Abstract
Recommendations for specimen collection and handling have been developed for adoption across breast cancer clinical trials conducted by the Breast International Group (BIG)-sponsored Groups and the National Cancer Institute (NCI)-sponsored North American Cooperative Groups. These recommendations are meant to promote identifiable standards for specimen collection and handling within and across breast cancer trials, such that the variability in collection/handling practices that currently exists is minimized and specimen condition and quality are enhanced, thereby maximizing results from specimen-based diagnostic testing and research. Three working groups were formed from the Cooperative Group Banking Committee, BIG groups, and North American breast cancer cooperative groups to identify standards for collection and handling of (1) formalin-fixed, paraffin-embedded (FFPE) tissue; (2) blood and its components; and (3) fresh/frozen tissue from breast cancer trials. The working groups collected standard operating procedures from multiple group specimen banks, administered a survey on banking practices to those banks, and engaged in a series of discussions from 2005 to 2007. Their contributions were synthesized into this document, which focuses primarily on collection and handling of specimens to the point of shipment to the central bank, although also offers some guidance to central banks. Major recommendations include submission of an FFPE block, whole blood, and serial serum or plasma from breast cancer clinical trials, and use of one fixative and buffer type (10% neutral phosphate-buffered formalin, pH 7) for FFPE tissue across trials. Recommendations for proper handling and shipping were developed for blood, serum, plasma, FFPE, and fresh/frozen tissue.
INTRODUCTION AND PURPOSE
The Breast International Group (BIG) and the North American Cooperative Groups encompass breast cancer clinical trials groups (“groups”) from across the world. The European-based BIG conducts major, multicenter trials in breast cancer across groups based in Europe, Australasia, Latin America, and Canada. In North America, major group clinical trials in breast cancer are performed by the Breast Cancer Intergroup of North America (TBCI), which unifies the efforts of several breast cancer research groups, and the National Surgical Adjuvant Breast and Bowel Project (NSABP); the Radiation Therapy Oncology Group (RTOG) conducts research in several cancers, including breast cancer, with a focus on radiation oncology. Thus, research conducted by BIG and the North American groups spans a large number of sites across the world, as well as a wide range of treatment modalities.
In 2005, BIG approached the North American breast cancer cooperative groups to initiate more in-depth discussions between these two major networks of breast cancer research groups. The following goals were determined for this international collaboration: (1) early sharing of trial designs for drugs and strategies; (2) discussion of combined analyses, where appropriate; (3) early sharing of ideas about translational research projects; and (4) collaboration on translational research projects.
During their first meeting, both sets of groups agreed that a strong need existed for identifiable standards for the collection and handling of specimens from group breast cancer clinical trials. This article, one of the first major efforts of the collaboration, was developed to identify and elucidate such a standard.
Variable methods of collection, processing, and storage of clinical trial biospecimens across these groups have resulted in variability of specimen condition and quality as well as subsequent research results. Indeed, data demonstrate that upfront handling and processing of specimens, including formalin-fixed, paraffin-embedded (FFPE) tissue has a significant impact on the quality of intercellular and intracellular components, which may affect results of analyses performed on those specimens.1-5
We hope that the following standards, or guidelines, will help to ensure an acceptable level of quality in group clinical trial specimens, both for research and diagnosis, and will enhance the reproducibility and comparability of test results derived from them. Given the high level of annotation that typifies clinical trial specimens, including outcome data as well as the relatively uniform treatments administered across cohorts of clinical trial patients, clinical trial specimens are indeed valuable to cancer research, particularly translational research.
Specifically, these guidelines were developed to provide a set of consensus procedures for the collection and handling of (1) blood and blood components (cells, serum, and plasma), (2) FFPE tissue, and (3) fresh/frozen tissue biospecimens collected from BIG and North American group breast cancer clinical trials. They focus primarily on specimen collection and processing to the point of specimen shipment to the central bank. Therefore, procedures discussed are mostly intended for procurement sites, as opposed to central banks and investigators processing and analyzing the specimen, although some guidance on those points is also provided.
These guidelines are not expected to be a comprehensive report for all biospecimen collection, processing, storage, distribution, and repository operations. For additional guidance, we refer readers to the National Cancer Institute Best Practices for Biospecimen Resources6 (these guidelines are also referenced at http://ctep.cancer.gov/guidelines/spec_bc_grptrials.html).
