Abstract
Purpose
To evaluate the prognostic and predictive value of Ki-67 labeling index (LI) in a trial comparing letrozole (Let) with tamoxifen (Tam) as adjuvant therapy in postmenopausal women with early breast cancer.
Patients and Methods
Breast International Group (BIG) trial 1-98 randomly assigned 8,010 patients to four treatment arms comparing Let and Tam with sequences of each agent. Of 4,922 patients randomly assigned to receive 5 years of monotherapy with either agent, 2,685 had primary tumor material available for central pathology assessment of Ki-67 LI by immunohistochemistry and had tumors confirmed to express estrogen receptors after central review. The prognostic and predictive value of centrally measured Ki-67 LI on disease-free survival (DFS) were assessed among these patients using proportional hazards modeling, with Ki-67 LI values dichotomized at the median value of 11%.
Results
Higher values of Ki-67 LI were associated with adverse prognostic factors and with worse DFS (hazard ratio [HR; high:low] = 1.8; 95% CI, 1.4 to 2.3). The magnitude of the treatment benefit for Let versus Tam was greater among patients with high tumor Ki-67 LI (HR [Let:Tam] = 0.53; 95% CI, 0.39 to 0.72) than among patients with low tumor Ki-67 LI (HR [Let:Tam] = 0.81; 95% CI, 0.57 to 1.15; interaction P = .09).
Conclusion
Ki-67 LI is confirmed as a prognostic factor in this study. High Ki-67 LI levels may identify a patient group that particularly benefits from initial Let adjuvant therapy.
INTRODUCTION
Recent St Gallen Guidelines for the selection of therapy in early breast cancer have increasingly stressed the importance of identifying primarily factors predictive of response to particular therapies, and secondarily prognostic factors for risk of recurrence.1 Tumor proliferation fraction is an established predictor of prognosis.2 The nuclear protein Ki-67, present in cycling cells,3 is an indicator of tumor proliferation4,5 and has been found to be a prognostic marker in breast cancer.6-11 High Ki-67 labeling index (LI) is reportedly predictive of responsiveness to preoperative chemotherapy.12,13 A fall in Ki-67 LI during preoperative endocrine therapy has been associated with pathologic tumor response,14 whereas Dowsett et al15 found that persistently higher Ki-67 LI after short-term preoperative endocrine therapy predicted shorter disease-free survival. We have recently described the role of Ki-67 LI as a prognostic factor in premenopausal and postmenopausal women with hormone receptor–positive, node-negative breast cancer, but we did not find Ki-67 LI to be predictive of differential responsiveness to the chemoendocrine or endocrine therapies studied in that adjuvant setting.16 To our knowledge, there are no reports of Ki-67 LI predicting responsiveness to postoperative cytotoxic or endocrine adjuvant therapies.
Breast International Group (BIG) trial 1-98 is an international, double-blind, four-arm, randomized phase III trial investigating the aromatase inhibitor letrozole (Let) compared with tamoxifen (Tam) in the adjuvant setting among postmenopausal women with endocrine-responsive, early invasive breast cancer. Both the primary analysis17 and a subsequent report limited to patients randomly assigned to the monotherapy treatment arms18 supported the improvement of disease-free survival (DFS) in patients assigned initial Let compared with Tam.
The purpose of this report is to examine the value of Ki-67 LI, as assessed in the International Breast Cancer Study Group (IBCSG) Central Pathology Laboratory, both as a prognostic factor and as a predictive factor for differential efficacy of Let versus Tam used as initial adjuvant therapy in postmenopausal women with endocrine-responsive breast cancer.
PATIENTS AND METHODS
Study Design
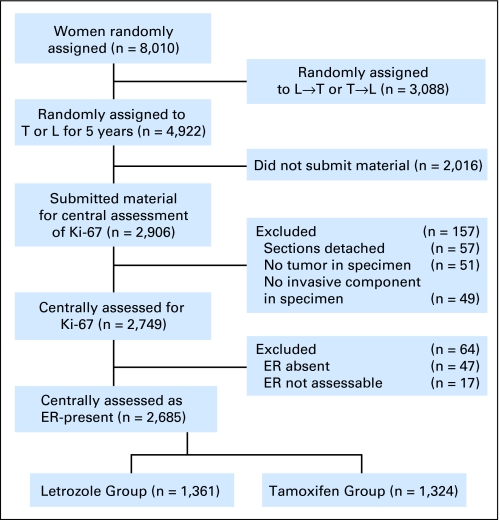
The BIG 1-98 patient population was defined as postmenopausal women with early invasive breast cancer whose tumors were assessed by local pathologists as hormone receptor (estrogen receptor [ER] and/or progesterone receptor [PgR]) positive. Between March 1998 and March 2000, patients were randomly assigned to receive adjuvant endocrine therapy in one of the monotherapy arms comprising either Let 2.5 mg/d or Tam 20 mg/d for 5 years, and from April 1999 to May 2003 to all four arms including the sequence of 2 years, Tam followed by 3 years of Let or 2 years of Let followed by 3 years of Tam. The primary efficacy analysis among 8,010 patients17 was updated as specified by protocol, and reported among the 4,922 patients who were randomly assigned to the monotherapy arms only at a median follow-up time of 51 months.18 This updated analysis, limited to patients assigned to 5 years of monotherapy with either Tam or Let, is used for the current report. Retrospective tissue collection was carried out in accordance with institutional guidelines and national laws. Tumor material from 2,906 (59%) of the 4,922 patients was submitted to the IBCSG Central Pathology Office for central pathology review (CPR) of Ki-67. The analysis cohort was further limited to patients for whom adequate tumor material was available, and for whom CPR confirmed expression of ER in the tumor (n = 2,685; Fig 1).
Fig 1.
Patients from the Breast International Group 1-98 trial included and excluded in this study according to treatment group and availability of tumor material. L, letrozole; T, tamoxifen; ER, estrogen receptor.
Pathology
The IBCSG Central Pathology Laboratory performed central review of paraffin-embedded primary tumor specimens for ER and PgR by immunohistochemistry (IHC),19 and for human epidermal growth factor receptor 2 (HER-2) by IHC and fluorescent in situ hybridization (FISH).20 Tumors were considered to express ER or PgR if they showed at least 1% of immunoreactive cells. Tumors were considered to be HER-2 positive if amplified by FISH, or in a few cases with nonassessable FISH results, if IHC was 3+.
Ki-67 was assessed by IHC using the Mib-1 monoclonal antibody (1:200 dilution; Dako, Glostrup, Denmark). Slides were cut and stained centrally using an automated immunostainer (Autostainer, Dako), and the results assessed without the use of an image analysis system. The percentage of cells showing definite nuclear immunoreactivity among 2,000 invasive neoplastic cells in randomly selected, high-power (magnification, ×400) fields at the periphery of the tumor was recorded. The CPR was performed without knowledge of patients’ treatment assignment or outcome. All of the assays were performed on whole tissue sections.
End Points and Statistical Considerations
A comparison of patients with and without material for CPR was previously described.21 Levels of Ki-67 LI were dichotomized as high (> 11%) and low (≤ 11%) for the primary analysis. The cutoff is the median of the distribution of Ki-67 LI among all BIG 1-98 trial patients’ material that was centrally assessed for Ki-67 (n = 4,399 of 8,010). Associations of other prognostic tumor features and Ki-67 LI levels were evaluated using χ2 tests.
