Abstract
Purpose
Late relapse (LR) of germ cell tumor (GCT) is a well recognized entity associated with poor survival. We report on our experience with LR and determine predictors of survival.
Patients and Methods
From 1990 to 2004, 75 patients were managed for LR of GCT at our institution. Clinical and pathologic parameters were reviewed. Estimates of cancer-specific survival were generated using the Kaplan-Meier method, and a Cox proportional hazards model was used to assess potential predictors of outcome.
Results
The median time to LR was 6.9 years (range, 2.1 to 37.7 years). Overall, 56 patients (75%) had LR in the retroperitoneum, including 25 (93%) of 27 patients initially managed without retroperitoneal lymph node dissection. The 5-year cancer-specific survival (CSS) was 60% (95% CI, 46% to 71%). Patients who underwent complete surgical resection at time of LR (n = 45) had a 5-year CSS of 79% versus 36% for patients without complete resection (n = 30; P < .0001). The 5-year CSS for chemotherapy-naive patients was significantly greater than patients with a prior history of chemotherapy as part of their initial management (5-year CSS, 93% v 49%, respectively). In multivariable analysis of pretreatment parameters available at the time of LR, the presence of symptoms (hazard ratio [HR] = 4.9) and multifocal disease (HR = 3.0) were associated with an inferior CSS.
Conclusion
The data suggest that meticulous control of the retroperitoneum is critical to prevent LR in the retroperitoneum. In multivariable analysis, patients with a symptomatic presentation and those with multifocal disease have a significantly decreased survival. Survival is greatly improved if complete surgical excision of disease is attained.
INTRODUCTION
When testicular germ cell tumor (GCT) recurs, it usually does so within the first 2 years after initial therapy. Late relapse (LR), defined as relapse in the absence of a contralateral tumor more than 2 years after a complete response (CR) to initial treatment,1-6 is an uncommon but well-recognized clinical entity. The incidence among patients who achieve a CR has been reported as 3% to 4%, and it seems to be increasing.1,7 A recent review compiling the data from seven studies and 5,880 patients at risk estimated the incidence of LR to be 3.2% in nonseminomatous GCT and 1.4% in seminoma.8 Viable GCT components of LR are characterized by relative chemotherapy resistance; in additon, chemotherapy-resistant teratoma and malignant transformation of teratomatous elements are relatively common in LR. For patients suffering an LR, overall survival ranges from 26% to 69%.1-6
In this study, we examine a large single-center experience with LR, describe the clinical and pathologic characteristics of patients with LR, and analyze predictors of survival. We also examine patient presentation and prior therapy in an effort to better characterize the etiology of LR and to identify methods that might decrease incidence, morbidity, and mortality.
PATIENTS AND METHODS
Patients
After an institutional review board waiver was obtained, a prospectively accrued database was reviewed to identify 75 patients who presented with LR of testicular GCT to Memorial Sloan-Kettering Cancer Center (New York, NY) between 1990 and 2004. LR was defined as relapse after a 2-year or greater disease-free interval after CR to initial therapy in the absence of second primary tumor in the contralateral testicle. Relapse was determined by biopsy, marker elevation without secondary cause, or radiographic findings of growth of a previously stable or undetected site of disease.
Data Collection
Clinical and pathologic parameters were obtained regarding initial stage and therapy for original testicular GCT, follow-up intervals, site(s) and clinical presentation of LR, therapy and response to treatment of LR, and disease-specific outcome. Initial clinical stage was determined according to International Union Against Cancer guidelines.9 Follow-up status of the patients was attained through clinic records or periodic mailings for patients who were observed elsewhere. The Social Security Death Index was used to corroborate survival data. Cause of death was determined by clinic notes, death certificates, and correspondence with treating physicians.
A patient was considered to have multiple sites of metastasis if he had radiographic or pathologic evidence of disease in more than one organ. Lymph node involvement in the retroperitoneum was considered a single site, regardless of number or size of involved nodes. Concomitant lymph node involvement in extra-retroperitoneal regions (eg, mediastinal, pelvic, retrocrural, or supraclavicular) was denoted as multiple sites of relapse. GCT histology was reported according to the most aggressive component within the specimen. Sarcoma, adenocarcinoma, and neuroectodermal tumors were categorized as malignant transformation of teratoma after clinicopathologic review. Histology at time of LR was reported using all histologic data available, whether before or after chemotherapy. For example, if the patient had a biopsy before chemotherapy showing viable GCT, then had fibrosis at time of surgical resection, the histology of the biopsy was evaluated. Complete surgical resection was defined as no gross unresected disease and negative surgical margins. For patients who received initial chemotherapy to treat LR, postchemotherapy surgery was performed whenever disease was considered to be completely resectable and serum tumor markers were stable.