OVERARCHING AIMS OF THESE GUIDELINES
There are four overarching aims of the guidelines: (1) to promote and ensure proper collection of high-quality research specimens such that each patient diagnosed with breast cancer can have a reliable, interpretable molecular diagnosis; (2) to provide a known baseline of standardization of specimen collection and handling procedures, to the extent possible, such that more global biomarker analysis across studies is possible; (3) to promote specimen collection that would allow for future technologies, particularly in the molecular arena, to be applied to these specimens for research; and (4) to provide guidelines that group trial leadership can incorporate into clinical trial protocols.
It should be noted that each clinical trial protocol should stress the importance of processing specimens in adherence to a standardized set of procedures. Furthermore, adequate patient consent and institutional review board approval for research on specimens is a prerequisite for all specimen collection described herein. Finally, universal precautions apply to all handling of human specimens: Human specimens should be handled as infectious agents (although FFPE tissue can be shipped through regular mail).
INFORMATION GATHERING FOR THESE GUIDELINES
Three working groups—a blood, serum, and plasma; FFPE; and fresh/frozen tissue—have met through many conference calls and one face-to-face meeting to discuss best practices for specimen collection and handling in group breast cancer trials. These working groups included representatives from BIG, the North American Groups, the National Cancer Institute (NCI) Cancer Therapy Evaluation Program (CTEP) and Cancer Diagnosis Program (CDP), and the NCI Cooperative Group Banking Committee (GBC), which was formed by NCI to develop specimen banking best practices for the North American cooperative groups, across all cancers.
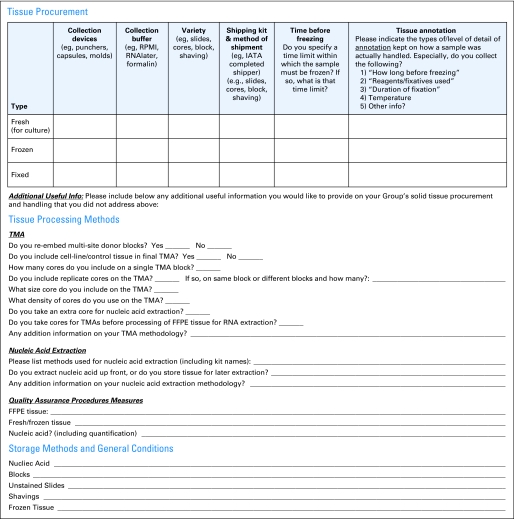
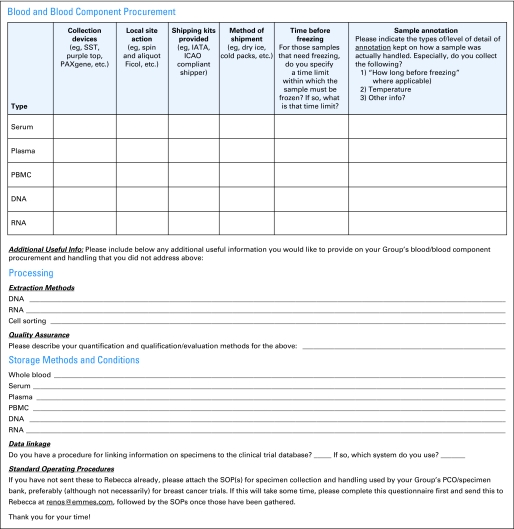
The working groups gathered information on the standard operating procedures (SOPs) of the specimen repositories of various North American and international groups through collecting group bank SOPs and administering a questionnaire to the GBC's Best Practices and Operations Subcommittee and BIG. The questionnaires shown in Figures 1 and 2 cover the following areas:
Procurement methods for fresh, frozen, and fixed solid tissue
Procurement methods for blood components (for serum, plasma, peripheral-blood mononuclear cells [PMBCs], DNA, and RNA)
Level of detail of annotation kept on how a sample was actually handled, especially time elapsed from extirpation to freezing, reagents and fixatives used, and duration and temperature of fixation
Actions required at the local site (eg, centrifugation, aliquoting)
Processing methodology for nucleic acid extraction from solid tissue and blood; cell sorting from blood; tissue microarray; and quality assurance (QA)
Storage of nucleic acid, FFPE blocks, unstained slides, shavings, fresh/frozen tissue, whole blood, serum, plasma, PBMC, DNA, and RNA
Data linkage procedures for linking information on specimens to the clinical trial database
Shipping from the collection site to the central repository
Fig 1.