The protocol-specified primary trial end point was DFS, which was defined as the time from random assignment to the earliest time of invasive recurrence in local, regional, or distant sites; a new invasive breast cancer in the contralateral breast; any second (nonbreast) malignancy; or death resulting from any cause. The distribution of DFS was summarized using the Kaplan-Meier method.22 Proportional hazards modeling,23 stratified by whether chemotherapy had been administered and by randomization option (two or four arm),17 was used to investigate predictors of DFS and to estimate hazard ratios (HRs), 95% CI, and to assess interactions of the treatment effect with Ki-67 LI and other prognostic variables. Subpopulation Treatment Effect Pattern Plot (STEPP)24 methodology was employed to further illustrate the relationship between Ki-67 LI and outcome across the continuum of Ki-67 LI levels. The STEPP method uses a sliding-window approach to define several overlapping subpopulations of patients according to Ki-67 LI. The values on the x-axis are the median values of Ki-67 LI for patients in a subpopulation, and the y-axis indicates the treatment effects, expressed as the Kaplan-Meier estimates of 4-year DFS. Each subpopulation contains approximately 200 patients and slides by approximately 50 patients.
To determine the most predictive Ki-67 LI cut point, separate proportional hazards models including treatment, Ki-67 LI, and their interaction as predictors were constructed with Ki-67 LI dichotomized at successive integer values between 1% and 55%. The best cut point was identified by determining the Ki-67 LI division that minimized the Wald χ2 P value of the interaction.
Statistical analyses used SAS version 9.1 (SAS Institute, Cary, NC) and S-PLUS version 6.1 (Insightful Corp, Seattle, WA). All statistical tests provided two-sided P values, and P ≤ .05 was considered statistically significant.
RESULTS
In univariate analyses, high (> 11%) Ki-67 LI was associated with larger tumors, higher tumor grade, peritumoral vascular invasion, and HER-2 positivity (each P < .01), but in this population, as distinct from our earlier trials,16 there was no association between Ki-67 LI and presence or absence of PgR expression (Table 1).
Table 1.
Association of Tumor Ki-67 LI With Other Tumor Features
| Feature | Ki-67 LI
|
P | |||
|---|---|---|---|---|---|
| Low ≤ 11%
|
High > 11%
|
||||
| No. | % | No. | % | ||
| No. of patients | 1,433 | 53.4 | 1,252 | 46.6 | |
| HER-2 overexpression | < .0001 | ||||
| No | 1396 | 97.4 | 1114 | 89.0 | |
| Yes | 37 | 2.6 | 138 | 11.0 | |
| Nodal status | .0004 | ||||
| N-/Nx | 971 | 67.8 | 767 | 61.3 | |
| N+ | 462 | 32.2 | 485 | 38.7 | |
| Tumor size, cm | < .0001 | ||||
| ≤ 2 | 1013 | 70.7 | 750 | 59.9 | |
| > 2 | 414 | 28.9 | 491 | 39.2 | |
| Unknown | 6 | .4 | 11 | 0.9 | |
| Tumor grade | < .0001 | ||||
| 1 | 561 | 39.1 | 192 | 15.3 | |
| 2 | 655 | 45.7 | 632 | 50.5 | |
| 3 | 89 | 6.2 | 326 | 26.0 | |
| Unknown | 128 | 8.9 | 102 | 8.1 | |
| Peritumoral vascular invasion | < .0001 | ||||
| No | 1127 | 78.6 | 870 | 69.5 | |
| Yes | 178 | 12.4 | 278 | 22.2 | |
| Unknown/not able to assess | 128 | 8.9 | 104 | 8.3 | |
| ER/PgR expressed (assessed centrally)* | .68 | ||||
| ER present/PgR absent | 149 | 10.4 | 119 | 9.5 | |
| ER present/PgR present | 1278 | 89.2 | 1129 | 90.2 | |
| Other | 6 | .4 | 4 | 0.3 | |
Abbreviations: LI, labeling index; HER-2, human epidermal growth factor receptor 2; ER, estrogen receptor; PgR, progesterone receptor.
ER and PgR each are considered as present if ≥1% immunoreactive cells.
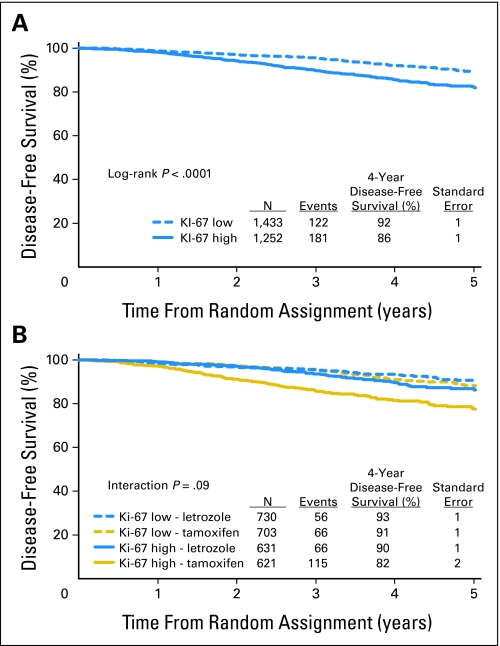
DFS was significantly lower for patients with tumors with high Ki-67 LI (HR [high:low] = 1.8; 95% CI, 1.4 to 2.3; P = .0001), confirming the prognostic value of Ki-67 LI in this cohort (Fig 2A). Four-year DFS estimates were 92.2% for low versus 85.6% for high Ki-67 LI. In a multivariable proportional hazards regression model adjusted for patient age, PgR status, tumor size, tumor grade, nodal status, HER-2 status, and presence of peritumoral vascular involvement, high Ki-67 LI remained an independent adverse prognostic factor (HR = 1.4; 95% CI, 1.1 to 1.9; P = .02).
Fig 2.
Kaplan-Meier estimates of disease-free survival according to (A) level of Ki-67 labeling index (high > 11% v low ≤ 11%) and (B) treatment assignment.
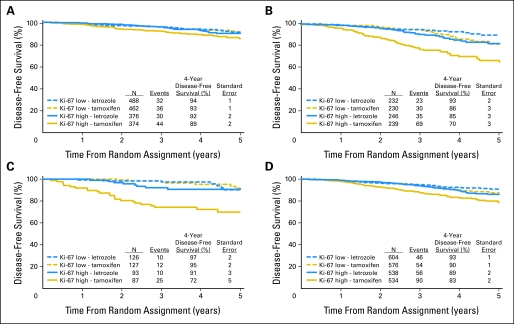
In this analytic cohort, as for the trial population as a whole, DFS was significantly better in patients randomly assigned to receive Let compared with Tam; (HR [Let:Tam] = 0.63, 95% CI, 0.50 to 0.80; P < .0001) and 4-year DFS estimates were 91.7% and 86.5%, respectively. There was a suggestion of heterogeneity in the treatment effect among patients with tumors having high versus low Ki-67 LI (P = .09 for interaction; Fig 2B). Within the subgroup having high tumor Ki-67 LI, the hazard for a DFS event for patients who received Let was approximately half the hazard of patients who received Tam (HR [Let:Tam] = 0.53, 95% CI, 0.39 to 0.72) which was a greater treatment effect than that observed among patients with low tumor Ki-67 LI (HR [Let:Tam] = 0.81; 95% CI, 0.57 to 1.15). The estimated 4-year DFS among patients in the subgroup having high tumor Ki-67 LI who received Let (89.6%) was comparable with those for patients with low Ki-67 LI who received either Let (93.4%) or Tam (90.9%). The overall pattern was similar whether patients had node-negative or node-positive disease or whether the tumors were ER expressing (1% to 79%) or strongly ER expressing (80% or higher; Fig 3).
Fig 3.
Kaplan-Meier estimates of disease-free survival according to level of Ki-67 labeling index (LI) (high > 11% v low ≤ 11%) and treatment assignment, separately for patients (A) with lymph node–negative disease and (B) node-positive disease, and with tumors that are (C) estrogen receptor expressing (1% to 79%) and (D) strongly estrogen receptor expressing (≥ 80%).
Through exploratory analyses of integer cut points of the Ki-67 LI distribution, the P value for the interaction of treatment and Ki-67 LI was minimized (P = .02) when Ki-67 LI was dichotomized at 14%. When Ki-67 was dichotomized as less than or equal to 14% versus greater than 14%, treatment comparisons yielded results similar to those reported above.