Statistical Analysis
The Kaplan-Meier method was used to analyze factors associated with survival and generate survival curves. A multivariable Cox proportional hazards regression model that included factors known at the time of LR was used to determine their association with 5-year cancer-specific survival (CSS).
RESULTS
Patient Characteristics
The median time from initial CR to LR was 6.9 years (interquartile range [IQR], 3.2 to 12.0 years). The longest time to LR in this series (and, to our knowledge, the longest time to LR yet reported) was 37.7 years. Duration of CR was less than 5 years in 28 patients (38%), between 5 and 10 years in 19 patients (26%), between 10 and 15 years in 18 patients (24%), and more than 15 years in nine patients (12%). Median age at presentation of LR was 38 years (IQR, 35 to 42 years). Table 1 lists the clinical characteristics at the time of LR. A single site of relapse was present in 40 patients (53%), and relapse in the retroperitoneum occurred in 54 patients (72%).
Table 1.
Clinical Characteristics of Patients Treated for Late Relapse (N = 75)
| Characteristic | No. | % |
|---|---|---|
| Age at LR, years | ||
| Median | 38 | |
| IQR | 35-42 | |
| Primary histology | ||
| Seminoma | 5 | 7 |
| Nonseminoma | 70 | 93 |
| UICC stage | ||
| I | 12 | 16 |
| IS | 3 | 4 |
| II | 32 | 43 |
| III | 26 | 35 |
| Unknown | 2 | 3 |
| Treatment for primary disease | ||
| Surveillance | 8 | 11 |
| Chemotherapy alone | 18 | 24 |
| Chemotherapy plus RPLND* | 34 | 45 |
| Chemotherapy plus other surgery† | 3 | 4 |
| Chemotherapy plus XRT | 2 | 3 |
| RPLND alone | 6 | 8 |
| RPLND plus other surgery† | 1 | 1 |
| RPLND plus XRT | 1 | 1 |
| XRT alone | 1 | 1 |
| XRT plus other surgery† | 1 | 1 |
| Time from complete response to LR, years | ||
| Median | 6.9 | |
| IQR | 3.2-12 | |
| Range | 2.1-37.7 | |
| No. of relapse sites | ||
| One | 40 | 53 |
| Multiple | 33 | 44 |
| Marker only | 2 | 3 |
| Site(s) of relapse | ||
| Retroperitoneum | 54 | 72 |
| Chest | 24 | 32 |
| Lung | 15 | 20 |
| Mediastinum | 9 | 12 |
| Retrocrural | 3 | 4 |
| Neck | 8 | 11 |
| Other visceral | 24 | 32 |
| Liver | 12 | 16 |
| Brain | 4 | 5 |
| Bone | 7 | 9 |
| Adrenal | 1 | 1 |
| Symptoms at presentation of LR | ||
| Yes | 45 | 60 |
| No | 30 | 40 |
| Markers at presentation of LR | ||
| Elevated AFP | 36 | 48 |
| Elevated β-HCG | 18 | 24 |
| Either positive | 46 | 61 |
| Both positive | 8 | 11 |
Abbreviations: IQR, interquartile range; LR, late relapse; UICC, International Union Against Cancer; RPLND, retroperitoneal lymph node dissection; XRT, radiation therapy; AFP, α-fetoprotein; β-HCG, β-human choriogonadotropin.
RPLND either before adjuvant chemotherapy or after induction chemotherapy.
Thoracotomy with wedge resection, resection of mediastinal mass, resection of liver metastases, supraclavicular node excision, neck dissection, or resection of spinal metastasis.