Quick questionnaire on group specimen collection, handling, and processing practices. IATA, International Air Transport Association; TMA, tissue microarray; FFPE, formalin fixed, paraffin embedded; QA, quality assurance.
Fig 2.
Fluid specimen questionnaire. SST, serum separator tube; IATA, International Air Transport Association; ICAO, International Civil Aviation Organisation; PBMC, peripheral-blood mononuclear cell; SOP, standard operating procedure; PCO, Pathology Coordinating Office.
The data gathered from this questionnaire, from a comparison of the submitted group banking SOPs, and from the working groups’ deliberations have been incorporated into these guidelines. A list of the groups who submitted questionnaires appears in the Appendix (online only).
SPECIMEN ACCESS AND USE
Central Banking of and Access to Research Specimens
Specimens from a clinical trial, particularly FFPE tissues, must be consolidated in a central bank independent from industry. Centralization of specimens will greatly facilitate access to specimens by investigators with meritorious proposals for specimen-based studies. Each central bank should have a disaster policy in place with details for carrying out this plan provided in the manual of operation.
The “honest broker” and “safe harbor” systems provide a high level of comfort to member sites, institutional pathologists, and patients. These intrinsic measures ensure confidentiality and privacy while providing researchers with necessary information and high-quality samples. Independence from industry is important because efforts by industry to claim intellectual property or other rights to research specimens may compromise research.
Intellectual property issues related to clinical trial tissue banks must be considered because many trials groups, particularly outside of the cooperative groups, are not legal entities and cannot own or govern such intellectual property. A material transfer agreement (MTA) that clearly identifies who owns the biologic material and its associated intellectual property is an important component of any specimen repository.
Researchers should be able to submit proposals for use of clinical trial specimens (which would include tissue microarray sections). The limited quantity and high value of clinical trial specimens warrant their careful and triaged use. A committee should be in place, or established, using prespecified policies, to vet proposals to prioritize specimen usage appropriately, and to screen out proposals that are without merit or that contain serious drawbacks.
International Trials
A central tissue bank should be established for each trial, with custodianship vested in the trial's steering group. An educational push is needed among international clinical trial groups for sites to send in biologic material for research, and then to centralize those biologic materials.
North American Trials
In North America, specimens for a given trial should be centralized at the level of the group leading the trial; this also pertains to specimens from intergroup trials. Retrospective as well as prospective consolidation of intergroup specimens is important to enable research to be carried out on those specimens. Especially, intergroup specimens from closed trials that are still stored at various groups or at various sites need to be consolidated in the lead group's bank.
FACILITIES, EQUIPMENT, AND SPACE
Common procedures for the collection of clinical trial specimens are compounded by the variable differences in the facilities, equipment, and expertise in personnel of the institutions submitting specimens. Specimen processing variability at submitting institutions can result in considerable heterogeneity in specimen likeness and quality.1-5 Biospecimen policy reviewing committees have concluded that efforts to harmonize specimen processing at institutional levels is a significant area of importance to improve the clinical trial specimen bank inventory.7 Additional guidelines reported in the NCI Best Practices for Biospecimens Resources6 and the International Society for Biological and Environmental Repositories Best Practices for Repositories8 also include comments on facilities and equipment.
PATHOLOGIST SUPPORT FOR SUBMITTING RESEARCH SPECIMENS
Inconsistencies in how pathologists are engaged in the clinical trial at the institutional level can greatly affect the consistency of specimen submission. Does the pathologist specifically know the trial and the specimen submission requirements? Will the pathologist provide time and effort to screen the best or acceptable material requested by the specimen submission requests of the trial? Is there established willingness through fee-for-service to the pathology department, academic credit, or official scientific engagement as part of a funded grant to secure the pathologist's effort? A great need exists to increase support for pathologists and their associates, who carry the major workload associated with submitting research tissue specimens.