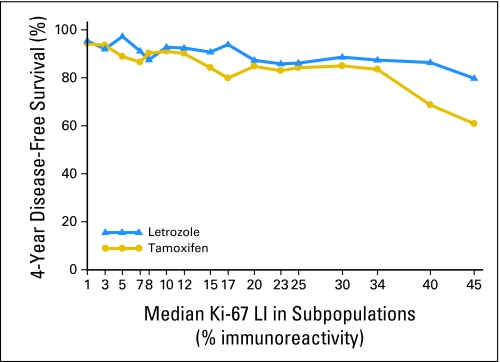
The STEPP analysis of 4-year DFS across the continuum of Ki-67 LI percentages (Fig 4) displays the suggested heterogeneity in the treatment effect across various levels of Ki-67 LI. For subpopulations with higher median Ki-67 LI greater than 10% (and especially above 30%), the separation of the curves suggests greatest benefit of Let relative to Tam for subpopulations with the highest levels of Ki-67 LI.
Fig 4.
Subpopulation Treatment Effect Pattern Plot analysis of the treatment effect of letrozole versus tamoxifen as measured by 4-year disease-free survival according to overlapping subpopulations defined by percentages of Ki-67 labeling index (LI). The x-axis indicates the median percentage of Ki-67 LI for patients in each of the overlapping subpopulations.
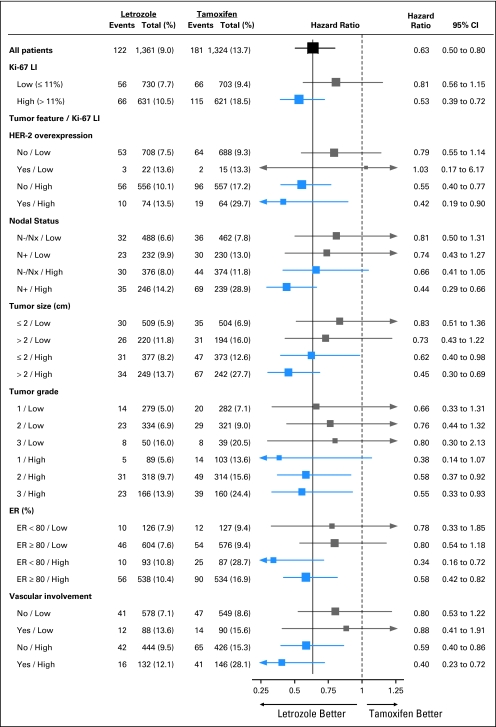
As a hypothesis-generating exercise, we further explored whether the suggested difference in the relative efficacy of Let versus Tam for high and low tumor Ki-67 LI might be modified by other prognostic tumor features (Fig 5). There was little evidence for any such interaction, and the overall impression from inspection of the forest plot is that the relative efficacy of Let is greater in subgroups with high tumor Ki-67 LI regardless of other tumor features.
Fig 5.
Proportional hazards model results of disease-free survival in subgroups. The size of each box is inversely proportional to the SE of the hazard ratio (HR). The solid vertical line is placed at HR = 0.63, which is the HR estimate for the overall analysis of letrozole compared with tamoxifen in this analytic cohort. HER-2, human epidermal growth factor receptor 2; LI, labeling index; ER, estrogen receptor.
DISCUSSION
This analysis supports previous reports that Ki-67 LI is a prognostic factor in early breast cancer.6-11 The median value of 11% for KI-67 LI in the CPR cohort from BIG 1-98 was lower than the 19% median we had observed in an earlier series in IBCSG Trial IX, which compared chemoendocrine with endocrine therapy among node-negative patients,16 but similar to the 10% cut point used in several series published by others.7,25 We16 and others6 have noted a correlation between Ki-67 LI and adverse prognostic factors including tumor differentiation, which may partly explain the lower median Ki-67 LI in the present series, because the earlier trials included more patients with high-grade tumors.16
More importantly, our analysis provides the first evidence to our knowledge suggesting that Ki-67 LI may have predictive value for the choice of an aromatase inhibitor rather than Tam as adjuvant endocrine therapy among postmenopausal women with endocrine-responsive early breast cancer. Comparison of Let and Tam by Ki-67 LI suggests that Let may be particularly beneficial at higher levels of Ki-67 LI. The hazard of a DFS event was reduced by approximately half in favor of Let for higher levels of Ki-67 LI, which was a treatment effect of greater magnitude than among patients with tumors having low levels of Ki-67 LI. The larger magnitude of benefit for higher Ki-67 LI is in contrast to the lack of such a differential efficacy of Let versus Tam, which we have previously described for HER-220 and PgR.21
Why should the magnitude of Let superiority over Tam be larger in patients with high tumor Ki-67 LI? One possibility is that among patients with a high tumor proliferation fraction, particularly if associated with overexpression of membrane growth factors (as we show in the present study for HER-2), patients receiving Tam would have higher residual circulating estrogen levels than those receiving an aromatase inhibitor. This residual estrogen, or indeed an agonistic action of Tam itself,26 may activate membrane ER and combine with high levels of growth factor receptors to worsen prognosis, whereas patients with profound estrogen deprivation induced by an aromatase inhibitor might be protected from tumor cell stimulation through membrane ER.27 Alternatively, the observed high Ki-67 LI may itself be a reflection of an established growth factor-driven stimulation by residual postmenopausal levels of estrogen through membrane ER crosstalk.27 In such a scenario, Tam would be less able than profound estrogen depletion to reverse the stimulus to tumor growth.
Particular interest has centered on the choice between initial use of an aromatase inhibitor, as in the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial28 and the results so far available from the BIG 1-98 trial,17,18 compared with a policy of switching to an aromatase inhibitor after 2 or 3 years of adjuvant Tam therapy.29,30 Pending availability of results from the sequential arms of BIG 1-98, and provided that our observations are confirmed in other studies, it may be that high tumor Ki-67 LI could identify patients in whom the superiority of Let over Tam as initial endocrine therapy is particularly marked.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: Beat Thürlimann, Novartis Honoraria: Beat Thürlimann, Novartis Research Funding: None Expert Testimony: None Other Remuneration: Alan S. Coates, Novartis
AUTHOR CONTRIBUTIONS
Conception and design: Giuseppe Viale, Alan S. Coates, Richard D. Gelber, Aron Goldhirsch
Administrative support: Beat Thürlimann, Karen N. Price, Monica Castiglione-Gertsch
Provision of study materials or patients: Giuseppe Viale, Mauro G. Mastropasqua, Patrizia Dell'Orto, Gaëtan MacGrogan, Stephen G. Braye, Christian Öhlschlegel, Patrick Neven, Zsolt Orosz, Wojciech P. Olszewski, Fiona Knox, Beat Thürlimann, Monica Castiglione-Gertsch, Barry A. Gusterson, Aron Goldhirsch
Collection and assembly of data: Giuseppe Viale, Mauro G. Mastropasqua, Patrizia Dell'Orto, Eugenio Maiorano
Data analysis and interpretation: Giuseppe Viale, Anita Giobbie-Hurder, Meredith M. Regan, Alan S. Coates, Richard D. Gelber
Manuscript writing: Giuseppe Viale, Anita Giobbie-Hurder, Meredith M. Regan, Alan S. Coates, Karen N. Price
Final approval of manuscript: Giuseppe Viale, Anita Giobbie-Hurder, Meredith M. Regan, Alan S. Coates, Mauro G. Mastropasqua, Patrizia Dell'Orto, Eugenio Maiorano, Gaëtan MacGrogan, Stephen G. Braye, Christian Öhlschlegel, Patrick Neven, Zsolt Orosz, Wojciech P. Olszewski, Fiona Knox, Beat Thürlimann, Karen N. Price, Monica Castiglione-Gertsch, Richard D. Gelber, Barry A. Gusterson, Aron Goldhirsch
Acknowledgments
We thank Rosita Kammler, Stefania Andrighetto, the pathologists, patients, physicians, nurses, and data managers who participated; Novartis; the International Breast Cancer Study Group (IBCSG), the French Fédération Nationale des Centres de Lutte Contre le Cancer (FNCLCC), and the other participating groups and centers listed in the Appendix (online only).
