chi et al. 2003), we also tested a syngeneic breast cancer allograft from the BXD mouse to study the staining pattern of newly formed vessels derived from the host. It was shown clearly that the neovasculature staining obtained with anti-CD31 antibody was superior to that obtained with anti-F VIII RAg antibody after corresponding AR methods (Fig. 5).
We observed also that SKOV3.ip1 ovarian cancer cell xenografts had neither anti-F VIII RAg nor anti-CD31 antibody positive staining, which argues that “stainability” with different endothelial markers is tumor type-specific (Norrby and Ridell 2003). Recent studies also have shown that some aggressive tumor cells can generate vessel-like channels, i.e., vasculogenic mimicry in the absence of endothelial cells (Shirakawa et al. 2002, Su et al. 2007), thus providing another pathway for tumor perfusion independent of angiogenesis (Folberg et al. 2000). Su et al. (2007) reported that the human ovarian cancer cell line SKOV3.ip1 may express some endothelium-specific markers after vasculogenic mimicry in vivo. Weak and focal CD31 staining lies along the channels of tumor cells in one such study (Su et al. 2007). A similar staining pattern also was observed in our study.
Other markers of endothelial cells used in angiogenesis research include CD105 (endoglin) and CD34. CD105 is a homodimeric cell surface component of the transforming growth factor β (TGF-β) receptor complex. It is highly expressed in proliferating endothelial cells and has been suggested to be a marker of angiogenesis (Behrem et al. 2005). CD34 is a transmembrane glycoprotein present on lymphohematopoietic stem cells and progenitor cells, leukemic cells, endothelial cells, and embryonic fibroblasts (Greaves et al. 1992). We attempted to stain formalin fixed, paraffin embedded xenografts described above with a mouse mAntibodyagainst human CD34 (clone QBEnd/10; Biogenex, San Ramon, CA), a widely used marker in clinical practice, and a rat mAntibodyagainst mouse CD105 (clone MJ7/18; BD Pharmingen, San Jose, CA). We found the cross-reaction between the anti-CD34 mAntibodyand mouse tissue was minimal. Anti-CD105 staining also failed to elicit positive staining, which may indicate that this is not an appropriate antibody for formalin fixed, paraffin embedded tissue (data not shown). Two monoclonal antibodies against mouse CD31, including rat anti-mouse CD31 clone MEC 13.3 and clone 390, also were tested. No microvasculature staining was obtained using these two monoclonal antibodies on the formalin fixed, paraffin embedded xenografts, although it has been reported that these two antibodies produced good staining of endothelium on either fresh frozen tissue with acetone fixation or paraffin embedded samples after zinc (formalin-free) fixation (Vecchi et al. 1994, Vanzulli et al. 1997). Collectively, these data suggest the importance of antibody selection for immunohistochemical evaluation of angiogenesis. Further investigation with a wider panel of antibodies against different endothelial markers for various experimental subjects and settings should be performed.
Our studies explored the “stainability” of vessels in tumor cell line-derived xenografts with anti-CD31 and anti-F VIII RAg antibodies. AR methods for immunohistochemical staining of endothelial markers should be considered in angiogenesis research. We also suggest that anti-CD31 is superior to anti-Factor VIII in terms of immunostaining. Evaluation of neovascularization requires case-optimized methodology including antibody selection, maximum AR testing, appropriate assessment of vessel density, and many other factors, because methodological differences significantly influence the interpretation of neovascularization based on the detection of endothelial markers. The approaches described here with the CD31 polyclonal antibody should permit a rigorous evaluation of both the neovasculature and changes in the neovasculature in zenograft tumors grown in mice and allografts.