FFPE RECOMMENDATIONS
Do policies in the pathology department fit the needs of the clinical trial or can they be arranged to accommodate trial requirements? Many pathology groups have instituted policies to not provide paraffin blocks. More often, tissue sections are provided. However, tissue sections are no longer desired for correlative science because of the potential loss or degradation of biomarkers of interest, especially if the period from sectioning to the time of analysis is greater than days or weeks, which is often the situation in long-accruing trials. More appropriately, paraffin tissue blocks, which patients consent to be used in research, are the acceptable requirement. Policies should be developed to accommodate FFPE tissue block submission when future clinical needs of the existing FFPE block(s) are no longer indicated. However, when there are few or single paraffin blocks as part of the clinical record of the patient, then tissue cores should be the alternative to tissue submission, provided that enough target tissue is available.
BLOOD, PLASMA, AND SERUM RECOMMENDATIONS
The common issues to procure quality blood, plasma, or serum center on whether the specimens need to be processed expediently onsite by the submitting institution or whether stability in the blood components can survive the transport process to a central laboratory for processing. There are both positive and negative results in either of these performance-based issues. When multiple institutions process specimens, procedures become more variable, as will specimen end products. The more time sensitive or detailed the specimen process the more variable the product. For example, if serum or plasma is required and this must be processed (centrifuged, removed, aliquoted, and frozen) within 8 hours of phlebotomy, most laboratories can manage this requirement well. However, if the specimens should be processed within 2 hours of phlebotomy, the percentage of trial specimens that fail to be processed in this timeline increases. The fact is that some institutions manage this well and others do not. The best practice is to balance the use of laboratories that are accustomed to performing these steps at any hour of the day or can quickly ship specimens to central bank locations where standard procedures can be better managed.
Other more complicated specimen processing procedures can be problematic for clinical trials and should be distinguished and discussed with scientist and pathologist when protocols are being developed. Education in the clinical trial development may be needed. For example, isolation of PBMCs as opposed to buffy-coat WBCs (WBCs), requires quite different processes and with much greater expertise for PBMC compared with WBC preparations. Clinical trial protocol developers and physician-scientists often inappropriately interchange these kinds of terms, but in fact, these specimens represent significant differences in processing requirements. Whereas academic institutions can be organized to perform PBMC isolations, most community hospitals cannot. Unless these specimens can be sent to a central processing location, more stringent requests for the collection of PBMCs limit specimen submission.
FRESH/FROZEN TISSUE RECOMMENDATIONS
Fresh/frozen tissue collection is susceptible to limited timelines for acceptable collection. However, as long as the general principles of rapid collection and freezing (or transfer to RNAlater), as well as seamless integration with standard pathologic assessment are respected, these collections can be accomplished. To determine if acceptable methods are being used, adopted methods should be employed as evidence of careful QA and should demonstrate feasibility in a variety of practice settings. Fresh/frozen tissue QA data should be completed and made available on submitted specimens within a relatively short timeline to provide feedback on the ability of each collecting site to submit quality specimens as well as to document that the protocol procedures are meeting the expectations of the clinical trial.
SPECIMEN COLLECTION KITS
Preassembled kits sent by the central group bank to sites can vastly expedite as well as standardize the collection of good-quality specimens from sites, reduce the variability in collection procedures, facilitate onsite processing (such as aliquoting), and increase compliance with the protocol-stipulated collection. Group banks should use preassembled collection kits to the broadest extent possible.
LABELING
Vials, including cryovials, should be labeled with the study name or number, a specimen ID number that is linked to the subject's study ID, contents of the vial, and date of collection. The latter is especially important if the sample is a serial specimen. The subject's study ID should not be on the vial unless patient confidentiality is determined to be secured according to the clinical trial protocol.
Specific procedures for labeling specimens should clearly be defined in the protocol. The central bank itself should have standardized labeling (printed or written) for archiving samples, such as unique sample IDs and/or barcodes. For specimens that will be placed into frozen storage, information on the label should be able to withstand −80°C and not become illegible. Alcohol-based permanent markers will smudge. Markers specifically made for cryotemperatures are recommended instead.
All levels of receptacles containing the specimen should be labeled, from the smallest unit (eg, tube), to large storage units. Labeling should be resistant to cold, solvents, and water (eg, cryomarker, cold-resistant label, waterproof and solvent-proof pen).
The information included on a sample label must not include patient identifying information, and should be compliant with the Health Insurance Portability and Accountability Act (HIPAA). The information should be sufficiently specific such that the encoded information (eg, tracking number) can be linked to the sample in the database.