Appendix
BIG 1-98 Collaborative Group Participants
Steering Committee.
B. Thürlimann (Chair), S. Aebi, L. Blacher, M. Castiglione-Gertsch, A.S. Coates, T. Cufer, J.F. Forbes, R.D. Gelber, A. Giobbie-Hurder A. Goldhirsch, A. Hiltbrunner, S.B. Holmberg, R. Maibach, A. Martoni, L. Mauriac, G. McGrogan, H.T. Mouridsen, R. Paridaens, K.N. Price, M. Rabaglio, B.B. Rasmussen, M.M. Regan, A. Santoro, I.E. Smith, A. Wardley, K. Vantongelen, G. Viale. Novartis: H.A. Chaudri-Ross, D. Emanuel, D.B. Evans, C. Sguotti, U. Trostmann.
IBCSG Scientific Committee.
A. Goldhirsch, A.S. Coates (Co-Chairs), L. Blacher, M. Castiglione-Gertsch, J.F. Forbes, R.D. Gelber, B.A. Gusterson, A. Hiltbrunner, C. Hürny, E. Murray, K.N. Price, M. Rabaglio, R. Studer, G. Viale, A. Wallgren.
IBCSG Foundation Council.
R. Stahel (President), M. Castiglione-Gertsch, A.S. Coates, J.P. Collins, R.D. Gelber, A. Goldhirsch, M. Green, A. Hiltbrunner, S.B. Holmberg, D.K. Hossfeld, P. Karlsson, I. Láng, M. de Stoppani, C.-M. Rudenstam, B. Thürlimann, A. Veronesi.
Coordinating Center (Bern, Switzerland).
A. Hiltbrunner (Director), M. Rabaglio, G. Egli, B. Cliffe, S. Ribeli-Hofmann, R. Kammler, R. Studer, B. Ruepp, R. Maibach, N. Munarini.
Coordinating Center (Bern, Switzerland).
M. Castiglione-Gertsch (CEO), A. Hiltbrunner (Director), M. Rabaglio, G. Egli, B. Cliffe, S. Ribeli-Hofmann, F. Munarini, R. Kammler, R. Studer, B. Ruepp, R. Maibach, N. Munarini.
Statistical Center (Dana-Farber Cancer Institute, Boston, MA).
R.D. Gelber (Group Statistician), K.N. Price (Director of Scientific Administration), A. Giobbie-Hurder (Trial Statistician), M. Regan, Z. Sun, A. Keshaviah, H. Litman, H. Huang, L.J. Somos, B. Timmers, L. Nickerson.
Data Management Center (Frontier Science & Technology Research Foundation, Amherst, NY).
L. Blacher (Director of Data Management), T. Heckman Scolese (Coordinating Data Manager), M. Belisle, M. Caporale, J. Celano, L. Dalfonso, L. Dooley, S. Fischer, K. Galloway, J. Gould, R. Hinkle, M. Holody, G. Jones, R. Krall, S. Lippert, J. Meshulam, L. Mundy, A. Pavlov-Shapiro, K. Scott, M. Scott, S. Shepard, J. Swick, L. Uhteg, D. Weinbaum, C. Westby, T. Zielinski.
Central Pathology Review Office (University of Glasgow, Glasgow, UK).
B.A. Gusterson, E. Mallon; (European Institute of Oncology, Division of Pathology, Milano, Italy): G. Viale, P. Dell'Orto, M. Mastropasqua, B. Del Curto.
Data and Safety Monitoring Committee.
D.F. Hayes, J.E. Garber, S.W. Lagakos, I. Lindgren.
Study Support (Novartis Corp. Basel, Switzerland).
E. Waldie, I. van Hoomissen, M. De Smet, W. Schmidt, A. Bolton, W. Hackl.
Breast International Group (BIG)
International Breast Cancer Study Group (IBCSG)
Australian New Zealand Breast Cancer Trials Group (ANZ BCTG).
Board Chair: J.H. Chirgwin, Group Co-ordinator: J.F. Forbes, Chair Scientific Advisory Committee: F.M. Boyle; ANZ BCTG Operations Office (Newcastle, Australia): D. Lindsay (Head Data Management), D. Preece (Senior Study Coordinator), K. Oleksyn.
Australia.
The Cancer Council Victoria, Melbourne, VIC: F. Abell, R. Basser, R. Bell, B. Brady, D. Blakey, P. Briggs, I. Burns, P. Campbell, M. Chao, J. Chirgwin, B. Chua, K. Clarke, J. Collins, R. De Boer, J.C. Din, R. Doig, A. Dowling, R. Drummond, N. Efe, S.T. Fan, M. Francis, P. Francis, V. Ganju, P. Gibbs, G. Goss, M. Green, P. Gregory, J. Griffiths, I. Haines, M. Henderson, R. Holmes, P. James, J. Kiffler, M. Lehman, M. Leyden, L. Lim, G. Lindeman, R. Lynch, B. Mann, J. McKendrick, S. McLachlan, R. McLennan, G. Mitchell, S. Mitra, C. Murphy, I. Parker, K. Phillips, I. Porter, G. Richardson, J. Scarlet, S. Sewak, J. Shapiro, R. Snyder, R. Stanley, C. Steer, D. Stoney, A. Strickland, G. Toner, C. Underhill, K. White, M. White, A. Wirth, S. Wong; W.P. Holman Clinic, Launceston General Hospital, Launceston, Tasmania: D. Byram, I. Byard; Liverpool Hospital, Sydney, NSW: S. Della-Fiorentina, A. Goldrick, E. Hovey, E. Moylan, E. Segelov; Mount Hospital, Perth, WA: A. Chan, M. Buck, D. Hastrich, D. Ingram, G. Van Hazel, P. Willsher; Nepean Cancer Care Centre, Sydney, NSW: N. Wilcken, C. Crombie; Calvary Mater Newcastle, Newcastle, NSW: J.F. Forbes, F. Abell, S. Ackland, A. Bonaventura, S. Cox, J. Denham, R. Gourlay, D. Jackson, R. Sillar, J. Stewart; Prince of Wales Hospital, Sydney, NSW: C. Lewis, B. Brigham, D. Goldstein, M. Friedlander; Princess Alexandra Hospital, Woollongabba, QLD: E. Walpole, D. Thompson; Royal Adelaide Hospital, Adelaide, SA: P.G. Gill, M. Bochner, J. Coventry, J. Kollias, P. Malycha, I. Olver; Royal Brisbane and Women's Hospital, Brisbane, QLD: M. Colosimo, R. Cheuk, L. Kenny, N. McCarthy, D. Wyld; Royal Hobart Hospital, Hobart, Tasmania: R. Young, R. Harrup, R. Kimber, R. Lowenthal; Royal Perth Hospital, Perth, WA: J. Trotter, E. Bayliss, A. Chan, D. Ransom; Sir Charles Gairdner Hospital, Perth, WA: M. Byrne, M. Buck, J. Dewar, A. Nowak, A. Powell, G. Van Hazel; Toowoomba Hospital, Toowoomba, QLD: E.A. Abdi, R. Brodribb, Z. Volobueva; Westmead Hospital, Sydney, NSW: P. Harnett, V. Ahern, H. Gurney, N. Wilcken.
New Zealand.
Auckland Hospital, Auckland: V.J. Harvey, B. Evans, W. Jones, M. McCrystal, D. Porter, P. Thompson, M. Vaughan; Christchurch Hospital, Christchurch: D. Gibbs, C. Atkinson, R. Burcombe, B. Fitzharris, B. Hickey, M. Jeffery, B. Robinson; Dunedin Hospital, Dunedin: B. McLaren, S. Costello, J. North, D. Perez; Waikato Hospital, Hamilton: I.D. Campbell, L. Gilbert, R. Gannaway, M. Jameson, I. Kennedy, J. Long, G. Round, L. Spellman, D. Whittle, D. Woolerton.