References
- Behrem S, Zarkovic K, Eskinja N, Jonjic N. Endoglin is a better marker than CD31 in evaluation of angiogenesis in glioblastoma. Croat Med J. 2005;46:417–22. [PubMed] [Google Scholar]
- Cattoretti G, Pileri S, Parravicini C, Becker MH, Poggi S, Bifulco C, Key G, D’Amato L, Sabattini E, Feudale E. Antigen unmasking on formalin-fixed, paraffin-embedded tissue sections. J Pathol. 1993;171:83–98. doi: 10.1002/path.1711710205. [DOI] [PubMed] [Google Scholar]
- Chi JT, Chang HY, Haraldsen G, Jahnsen FL, Troyanskaya OG, Chang DS, Wang Z, Rockson SG, van de Rijn M, Botstein D, Brown PO. Endothelial cell diversity revealed by global expression profiling. Proc Natl Acad Sci U S A. 2003;100:10623–8. doi: 10.1073/pnas.1434429100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folberg R, Hendrix MJ, Maniotis AJ. Vasculogenic mimicry and tumor angiogenesis. Am J Pathol. 2000;156:361–81. doi: 10.1016/S0002-9440(10)64739-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox SB. Tumour angiogenesis and prognosis. Histopathology. 1997;30:294–301. doi: 10.1046/j.1365-2559.1997.d01-606.x. [DOI] [PubMed] [Google Scholar]
- Fulzele SV, Chatterjee A, Shaik MS, Jackson T, Singh M. Inhalation delivery and anti-tumor activity of celecoxib in human orthotopic non-small cell lung cancer xenograft model. Pharm Res. 2006;23:2094–106. doi: 10.1007/s11095-006-9074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giatromanolaki A, Koukourakis MI, Theodossiou D, Barbatis K, O’Byrne K, Harris AL, Gatter KC. Comparative evaluation of angiogenesis assessment with anti-factor-VIII and anti-CD31 immunostaining in non-small cell lung cancer. Clin Cancer Res. 1997;3:2485–92. [PubMed] [Google Scholar]
- Greaves MF, Brown J, Molgaard HV, Spurr NK, Robertson D, Delia D, Sutherland DR. Molecular features of CD34: a hemopoietic progenitor cell-associated molecule. Leukemia. 1992;6:31–6. [PubMed] [Google Scholar]
- Grizzle WE, Mountz JD, Yang PA, Xu X, Sun S, Van Zant GE, Williams RW, Hsu HC, Zhang HG. BXD recombinant inbred mice represent a novel T cell-mediated immune response tumor model. Int J Cancer. 2002;101:270–9. doi: 10.1002/ijc.10606. [DOI] [PubMed] [Google Scholar]
- Hlatky L, Hahnfeldt P, Folkman J. Clinical application of antiangiogenic therapy: microvessel density, what it does and doesn’t tell us. J Natl Cancer Inst. 2002;94:883–93. doi: 10.1093/jnci/94.12.883. [DOI] [PubMed] [Google Scholar]
- Kim SH, Kook MC, Shin YK, Park SH, Song HG. Evaluation of antigen retrieval buffer systems. J Mol Histol. 2004a;35:409–16. doi: 10.1023/b:hijo.0000039854.17808.e0. [DOI] [PubMed] [Google Scholar]
- Kim SH, Kook MC, Song HG. Optimal conditions for the retrieval of CD4 and CD8 antigens in formalin-fixed, paraffin-embedded tissues. J Mol Histol. 2004b;35:403–8. doi: 10.1023/b:hijo.0000039856.43632.66. [DOI] [PubMed] [Google Scholar]
- Lehr HA, Skelly M, Buhler K, Anderson B, Delisser HM, Gown AM. Microvascular endothelium of human tumor xenografts expresses mouse (= host) CD31. Int J Microcirc Clin Exp. 1997;17:138–42. doi: 10.1159/000179221. [DOI] [PubMed] [Google Scholar]
- Leong AS. Pitfalls in diagnostic immunohistology. Adv Anat Pathol. 2004;11:86–93. doi: 10.1097/00125480-200403000-00002. [DOI] [PubMed] [Google Scholar]
- Meert AP, Paesmans M, Martin B, Delmotte P, Berghmans T, Verdebout JM, Lafitte JJ, Mascaux C, Sculier JP. The role of microvessel density on the survival of patients with lung cancer: a systematic review of the literature with meta-analysis. Br J Cancer. 2002;87:694–701. doi: 10.1038/sj.bjc.6600551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller AM, Hermanns MI, Skrzynski C, Nesslinger M, Müller KM, Kirkpatrick CJ. Expression of the endothelial markers PECAM-1, vWf, and CD34 in vivo and in vitro. Exp Mol Pathol. 2002;72:221–9. doi: 10.1006/exmp.2002.2424. [DOI] [PubMed] [Google Scholar]
- Muruganandham M, Lupu M, Dyke JP, Matei C, Linn M, Packman K, Kolinsky K, Higgins B, Koutcher JA. Preclinical evaluation of tumor microvascular response to a novel antiangiogenic/antitumor agent RO0281501 by dynamic contrast-enhanced MRI at 1.5 T. Mol Cancer Ther. 2006;5:1950–7. doi: 10.1158/1535-7163.MCT-06-0010. [DOI] [PubMed] [Google Scholar]