Primary Treatment of Initial GCT
Treatment for the initial testicular cancer was surveillance for eight patients, radiation therapy for two patients, and primary retroperitoneal lymph node dissection (RPLND; at various hospitals) for 19 patients. Overall, 18 of these patients did not receive chemotherapy as part of their initial clinical management. Of the 19 patients who underwent primary RPLND, 11 patients (58%) received chemotherapy before LR. Twelve (63%) of the 19 patients experienced relapse within the retroperitoneum, and eight (67%) of these 12 patients had retroperitoneal relapse within the primary landing zone. Among the 19 patients treated with primary RPLND, 5-year CSS after LR was estimated at 58% (95% CI, 31% to 77%).
Primary treatment for the initial testicular cancer was induction chemotherapy for 45 patients. Of these, 34 patients (76%) suffered LR in the retroperitoneum. Postchemotherapy RPLND was not performed in 18 of the 45 patients, 17 (94%) of whom presented with LR in the retroperitoneum. Conversely, postchemotherapy RPLND was performed in 27 patients (60%), 17 (63%) of whom had a retroperitoneal LR. The 5-year CSS rate after LR among these 45 patients was 53% (95% CI, 36% to 68%).
Treatment of Late Relapse of GCT
Treatment for LR and histology of LR is depicted in Table 2. Overall, 55 patients (73%) underwent surgery and 59 patients (79%) had chemotherapy as part of their treatment. The histology at time of LR was viable GCT in 54 patients (78%). Teratoma was present in 41 patients (59%), and malignant transformation was present in 14 patients (20%).
Table 2.
Treatment and Histology of Late Relapse
| Characteristic | No. | % |
|---|---|---|
| Treatment | ||
| RPLND alone | 12 | 16 |
| Chemotherapy alone | 18 | 23 |
| Chemotherapy plus other surgery* | 7 | 9 |
| Chemotherapy plus XRT | 2 | 3 |
| Chemotherapy plus other surgery* and XRT | 3 | 4 |
| Chemotherapy plus RPLND | 23 | 31 |
| RPLND plus other surgery* | 2 | 3 |
| RPLND plus XRT | 2 | 3 |
| RPLND plus other surgery* and XRT | 2 | 3 |
| Other surgery* | 4 | 7 |
| Histology† | ||
| Viable cancer‡ | 54 | 78 |
| Teratoma (any) | 41 | 59 |
| Only teratoma | 13 | 19 |
| Malignant transformation§ | 14 | 20 |
| Fibrosis‖ | 2 | 3 |
Abbreviations: RPLND, retroperitoneal lymph node dissection; XRT, radiation therapy.
Thoracotomy with wedge resection, resection of mediastinal mass, resection of liver metastases, supraclavicular node excision, neck dissection, or resection of spinal metastasis.
Histology of late relapse available for 69 (92%) of 75 patients; histology may be via RPLND, tumor excision, or needle biopsy.
Yolk sac tumor most common.
Malignant transformation to sarcoma, adenocarcinoma, or neuroectodermal tumor.
Fibrosis was noted in two patients via postchemotherapy RPLND after chemotherapy for late relapse. No prechemotherapy pathology is available for either of these patients.
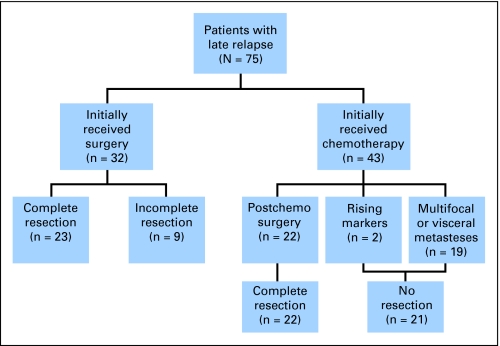
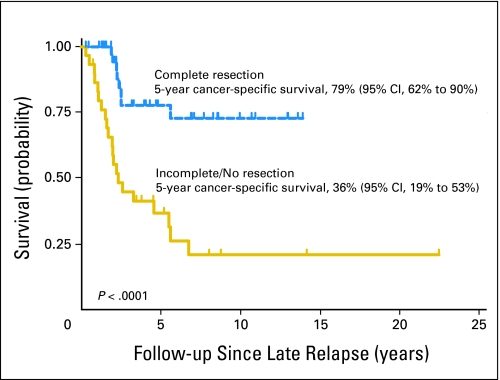
Overall, 40 patients (53%) were without evidence of disease at last follow-up, five patients (7%) were alive with residual stable radiographic disease, 29 patients (39%) were dead from testicular cancer, and one patient died from a presumptive pulmonary embolism 7 days after a redo RPLND and radical neck dissection. Median follow-up from initial diagnosis of GCT (to death or last follow-up) was 12.9 years (IQR, 7.6 to 17.6 years). Median follow-up from LR for survivors was 4.5 years (IQR, 2.1 to 8.5 years). The Kaplan-Meier estimate of 5-year CSS was 60% (95% CI, 46% to 71%). Figure 1 shows the treatment received when patients presented with LR, subdivided by those who underwent complete surgical resection. Five-year CSS was significantly higher in the 45 patients who underwent complete surgical resection (79%; 95% CI, 62% to 90%) as compared with the 30 patients for whom resection was incomplete, not possible, or not attempted (36%; 95% CI, 19% to 53%; P < .0001; Fig 2).