Brazil.
Hospital de Clinicas de Porto Alegre, Porto Alegre: C. Menke, J. Biazús, R. Cericatto, J. Cavalheiro, N. Xavier, A. Bittelbrunn, E. Rabin.
Chile.
Chilean Cooperative Group for Oncologic Research, GOCCHI. J. Gutiérrez (Chairman), R. Arriagada (Scientific Adviser), L. Bronfman (Principal Investigator), M. Zuñiga (Data Manager); Clinica Las Condes, Santiago: J. Gutiérrez, J.C. Acevedo, S. Torres, A. León, E. Salazar; Hospital DIPRECA, Las Condes, Santiago: L. Soto Diaz, R. Duval, N. Oddeshede, M.C. Venti; Hospital San Juan de Dios, Santiago: K. Peña, L. Puente, V. Maidana; IRAM/Instituto de Radiomedicina, Vitacura, Santiago: R. Baeza, R. Arriagada, P. Olfos, J. Solé, E. Vinés, C. Mariani.
Hungary.
National Institute of Oncology, Budapest. I. Láng, E. Hitre, E. Szabó, Z. Horváth, E. Ganofszky, E. Juhos.
Italy.
Centro di Riferimento Oncologico, Aviano. A. Veronesi, D. Crivellari, M.D. Magri, A. Buonadonna, F. Coran, E. Borsatti, E. Candiani, S. Massarut, M. Roncadin, M. Arcicasa, A. Carbone, T. Perin, A. Gloghini; Ospedali Riuniti di Bergamo, Bergamo: C. Tondini, R. Labianca, P. Poletti, A. Bettini; Ospedale degli Infermi, Biella: M. Clerico, M. Vincenti, A. Malossi, E. Seles, E. Perfetti, B. Sartorello; Spedali Civili, Brescia: E. Simoncini, G. Marini, P. Marpicati, R. Farfaglia, A.M. Bianchi, P. Grigolato, L. Lucini, P. Frata, A. Huscher, E. Micheletti, C. Fogazzi; U.O. Medicina Oncologica, Ospedale Carpi, Ospedale Mirandola: F. Artioli, K. Cagossi, L. Scaltriti, E. Bandieri, L. Botticelli, G. Giovanardi; Ospedale di Cattolica “Cervesi,” Cattolica: A. Ravaioli, E. Pasquini, B. Rudnas; Ospedale Civile, Gorizia: L. Foghin; Ospedale “A. Manzoni” Lecco, Lecco: M. Visini, L. Zavallone, G. Ucci; Istituto Europeo di Oncologia, Milano: M. Colleoni, G. Viale, P. Veronesi, G. Peruzzotti, L. Corsetto, R. Ghisini, G. Renne, A. Luini, L. Orlando, R. Torrisi, A. Rocca, T. De Pas, E. Munzone, V. Galimberti, S. Zurrida, M. Intra, F. Nolé, R. Orecchia, G. Martinelli, F. de Braud, A. Goldhirsch; Ospedale Infermi, Rimini: A. Ravaioli, L. Gianni.
Peru.
Instituto de Enfermedades Neoplásicas, Lima. H. Gome.
Slovenia.
Institute of Oncology, Ljubljana. T. Cufer, B. Pajk, J. Cervek.
South Africa.
Groote Schuur Hospital and University of Cape Town, Cape Town: I.D. Werner, E. Murray, D. Govender, S. Dalvie, T. Erasmus, B. Robertson, B. Read, E. Nel, J. Toop, N. Nedeva, E. Panieri; Sandton Oncology Centre, Johannesburg: D. Vorobiof, M. Chasen, G. McMichael, C. Mohammed. Local funding provided by the Cancer Association of South Africa.
Sweden.
West Swedish Breast Cancer Study Group: S.B. Holmberg; Sahlgrenska U Hospital, Moelndal: S.B. Holmberg, J. Mattsson; Boras Hospital, Boras; Karlstads Hospital, Karlstads: H. Sellström; Kungalvs Hospital, Kungalvs: B. Lindberg.
Switzerland.
Swiss Group for Clinical Cancer Research (SAKK). A. Goldhirsch (up to January 2004), R. Herrmann (from June 2004): Kantonsspital Aarau, Zentrum f. Onkologie, Aarau: A. Schönenberger, W. Mingrone, Ch. Honegger, E. Bärtschi, M. Neter, M. Rederer, G. Schär; University Hospital Basel, Basel: C. Rochlitz, R. Herrmann, D. Oertli, E. Wight, H. Moch; Institute of Oncology of Southern Switzerland: Ospedale San Giovanni, Bellinzona: J. Bernier, L. Bronz, F. Cavalli, E. Gallerani, A. Richetti, A. Franzetti; Ospedale Regionale di Lugano (Civico & Italiano), Lugano: M. Conti-Beltraminelli, M. Ghielmini, T. Gyr, S. Mauri, P.C. Saletti; Ospedale Regionale Beata Vergine, Mendrisio: A. Goldhirsch, O. Pagani, R. Graffeo, M. Locatelli, S. Longhi, P.C. Rey, M. Ruggeri; Ospedale Regionale La Carità, Locarno: E. Zucca, D. Wyss; Istituto Cantonale di Patologia, Locarno: L. Mazzucchelli, E. Pedrinis, T. Rusca; Inselspital, Bern: S. Aebi, M.F. Fey, M. Castiglione-Gertsch, M. Rabaglio; Kantonsspital Olten, Olten: S. Aebi, M.F. Fey, M. Zuber, G. Beck; Bürgerspital, Solothurn: S. Aebi, M.F. Fey, R. Schönenberger; Spital Thun-Simmental AG Thun: J.M. Lüthi, D. Rauch; Hôpital Cantonal Universitaire HCUG, Geneva: H. Bonnefoi; Rätisches Kantons- und Regionalspital, Chur: F. Egli, R. Steiner, P. Fehr; Centre Pluridisciplinaire d'Oncologie, Lausanne: L. Perey, P. de Grandi, W. Jeanneret, S. Leyvraz, J.-F. Delaloye; Kantonsspital St Gallen, St Gallen: B. Thürlimann, D. Köberle, F. Weisser, S. Mattmann, A. Müller, T. Cerny, B. Späti, M. Höfliger, G. Fürstenberger, B. Bolliger, C. Öhlschlegel, U. Lorenz, M. Bamert, J. Kehl-Blank, E. Vogel; Kantonales Spital Herisau, Herisau: B. Thürlimann, D. Hess, I. Senn, D. Köberle, A. Ehrsam, C. Nauer, C. Öhlschlegel, J. Kehl-Blank, E. Vogel; Stadtspital Triemli, Zürich: L. Widmer, M. Häfner; Universitätsspital Zürich, Zürich: B.C. Pestalozzi, M. Fehr, R. Caduff, Z. Varga, R. Trüb, D. Fink.
Swiss Private MDs.
Private Praxis, Zürich: B.A. Bättig; Sonnenhof-Klinik Engeried, Bern: K. Buser; Frauenklinik Limmattalspital, Schlieren: N. Bürki; Private Praxis, Birsfelden: A. Dieterle; Private Praxis, Biel: L. Hasler; Private Praxis, Baar: M. Mannhart-Harms; Brust-Zentrum, Zürich: C. Rageth; Private Praxis, Bern: J. Richner; Private Praxis, Bellinzona: V. Spataro; Private Praxis, Winterthur: M. Umbricht.
United Kingdom: King's College Hospital/Breast Unit, London.