SHIPPING
In addition to the following recommendations, please also see the recommendations on shipping contained in the NCI Best Practices for Biospecimens Resources, under Section B.1.5, Shipping Samples, found on page 6 of that document.6
Shipping personnel must receive training and be certified for biologic specimen shipping. Before choosing a courier for frozen tissue, ensure that they (1) handle dry ice shipments and (2) service the town or city in which the central bank is located. Dry ice should not be shipped with couriers who have extremely restrictive policies concerning shipments of hazardous materials.
For international studies, each country should consider identifying a tissue bank where tissue can be held before final shipping to a central bank across borders. A site should consult the central bank to determine the best times to ship samples that are frozen. This will help to avoid inadvertent thawing caused by the evaporation of dry ice.
Ideally, the central bank will have included shipping materials in a kit sent to the collection site. Batch shipping of samples will help to reduce the time required for organizing shipments and, in the case of frozen samples, dry-ice shipping costs. A good guideline for the interval of time between procurement and shipment is 1 month.
PACKAGING FOR ALL SPECIMENS
Packaging should comply with International Air Transport Association (IATA) criteria (http://www.iata.org). If ground overnight is used for FFPE samples, then shipment should conform to ground transportation standards (eg, Department of Transportation packaging standards, if in the US). The shipping box should be secured and appropriate stickers should be placed, such as “Biologic Substance, Category B UN 3373,” and the type of shipment (eg, next day). The IATA shipping category appropriate to the specimens collected should be used, both in labeling and in the training required for packaging. In addition to “Biologic Substance, Category B,” other IATA categories include “Exempt Human Specimens” and “Infectious Substance, Category A.”
Consult the latest IATA regulations to determine which category best applies to the specimens. Do not write the term “diagnostic specimens” on the box: revision of the IATA regulations has replaced the term “diagnostic specimens” with “Biologic Substance, Category B.” Biologic substances and specimens are subject to specific packaging requirements and there should be no misunderstanding about the contents of the shipment, particularly with regard to risk for infection of humans or animals. A completed material submission form from the trial needs to be submitted along with the specimens.
CONCLUSION
This article reflects time, effort, and experience from a substantial number of contributors during the last 3 years. It reflects the importance of deriving unified standards for specimen collection in global clinical trials; indeed it represents the first attempt at such a process. All three committees (blood, plasma, and serum; fresh/frozen tissue; and FFPE) comprise international participation; the extent of detail in the SOPs tries to embrace the diversity of multinational approaches to the same problems.
Key participants are committed to semi-annual meetings to formally update the recommendations herein. However, all of the key participants are requested to update the Web site of any important advances/policy changes in real time. Hence, the Web site should reflect the latest state-of-the-art specimen collection policies at any given moment.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Brian R. Leyland-Jones, Christine B. Ambrosone, John Bartlett, Matthew J.C. Ellis, Adekunle Raji, Michael R. Pins, Jo Anne Zujewski, Stephen M. Hewitt, John F. Forbes, Mark Abramovitz, Sofia Braga, Fatima Cardoso, Nadia Harbeck, Carsten Denkert, Scott D. Jewell
Administrative support: Brian R. Leyland-Jones, Rebecca A. Enos, Jo Anne Zujewski, Stephen M. Hewitt, Scott D. Jewell
Collection and assembly of data: Brian R. Leyland-Jones, Christine B. Ambrosone, John Bartlett, Matthew J.C. Ellis, Rebecca A. Enos, Adekunle Raji, Michael R. Pins, Jo Anne Zujewski, Stephen M. Hewitt, John F. Forbes, Mark Abramovitz, Sofia Braga, Fatima Cardoso, Nadia Harbeck, Carsten Denkert, Scott D. Jewell
Manuscript writing: Brian R. Leyland-Jones, Christine B. Ambrosone, John Bartlett, Matthew J.C. Ellis, Rebecca A. Enos, Adekunle Raji, Michael R. Pins, Jo Anne Zujewski, Stephen M. Hewitt, John F. Forbes, Mark Abramovitz, Sofia Braga, Fatima Cardoso, Nadia Harbeck, Carsten Denkert, Scott D. Jewell
Final approval of manuscript: Brian R. Leyland-Jones, Christine B. Ambrosone, John Bartlett, Matthew J.C. Ellis, Adekunle Raji, Michael R. Pins, Jo Anne Zujewski, Stephen M. Hewitt, John F. Forbes, Mark Abramovitz, Sofia Braga, Fatima Cardoso, Nadia Harbeck, Carsten Denkert, Scott D. Jewell
Supplementary Material
Acknowledgments
We thank Martine Piccart-Gebhart, MD, PhD; William C. Wood, MD; Larry Norton, MD; the Breast Cancer Research Foundation; specimen banks of BIG, TRANSBIG, and the Cooperative Groups; the EORTC PathoBiologyGroup; Manfred Schmitt, MD; the Group Banking Committee Best Practices and Operations Subcommittee; and Leah Kamin. We also thank the North Central Cancer Therapy Group (NCCTG) for providing comments on this article.