Fig 1.
CONSORT diagram.
Fig 2.
Kaplan-Meier analysis of cancer-specific survival according to complete resection of late relapse (LR) versus incomplete or no resection of LR.
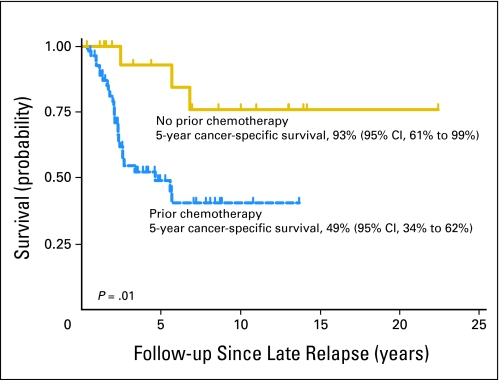
Chemotherapy-Naive Patients Have a Favorable Outcome
Eighteen chemotherapy-naive patients presented with LR after initial treatment with primary RPLND or surveillance. The 5-year CSS after therapy for LR in chemotherapy-naive patients was 93% (95% CI, 61% to 99%). This was significantly higher compared with patients who received chemotherapy as part of their initial management (Fig 3). Furthermore, on multivariable analysis, evaluating pretreatment parameters predicting for CSS at LR, the absence of prior chemotherapy was associated with a statistically significant improvement in outcome (patients initially treated with prior chemotherapy had a four-fold risk of death from cancer, hazard ratio [HR] = 4.0; 95% CI 1.2 to 13.6; P = .03). Given that chemotherapy-naive patients at LR have disease that is clinically and biologically different from that of patients with a history of prior chemotherapy, we chose to exclude these patients from our further analyses. Additionally, we propose that the definition of LR should include only patients with a history of prior chemotherapy as part of their initial management.
Fig 3.
Kaplan-Meier analysis of cancer-specific survival from time of late relapse according to prior chemotherapy.
Factors Associated With CSS
Predictors of CSS were analyzed for patients suffering a LR with a prior history of chemotherapy (n = 57). Patients who were chemotherapy-naive at LR were excluded from the analyses. In univariate analysis, factors associated with improved CSS were teratoma or fibrosis at LR, complete resection, single site of relapse, normal serum tumor markers, and asymptomatic presentation (Table 3). Disease-free interval was analyzed as a categoric variable; patients whose intervals were less than 5 years had greater 5-year CSS than patients with longer intervals (75% v 50%), although this difference was not statistically significant (P = .19). A pretreatment multivariable model was constructed to analyze variables that may be predictive for a worse CSS. Variables included in the multivariable analysis were limited to those known at time of LR: multifocality, elevated serum tumor markers, and presence of symptoms. Results of the multivariable analysis are shown in Table 4. Patients with a symptomatic presentation (HR = 4.9; 95% CI, 1.6 to 15.2) and multifocal sites of disease (HR = 3.0; 95% CI, 1.3 to 6.8) were independently predictive of decreased survival. The presence of elevated serum tumor markers at time of LR was not associated with survival in this model.
Table 3.