P. Ellis, S. Harris, N. Akbar, H. McVicars, C. Lees, R. Raman, G. Crane.
Danish Breast Cancer Cooperative Group (DBCG)
H.T. Mouridsen; Rigshospitalet, Copenhagen: H.T. Mouridsen; Vejle Hospital, Vejle: E. Jakobsen; Odense University Hospital, Odense: S. Cold; KAS Herlev/Herlev University Hospital, Herlev: C. Kamby; Aalborg Sygehus Syd, Aalborg: M. Ewertz; Hilleroed Hospital, Hilleroed: P.M. Vestlev; Aarhus University Hospital, Aarhus: J. Andersen; Roskilde County Hospital, Roskilde: P. Grundtvig; Esbjerg Central Hospital, Esbjerg: E. Sandberg; Naestved Central Hospital, Naestved: P. Philip; Soenderborg Sygehus, Soenderborg: E.L. Madsen; Herning Central Hospital, Herning: K.A. Moeller; Viborg Sygehus, Viborg: V. Haahr; Landspitali University Hospital, Reykjavik, Iceland: J. Johansson.
French Group (FNCLCC)
Institut Bergonié, Bordeaux.
L. Mauriac, M. Debled, P. Campo; Centre Hospitalier de la Côte Basque, Bayonne D. Larregain-Fournier, S. Remy, Centre Jean Perrin, Clermont-Ferrand: H. Auvray; Centre Georges François Leclerc, Dijon: C. De Gislain, F. Delille, M.-C. Porteret; Centre Oscar Lambret, Lille: V. Servent, M. Chapoutier; CHRU, Limoges: N. Tubiana-Mathieu, S. Lavau-Denes, P. Bosc; Centre Léon Bérard, Lyon: J.P. Guastalla, Th. Bachelot, C. Arbault; Centre Hospitalier Meaux, Meaux: G. Netter-Pinon; C.H.G. André Boulloche, Montbéliard: V. Perrin, A. Monnier, Y. Hammoud; Centre Paul Lamarque, Montpellier: G. Romieu, L. Culine, V. Pinosa; Clinique Francheville, Périgueux: L. Cany, C. Maguire; Hôpital de la Milétrie, Poitiers: A. Daban, M. Le Saux, C. Grandon; Centre Eugène Marquis, Rennes: P. Kerbrat, C. Catheline; Centre Henri Becquerel, Rouen: C. Veyret, E. Jugieau, V. Talon; Centre René Gauducheau, Saint-Herblain: A. Le Mevel, S. Maury; Centre Claudius Régaud, Toulouse: L. Gladieff, N. Lignon.
North Yorkshire Group.
D. Dodwell; Harrogate District Hospital, Harrogate, North Yorkshire: D. Dodwell; Huddersfield Royal Infirmary, Huddersfield: J. Joffe; Castlehill Hospital, Hull: P. Drew; Airedale General Hospital, Keighley, W. Yorkshire: A. Nejim; Leeds General Infirmary, Leeds: D. Dodwell, K. Horgan; St James's University Hospital, Leeds: M. Lansdown, T. Perren; Weston Park Hospital, Sheffield: R.E. Coleman.
Independent Centers/Groups
Argentina.
Centro Oncológico Confidence, Buenos Aires: D. Campos; Hospital Allemán, Buenos Aires: F. Cóppola; Hospital Británico, Buenos Aires: J. Martinez; Hospital Evita, Buenos Aires: M. Freue; Hospital Posadas, Buenos Aires: C. Wainstein; Hospital Zubizarreta, Buenos Aires: A. Zori Comba; Instituto Dr Estevez, Buenos Aires: E. Cazap; Instituto Oncológico Dr Angel H. Roffo, Buenos Aires: E. Mickiewicz; Sanatorio Municipal Julio A. Mendez, Buenos Aires: L. Balbiani; Centro Privado de Ginecología, Córdoba: A. Osuna; Hospital Privado de Córdoba, Córdoba: E. Palazzo; Instituto Modelo de Ginecología y Obstetricia, Córdoba: M. de Romedis; Fundación Mainetti-Centro Oncológico de Excelencia, La Pllata: S. Cagnolati; Hospital Privado de la Comunidad, Mar del Plata: C.A. Delfino, G. Caccia; Escuela de Medicina Nuclear (COIR), Mendoza: R.L. de Angelis; Centro Oncológico de Rosario, Rosario: L. Fein, R. Sala; Hospital Provincial de Rosario, Rosario: C. Nassurdi, A. Colombo Berra; Clínica Especializada ISIS, Santa Fe: R. Viroglio, C. Blajman; Hospital Regional de Concepción, Tucumán: H. Requejo; Instituto de Maternidad y Ginecología Nuestra Señoras de las Mercedes, Tucumán: L. Silberman.
Australia.
Flinders Medical Centre, Adelaide, SA: S. Birrell, M. Eaton, C. Hoffman; Queen Elizabeth Hospital, Adelaide, SA: V. Humeniuk; The Canberra Hospital, Canberra, ACT; P. Craft, R. Stuart-Harris, D. Yip; The Geelong Hospital, Geelong, VIC: R. Bell, F. Abell, M. Francis, J. Kiffer, R. Lynch, R. McLennan, K. White; Royal Melbourne Hospital, Melbourne, VIC: M. Green, R. Basser, J. Collins, R. De Boer, J. C. Din, N. Efe, S. T. Fan, G. Lindeman, S. Wong; Western General Hospital, Melbourne, VIC: M. Green, R. Basser, J. Collins, R. De Boer, J. C. Din, N. Efe, S. T. Fan, G. Lindeman, S. Wong; Newcastle Mater Hospital, Newcastle, NSW: J. Stewart, F. Abell, S. Ackland, A. Bonaventura; Royal Perth Hospital, Perth, WA: J. Trotter, E. Bayliss, A. Chan, D. Ransom, A. Redfern; St George Hospital, Sydney, NSW: P. de Souza, M. Links; St Vincent's Hospital, Sydney, NSW: D. Dalley, J. Grygiel, R. Ward; Murray Valley Private Hospital, Wodonga, VIC: C. Underhill, K. Clarke, C. Steer; Princess Alexandra Hospital, Woolloongabba, QLD: E. Walpole, D. Thompson.
Belgium.
Institut Jules Bordet, Bruxelles. J.M. Nogaret; University Hospitals Leuven, Leuven: M.R. Christiaens, P. Neven, R. Paridaens, A. Smeets, I. Vergote, C. Weltens, H. Wildiers; Les Cliniques Saint-Joseph ASBL, Liège: C. Focan; Clinique du Parc Léopold, Bruxelles: L. Marcelis; C.H. Etterbeek-Ixelles, Bruxelles: J.P. Kains; Service d'Oncologie Clinique Notre-Dame, Charleroi: J.-L. Canon; C.H. . André Vèsale, Montigny-Le Tilleul: D. Brohèe.
Canada.
Cambridge Memorial Hospital, Cambridge: J. Gowing; CHUM-Campus Notre-Dame, Montreal. L. Yelle; Hôpital Maisonneuve-Rosemont, Montreal: P. Dubé.
Chile.
Fundacion Lopez Perez, Santiago. C. Vogel; Hospital Carlos Van Buren, Valparaiso: M. León Prieto.
Czech Republic.
Institute of Oncology, Brno. K. Petrakova, M. Palacova, R. Demlova; Dept. of Clinical and Radiation Oncology, Ceske Budejovice: H. Siffnerova, J. Fischer, I. Bustova; Centre of Breast Diseases, Prague: H. Kankova, M. Pintova; Institute of Radiation Oncology, Prague: P. Vitek; University Hospital, Prague: J. Abrahamova, D. Kordikova; University Hospital Prague: L. Petruzelka, E. Sedlackova, H. Honova.
Germany.