Appendix
The groups of the Breast International Group (BIG) and TRANSBIG contributed to this article, along with the National Cancer Institute (NCI). The groups of the NCI Cooperative Group Banking Committee (GBC) include the following: Cooperative Groups of the Breast Cancer Intergroup of North America (TBCI)—American College of Surgeons Oncology Group (ACOSOG), Cancer and Leukemia Group B (CALGB), Eastern Cooperative Oncology Group (ECOG), North Central Cancer Treatment Group (NCCTG), National Cancer Institute of Canada Clinical Trials Group (NCIC CTG), Southwest Oncology Group (SWOG), National Surgical Adjuvant Breast and Bowel Project (NSABP), Radiation Oncology Group (RTOG), Gynecologic Oncology Group (GOG), and Children's Oncology Group (COG).
Standard operating procedures and completed questionnaires on specimen handling were collected from the following research groups in 2006: Groups of the Breast International Group and TRANSBIG, including Australian New Zealand Breast Cancer Trials Group (ANZ BCTG); Growth Factor Laboratory of Cancer Research UK; Jules Bordet Institute, Brussels, Belgium; TRANSBIG Proteomics GSF, Munich, Germany; Vall d'Hebron Hospital, Barcelona, Spain; National Cancer Institute Tissue Array Research Program; North American Cooperative Groups, including American College of Surgeons Oncology Group, Cancer and Leukemia Group B, Children's Oncology Group, Eastern Cooperative Oncology Group, Gynecologic Oncology Group, National Cancer Institute of Canada Clinical Trials Group, National Surgical Adjuvant Breast and Bowel Project, Radiation Oncology Group, and Southwest Oncology Group.
published online ahead of print at www.jco.org on October 27, 2008.
Supported by the Breast Cancer Research Foundation, the National Cancer Institute (NCI), the Breast International Group, and NCI-supported Cooperative Groups.
These guidelines are referenced on the World Wide Web at http://ctep.cancer.gov/guidelines/spec_bc_grptrials.html.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Dash A, Maine IP, Varambally S, et al: Changes in differential gene expression because of warm ischemia time of radical prostatectomy specimens. Am J Pathol 161:1743-1748, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Marzo AM, Fedor HH, Gage WR, et al: Inadequate formalin fixation decreases reliability of p27 immunohistochemical staining: Probing optimal fixation time using high-density tissue microarrays. Hum Pathol 33:756-760, 2002 [DOI] [PubMed] [Google Scholar]
- 3.DiVito KA, Charette LA, Rimm DL, et al: Long-term preservation of antigenicity on tissue microarrays. Lab Invest 84:1071-1078, 2004 [DOI] [PubMed] [Google Scholar]
- 4.O'Leary TJ, Edmonds P, Floyd AD, et al: Quality Assurance for Immunocytochemistry; Approved Guideline. NCCLS document MM4-AC. http://www.clsi.org/source/orders/free/MM4-a.pdf
- 5.Chung JY, Braunschweig T, Williams R, et al: Factors in tissue handling and processing that impact RNA obtained from formalin-fixed, paraffin-embedded tissue. J Histochem Cytochem [epub ahead of print on August 18, 2008] [DOI] [PMC free article] [PubMed]
- 6.National Cancer Institute Best Practices for Biospecimen Resources. National Cancer Institute Office of Biorepositories and Biospecimen Research, http://biospecimens.cancer.gov
- 7.National Biospecimen Blueprint. http://www.c-changetogether.org/pubs/pubs/FINAL_NBN_Blueprint.pdf
- 8.International Society for Biological and Environmental Repositories: Best Practices for Repositories I. http://www.isber.org/ibc.html
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.