Univariate Analysis: Factors Predictive of 5-Year CSS From Time of LR for Men With a History of Prior Chemotherapy (n = 57)
| Variable | 5-Year CSS
|
P (log-rank) | |
|---|---|---|---|
| % | 95% CI | ||
| Time to LR, years | |||
| < 5 | 60 | 32 to 80 | .54 |
| ≥ 5 | 44 | 26 to 60 | |
| Histology of LR | |||
| Viable cancer/TMT | 41 | 25 to 57 | .03 |
| Teratoma/fibrosis | 88 | 49 to 98 | |
| Complete resection | |||
| Yes | 73 | 49 to 87 | .0003 |
| No | 28 | 12 to 46 | |
| Multifocality | |||
| Yes | 28 | 12 to 46 | .0001 |
| No | 75 | 50 to 89 | |
| Elevated STM at LR | |||
| Yes | 39 | 23 to 55 | .12 |
| No | 71 | 41 to 88 | |
| Symptoms | |||
| Yes | 33 | 18 to 49 | .003 |
| No | 81 | 51 to 93 | |
Abbreviations: CSS, cancer-specific survival; LR, late relapse; TMT, malignant transformation of teratoma; STM, serum tumor markers (α-fetoprotein or β-human choriogonadotropin).
Table 4.
Multivariable Cox Proportional Hazards Model: Analysis of Pretreatment Factors Predictive of 5-Year Cancer-Specific Survival From Time of LR for Men With a History of Prior Chemotherapy (n = 57)
| Variable | Hazard Ratio | 95% CI | P |
|---|---|---|---|
| Symptoms, yes v no | 4.9 | 1.6 to 15.2 | .006 |
| Multifocal, yes v no | 3.0 | 1.3 to 6.8 | .007 |
| Elevated STM at LR, yes v no | 1.3 | 0.5 to 3.5 | .6 |
Abbreviation: LR, late relapse; STM, serum tumor markers (α-fetoprotein or β-human choriogonadotropin).
DISCUSSION
LR of testicular cancer is a rare but distinct clinical entity, and our single-institutional experience in treating this disease improves the understanding of this growing problem. First, LR may happen at any time after initial treatment of testicular GCT; 36% of patients had LR more than 10 years from initial CR. This finding demonstrates the need for life-long surveillance after initial treatment. Second, the retroperitoneum is the most common site of recurrence in patients with LR (72%), emphasizing the critical role for bilateral RPLND in the management of GCT. Third, patients who are chemotherapy-naive at the time of LR have a favorable outcome compared with patients with a prior history of chemotherapy. The presence of multifocal disease or symptoms at the presentation of LR portends an inferior CSS. Finally, 5-year CSS is substantially higher in patients with complete resection (79%) than in those without complete resection (36%); therefore, treatment for LR should be approached with the eventual goal of complete surgical resection.
Data from this and previous studies suggest that control of the retroperitoneum is critical in the treatment of patients with GCT of the testis. The retroperitoneum is the most common site of late recurrence in this series (72% of patients overall and 89% of patients without prior RPLND). Patients with nonseminomatous GCT who receive chemotherapy alone for their initial treatment or undergo an incomplete RPLND remain at risk for LR. The use of modified templates, techniques (laparoscopic or open) that fail to dissect posterior to the great vessels, and RPLND done without therapeutic intent may place patients at risk for potential LR. Eggener et al10 demonstrated that, for patients with clinical stage I or IIa disease, the use of modified RPLND templates potentially result in residual extra-template disease left behind in 3% to 23% of patients, depending on the template applied. It has been reported that for patients undergoing a primary bilateral RPLND by expert surgeons, the retroperitoneal relapse rate is less than 1%.11,12 Additionally, Carver et al13 demonstrated that for men undergoing a postchemotherapy RPLND for nonseminomatous GCT, extra-template disease is found in 4% to 32% of patients, depending on the anatomic boundaries of the modified template. Furthermore, in a nonseminomatous GCT series in which surgery included resection of postchemotherapy masses alone rather than full bilateral RPLND, 37 (10%) of 369 patients had a retroperitoneal recurrence.14 In our series at Memorial Sloan-Kettering Cancer Center (1989 to 2002), for men undergoing an initial postchemotherapy bilateral RPLND, four (0.8%) of 482 patients suffered a retroperitoneal recurrence. Three of these four patients had high-volume retroperitoneal disease at the time of RPLND and one underwent an RPLND in the presence of positive serum tumor markers despite multiple regimens of chemotherapy.