- Norrby K, Ridell B. Tumour-type-specific capillary endothelial cell stainability in malignant B-cell lymphomas using antibodies against CD31, CD34 and Factor VIII. Apmis. 2003;111:483–9. doi: 10.1034/j.1600-0463.2003.1110406.x. [DOI] [PubMed] [Google Scholar]
- Ragel BT, Jensen RL, Gillespie DL, Prescott SM, Couldwell WT. Celecoxib inhibits meningioma tumor growth in a mouse xenograft model. Cancer. 2007;109:588–97. doi: 10.1002/cncr.22441. [DOI] [PubMed] [Google Scholar]
- Shi SR, Imam SA, Young L, Cote RJ, Taylor CR. Antigen retrieval immunohistochemistry under the influence of pH using monoclonal antibodies. J Histochem Cytochem. 1995;43:193–201. doi: 10.1177/43.2.7822775. [DOI] [PubMed] [Google Scholar]
- Shi SR, Cote RJ, Yang C, Chen C, Xu HJ, Benedict WF, Taylor CR. Development of an optimal protocol for antigen retrieval: a ‘test battery’ approach exemplified with reference to the staining of retinoblastoma protein (pRB) in formalin-fixed paraffin sections. J Pathol. 1996;179:347–52. doi: 10.1002/(SICI)1096-9896(199607)179:3<347::AID-PATH559>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Shirakawa K, Kobayashi H, Heike Y, Kawamoto S, Brechbiel MW, Kasumi F, Iwanaga T, Konishi F, Terada M, Wakasugi H. Hemodynamics in vasculogenic mimicry and angiogenesis of inflammatory breast cancer xenograft. Cancer Res. 2002;62:560–6. [PubMed] [Google Scholar]
- Stirling J. Chapter5 : Antigen Retrieval and Unmasking for Immunoelectron Microscopy. In: Shi SR, Gu J, Taylor CR, editors. Antigen Retrieval Techniques: Immunohistochemistry and Molecular Morphology. Eaton Publishing; Natick, MA: 2000. p. 106. [Google Scholar]
- Su M, Feng YJ, Yao LQ, Cheng MJ, Xu CJ, Huang Y, Zhao YQ, Jiang H. Plasticity of ovarian cancer cell SKOV3ip and vasculogenic mimicry in vivo. Int J Gynecol Cancer. 2008;18:476–86. doi: 10.1111/j.1525-1438.2007.01034.x. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Bucana CD, Cleary KR, Ellis LM. p53, vessel count, and vascular endothelial growth factor expression in human colon cancer. Int J Cancer. 1998;79:34–8. doi: 10.1002/(sici)1097-0215(19980220)79:1<34::aid-ijc7>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Thorin E, Shreeve SM. Heterogeneity of vascular endothelial cells in normal and disease states. Pharmacol Ther. 1998;78:155–66. doi: 10.1016/s0163-7258(98)00005-9. [DOI] [PubMed] [Google Scholar]
- Tsuji T, Sasaki Y, Tanaka M, Hanabata N, Hada R, Munakata A. Microvessel morphology and vascular endothelial growth factor expression in human colonic carcinoma with or without metastasis. Lab Invest. 2002;82:555–62. doi: 10.1038/labinvest.3780450. [DOI] [PubMed] [Google Scholar]
- Ushijima C, Tsukamoto S, Yamazaki K, Yoshino I, Sugio K, Sugimachi K. High vascularity in the peripheral region of non-small cell lung cancer tissue is associated with tumor progression. Lung Cancer. 2001;34:233–41. doi: 10.1016/s0169-5002(01)00246-x. [DOI] [PubMed] [Google Scholar]
- Vanzulli S, Gazzaniga S, Braidot MF, Vecchi A, Mantovani A, Wainstok de Calmanovici R. Detection of endothelial cells by MEC 13.3 monoclonal antibody in mice mammary tumors. Biocell. 1997;21:39–46. [PubMed] [Google Scholar]
- Vecchi A, Garlanda C, Lampugnani MG, Resnati M, Matteucci C, Stoppacciaro A, Schnurch H, Risau W, Ruco L, Mantovani A. Monoclonal antibodies specific for endothelial cells of mouse blood vessels. Their application in the identification of adult and embryonic endothelium. Eur J Cell Biol. 1994;63:247–54. [PubMed] [Google Scholar]
- Vermeulen PB, Gasparini G, Fox SB, Toi M, Martin L, McCulloch P, Pezzella F, Viale G, Weidner N, Harris AL, Dirix LY. Quantification of angiogenesis in solid human tumours: an international consensus on the methodology and criteria of evaluation. Eur J Cancer. 1996;32A:2474–84. doi: 10.1016/s0959-8049(96)00379-6. [DOI] [PubMed] [Google Scholar]
- Weidner N. Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Res Treat. 1995;36:169–80. doi: 10.1007/BF00666038. [DOI] [PubMed] [Google Scholar]
- Yamashita S. Heat-induced antigen retrieval: mechanisms and application to histochemistry. Prog Histochem Cytochem. 2007;41:141–200. doi: 10.1016/j.proghi.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Zocchi MR, Ferrero E, Leone BE, Rovere P, Bianchi E, Toninelli E, Pardi R. CD31/PECAM-1-driven chemokine-independent transmigration of human T lymphocytes. Eur J Immunol. 1996;26:759–67. doi: 10.1002/eji.1830260406. [DOI] [PubMed] [Google Scholar]