Onkologische Gemeinschaftspraxis, Augsburg: B. Heinrich; Zentralklinikum/Frauenklinik, Augsburg: A. Wischnik; Universitätsklinikum Essen, Essen: C. Oberhoff, A.E. Schindler; Universitäts-Frauenklinik d. JLU Giessen, Giessen: K. Münstedt; Onkologische Gemeinschaftspraxis, Göttingen: D. Meyer; Martin-Luther-Universität Halle-Wittenberg, Halle: R. Grosse, H. Kölbl; Universitätskliniken des Saarlandes, Homburg: W. Schmidt, D. Mink; Universitäts-Frauenklinik und Poliklinik Universitätskrankenhaus Eppendorf, Hamburg: F. Jänicke; Kliniken d. Med. Hochschule, Frauenklinik, Hannover: H.J. Lück; Krankenanstalt Mutterhaus der Borromäerinnen, Trier: W. Dornoff; Gynäkologische Abteilung des St Josefshospital, Wiesbaden: G. Hoffmann; Gynäkologische Abteilung d. Marienhospitals, Universität Witten-Herdecke, Witten: J. Hackmann, W. Bader.
Hungary.
SZOTE Onkoterápiás Klinika, Szeged. Z. Kahan; BM Központi Kórház, Budapest: G. Pajkos, K. Kristo; SOTE Radiológiai és Onkoterápiás Klinika, Budapest: M. Dank; Uzsoki Utcai Kórház, Budapest: T. Nagykalnai, L. Landherr; Almási Balogh Pál Kórház, Ózd: E. Kner; Területi Kórház Onkologia, Szentes: M. Kispál; Szent Borbála Kórház, Megyei Onkológiai Gondozó, Tatabánya: Á. Dani.
Italy.
Policlinico S. Orsola-Malpighi, Bologna. A. Martoni, C. Zamagni, S. Giaquinta, E. Piana; Ospedale S. Croce, Fano: R. Mattioli, L. Imperatori; Istituto Clinica Humanitas, Milan/Rozzano: A. Santoro, C. Carnaghi, L. Rimassa; Azienda Ospedaliera San Filippo Neri, Rome: G. Gasparini, G. Sciarretta, A. Morabito; Az. Ospedaliera Treviglio-Caravaggio, Treviglio: S. Barni, M. Cazzaniga, M. Cabiddu; Policlinico Universitario (PUDG), Udine: F. Puglisi; Ospedale di Torrette, Ancona: R. Cellerino, S. Antognoli, F. Freddari; Universitiy of Cagliari, Policlinico Universitario, Cagliari: G. Mantovani, E. Massa, G. Astara; Ospedale Civile Feltre, Feltre: R. Segati; Istituto Nazionali Ricerca Cancro, Genova: R. Rosso, L. Del Mastro, M. Venturini, C. Bighin; Istituto Nazionale dei Tumori, Milano: E. Bajetta, N. Zilembo, D. Paleari, G. Procopio; Azienda Ospedaliera di Parma, Parma: S. Salvagni, M.A. Perrone, V. Franciosi; Azienda Ospedaliera “S. Salvatore,” Pesaro: G. Catalano, S. Luzi Fedeli; Azienda Ospedaliera “Ospedale di Circolo e Fondazione Macchi” Varese: G. Pinotti, G. Giardina, I. Vallini; Universitiy of Cagliari, Policlinico Universitario, Cagliari: B. Massidda, M.T. Ionta, M.C. Deidda; Ospedale Maggiore, Lodi: G. Nalli, G. Sita; Policlinico Universitario, Palermo: I. Carreca, S. Cucciarré, D. Burgio; Ospedale Civile dello Spirito Santo, Pescara: M. Lombardo, G. Pandoli, P. Di Stefano; Azienda Ospedaliera Santa Maria Nuova, Reggio Emilia: C. Boni, G. Bisagni, M.C. Banzi, P. Linarello; Azienda Ospedaliera Desenzano del Garda, Manerbio: G. Colosini, A. Spasiano, A. Caldonazzo; Ospedale Civile ASL 20, Tortona: M. G. Pacquola.
Netherlands.
Ziekenhuis Leyenburg, Den Haag. H.P. Sleeboom; Catharina Ziekenhuis, Eindhoven: H.J.T. Rutten; St Anna Ziekenhuis, Geldrop: E.J.T. Luiten; Tweesteden Ziekenhuis, Tilburg: H.Th.J. Roerdink; Maxima Medisch Centrum, Veldhoven: R.H.M. Roumen.
New Zealand.
Dunedin Hospital, Dunedin: B. McLaren, S. Costello, J. North, D. Perez, K., Bayston, M. Pfieffer; Waikato Hospital, Hamilton: I. Kennedy, I.D. Campbell, L. Gilbert, R. Gannaway, M. Jameson, J. Long, G. Round, L. Spellman, D. Whittle, D. Woolerton.
Poland.
Department of Oncology and Radiotherapy, Medical University of Gdansk, Gdansk: J. Jassem, M. Welnicka-Jaskiewicz, E. Senkus-Konefka, K. Matuszewska; Rydygier's Memorial Hospital, Krakow-Nova Huta: P. Koralewski, J. Pernal; Klinika Nowotworów Piersi i, Chirurgii Rekonstrukcyjnej-Warszawa, Warszawa: T. Pienkowski, E. Brewczynska, B. Bauer-Kosinska, R. Sienkiewicz-Kozlowska, A. Jagiello-Gruszfeld, K. Sudol.; Centrum Onkologii w Bydgoszczy, Oddzial Onkologii Klinicznej, Bydgoszcz: J. Tujakowski, B. Zurawski; Collegium Medicum Jagiellonian University, Krakow: J. Pawlega, E. Jablonska, A. Zygulska; Oddzial Kliniczny Onkologiczny, Centralnego Szpitala Klinicznego Wojskowej, Akademii Medycznej-Warszawa, Warszawa: M. Górnasiowa; Dolnoslaskie Centrum Onkologii, Wroclaw: E. Filypczyk-Cisarz, K. Pajak.
Portugal.
Hospital de S. João, Porto. M. Damasceno; Instituto Português de Oncologia de Coimbra, Coimbra: J.Q. Albano; Hospital de Santa Maria, Lisboa: B. da Costa, L. Costa; Instituto Português de Oncologia de Lisboa, Lisboa: A. Henriques, H. Amaral; Hospital Geral de Santo António, Porto: F. Marques.
Russia.
Cancer Research Centre, Moscow. D.V. Komov, S.B. Polikarpova; Moscow Municipal Hospital No. 62, Moscow: A.N. Makhson, N.V. Zabaznyi; Moscow Research Institute of Diagnostics and Surgery, Moscow: E.K. Vozny, N.Y. Dobrovolskaya, S. Bolshakova, O.V. Yurgina; N.M. Emmanuel Institute of Biochemical Physics, Moscow: D.B. Korman, I.A. Maslova; N.N. Petrov Research Institute of Oncology, St Petersburg: V. Semiglazov, V. Ivanov; Saint-Petersburg City Oncological Dispensary, St Petersburg: G. Manikhas, G. Dolmatov.
South Africa.
Mamma Clinic, Tygerberg Hospital, Cape Town. J. Apffelstaedt; Southern Cross Hospital, Cape Town: D. Eedes; Pretoria Academic Hospital, Pretoria: C. Slabber; Pretoria East Hospital, Pretoria: M.A. Coccia-Portugal; Eastern Cape Oncology Centre, Port Elizabeth: K. Maart.
Spain.
Hospital Ruber Internacional, Madrid. J.E. Alés Martinez, P. Aramburo, R. Sánchez; Hospital Son Dureta, Palma del Mallorca: J. Rifa, J. Martin; Centro Oncológico Integral de Madrid (CONIM), Madrid: R. Pérez-Carrión, J.L. González Larriba, A. Cubillo; Hospital Universitario San Carlos, Madrid: M.M. Jiménez, A. Casado; Hospital Central de Asturias, Oviedo: J. Fra, J.M. Vieitez, E. Esteban, A.J. Lacave.
Switzerland.
Universitätsfrauenklinik, Basel. E. Wight, S. Bartens, R. Decio, U. Güth; Klinik am Park, Zürich: U. Breitenstein.
Turkey.
Ankara University Ibni Sina Hospital, Ankara: F. Icli, D. Dincol; Hacettepe University Oncology Institute, Ankara: E. Baltali, Y. Ozisik; Istanbul University Oncology Institute, Istanbul: E. Topuz, M. Basaran, A. Aydiner; Ege University Medical School, Izmir: E. Ozdedeli; 9 Eylul University Medical School, Izmir: O. Harmancioglu, A.U. Yilmaz.
United Kingdom.
The Royal Marsden Hospital, London, Royal Marsden NHS Trust, Surrey. I.E. Smith; University of Dundee, Dundee: A.M. Thompson; Christie Hospital NHS Trust, South Manchester University Hospital Trust, Manchester: A. Wardley; Royal Bournemouth Hospital, Bournemouth: T. Hickish; North Middlesex Hospital, London: F. Neave.
Uruguay.
Hospital de Clinicas Dr Manuel Quintela, Montevideo, Uruguay: G. Sabini.
published online ahead of print at www.jco.org on November 3, 2008
Supported by Novartis, Swedish Cancer Society, Cancer Council Australia, Australian New Zealand Breast Cancer Trials Group, Frontier Science and Technology Research Foundation, Swiss Group for Clinical Cancer Research (SAKK), National Cancer Institute Grant No. CA-75362, Cancer Research Switzerland/Oncosuisse, and the Foundation for Clinical Cancer Research of Eastern Switzerland (OSKK).
Presented in part at the 30th Annual San Antonio Breast Cancer Symposium, December 13-16, 2007.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Goldhirsch A, Wood WC, Gelber RD, et al: Progress and promise: Highlights of the international expert consensus on the primary therapy of early breast cancer 2007. Ann Oncol 18:1133-1144, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Clahsen PC, Van de Velde CJ, Duval C, et al: The utility of mitotic index, oestrogen receptor and Ki-67 measurements in the creation of novel prognostic indices for node-negative breast cancer. Eur J Surg Oncol 25:356-363, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Gerdes J, Schwab U, Lemke H, et al: Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer 31:13-20, 1983 [DOI] [PubMed] [Google Scholar]
- 4.Lehr HA, Hansen DA, Kussick S, et al: Assessment of proliferative activity in breast cancer: MIB-1 immunohistochemistry versus mitotic figure count. Hum Pathol 30:1314-1320, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Thor AD, Liu S, Moore DH, et al: Comparison of mitotic index, in vitro bromodeoxyuridine labeling, and MIB-1 assays to quantitate proliferation in breast cancer. J Clin Oncol 17:470-477, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Sahin AA, Ro J, Ro JY, et al: Ki-67 immunostaining in node-negative stage I/II breast carcinoma: Significant correlation with prognosis. Cancer 68:549-557, 1991 [DOI] [PubMed] [Google Scholar]
- 7.Domagala W, Markiewski M, Harezga B, et al: Prognostic significance of tumor cell proliferation rate as determined by the MIB-1 antibody in breast carcinoma: Its relationship with vimentin and p53 protein. Clin Cancer Res 2:147-154, 1996 [PubMed] [Google Scholar]
- 8.Pietiläinen T, Lipponen P, Aaltomaa S, et al: The important prognostic value of Ki-67 expression as determined by image analysis in breast cancer. J Cancer Res Clin Oncol 122:687-692, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Trihia H, Murray S, Price K, et al: Ki-67 expression in breast carcinoma. Cancer 97:1321-1331, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Jansen RL, Hupperets PS, Arends JW, et al: MIB-1 labelling index is an independent prognostic marker in primary breast cancer. Br J Cancer 78:460-465, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Azambuja E, Cardoso F, de Castro G Jr, et al: Ki-67 as prognostic marker in early breast cancer: A meta-analysis of published studies involving 12 155 patients. Br J Cancer 96:1504-1513, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang J, Ormerod M, Powles TJ, et al: Apoptosis and proliferation as predictors of chemotherapy response in patients with breast carcinoma. Cancer 89:2145-2152, 2000 [PubMed] [Google Scholar]
- 13.Archer CD, Parton M, Smith IE, et al: Early changes in apoptosis and proliferation following primary chemotherapy for breast cancer. Br J Cancer 89:1035-1041, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller WR, White S, Dixon JM, et al: Proliferation, steroid receptors and clinical/pathological response in breast cancer treated with letrozole. Br J Cancer 94:1051-1056, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dowsett M, Smith IE, Ebbs SR, et al: Prognostic value of Ki67 expression after short-term presurgical endocrine therapy for primary breast cancer. J Natl Cancer Inst 99:167-170, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Viale G, Regan MM, Mastropasqua MG, et al: Predictive value of tumor Ki-67 in two randomized trials of adjuvant chemo-endocrine therapy for node-negative breast cancer. J Natl Cancer Inst 100:207-212, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Thürlimann B, Keshaviah A, Coates AS, et al: BIG 1-98 Collaborative Group: A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med 353:2747-2757, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Coates AS, Keshaviah A, Thurlimann B, et al: Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: Update of study BIG 1-98. J Clin Oncol 25:486-492, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Regan MM, Viale G, Mastropasqua MG, et al: Re-evaluating adjuvant breast cancer trials: Assessing hormone receptor status by immunohistochemical versus extraction assays. J Natl Cancer Inst 98:1571-1581, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Rasmussen BB, Regan MM, Lykkesfeldt AE, et al: Adjuvant letrozole versus tamoxifen according to centrally-assessed ERBB2 status for postmenopausal women with endocrine-responsive early breast cancer: Supplementary results from the BIG 1-98 randomised trial. Lancet Oncol 9:23-28, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Viale G, Regan MM, Maiorano E, et al: Prognostic and predictive value of centrally reviewed expression of estrogen and progesterone receptors in a randomized trial comparing letrozole and tamoxifen adjuvant therapy for postmenopausal early breast cancer: BIG 1-98. J Clin Oncol 25:3846-3852, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Kaplan EL, Meier P: Nonparametric estimation from incomplete observation. J Am Stat Assoc 53:457-481, 1958 [Google Scholar]
- 23.Cox DR: Regression models and life tables (with discussion). J Roy Stat Soc B 34:187-220, 1972 [Google Scholar]
- 24.Bonetti M, Gelber RD: A graphical method to assess treatment-covariate interactions using the Cox model on subsets of the data. Stat Med 19:2595-2609, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Pinto AE, Andre S, Laranjeira C, et al: Correlations of cell cycle regulators (p53, p21, pRb and mdm2) and c-erbB-2 with biological markers of proliferation and overall survival in breast cancer. Pathology 37:45-50, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Lewis-Wambi JS, Jordan VC: Treatment of postmenopausal breast cancer with selective estrogen receptor modulators (SERMs). Breast Dis 24:93-105, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Osborne CK, Shou J, Massarweh S, et al: Crosstalk between estrogen receptor and growth factor receptor pathways as a cause for endocrine therapy resistance in breast cancer. Clin Cancer Res 11:865s-8870, 2005 [PubMed] [Google Scholar]
- 28.Baum M, Budzar AU, Cuzick J, et al: Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: First results of the ATAC randomised trial. Lancet 359:2131-2139, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Coombes RC, Kilburn LS, Snowdon CF, et al: Survival and safety of exemestane versus tamoxifen after 2-3 years’ tamoxifen treatment (Intergroup Exemestane Study): A randomised controlled trial. Lancet 369:559-570, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Kaufmann M, Jonat W, Hilfrich J, et al: Improved overall survival in postmenopausal women with early breast cancer after anastrozole initiated after treatment with tamoxifen compared with continued tamoxifen: The ARNO 95 study. J Clin Oncol 25:2664-2670, 2007 [DOI] [PubMed] [Google Scholar]