The incidence of teratoma and somatic malignant transformation of teratoma is increased in LR and was found in 59% and 20% of patients in this series, respectively. The incidence of malignant transformation of teratoma is greater than that noted in our primary RPLND series (two of 550 patients, or 0.4%) and postchemotherapy series (18 of 532 patients, or 3%).15 These histologies are chemotherapy resistant, and surgical excision is requied for cure. Some teratomas may have an indolent course, but others may grow and invade locally or dedifferentiate to secondary somatic malignancy. These findings, in addition to the relative chemotherapy resistance of viable GCT in patients with prior chemotherapy, underscore the critical role for complete surgery in patients with LR.
Chemotherapy before LR is a statistically significant and clinically relevant predictor of inferior CSS.4,5 The chemotherapy resistance that underlies the biology of LR does not seem apply to chemotherapy-naive patients. At our institution, chemotherapy-naive patients who suffer LR after initial treatment of testicular cancer are treated according to clinical stage and International Germ Cell Consensus Classification Group risk criteria as any other previously untreated patient, with comparable outcomes to patients not suffering an LR. Thus we believe that the definition of LR should be limited to patients who have had prior chemotherapy, because the 5-year CSS is significantly higher in patients who are chemotherapy-naive (93%; 95% CI, 61% to 99%) than in patients who received chemotherapy before LR (49%; 95% CI, 34% to 62%). Taking this into consideration, we evaluated patients suffering an LR with a history of prior chemotherapy to determine which pretreatment variables may predict for CSS. On multivariable analysis, patients presenting with symptoms and multifocal disease at time of LR of GCT were associated with significantly inferior CSS.
Complete surgical resection is central to the management of LR. Our study and others have demonstrated that attaining complete resection should be the goal of treatment for any patient presenting with LR. We acknowledge that patients with LR in whom a complete resection is attainable are likely to have lower burden of disease at the time of LR; thus it is impossible from this data set to separate the effects of surgical complete resection from extent and aggressiveness of disease at LR. Our treatment paradigm for patients with LR who have had prior chemotherapy consists of initial surgery if a complete resection seems feasible after radiographic review, tumor markers are not increasing, and the disease is unifocal. Chemotherapy for LR is offered first if disease is multifocal or deemed unresectable, or markers are increasing at the time of LR. In a recent study, Ronnen et al7 report that the only chemotherapy regimen that produced durable responses in patients with LR was paclitaxel, ifosfamide, and cisplatin (TIP), with seven (50%) of 14 patients who received TIP achieving a continuous CR. TIP remains our regimen of choice for patients with a history of prior chemotherapy who suffer an LR.16 Consolidative postchemotherapy surgery is then suggested for all patients who have resectable disease.
In conclusion, patients with LR have an overall 5-year CSS of 60% (95% CI, 46% to 71%). Factors that predict improved survival at time of LR include no prior chemotherapy, asymptomatic presentation, and a single site of recurrence. Complete resection during treatment for LR is associated with significantly improved survival. This observation, along with the preponderance of retroperitoneal recurrence and high prevalence of chemotherapy-resistant teratoma and malignant transformation of teratoma, highlight the importance of surgery in the treatment of LR.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: David S. Sharp, Brett S. Carver, Joel Sheinfeld
Provision of study materials or patients: G. Varuni Kondagunta, Robert J. Motzer, George J. Bosl, Joel Sheinfeld
Collection and assembly of data: David S. Sharp, Scott E. Eggener, G. Varuni Kondagunta
Data analysis and interpretation: David S. Sharp, Brett S. Carver, Scott E. Eggener, Joel Sheinfeld
Manuscript writing: David S. Sharp, Joel Sheinfeld
Final approval of manuscript: David S. Sharp, Brett S. Carver, Scott E. Eggener, G. Varuni Kondagunta, Robert J. Motzer, George J. Bosl, Joel Sheinfeld
published online ahead of print at www.jco.org on October 20, 2008
Supported by a National Institute of Health Research Service Award (Grant No. T32-CA82088 to D.S.S.).
Presented in part at the 42nd Annual Meeting of the American Society of Clinical Oncology, June 2-6, 2006 Atlanta, GA.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Baniel J, Foster RS, Gonin R, et al: Late relapse of testicular cancer. J Clin Oncol 13:1170-1176, 1995 [DOI] [PubMed] [Google Scholar]
- 2.Gerl A, Clemm C, Schmeller N, et al: Late relapse of germ cell tumors after cisplatin-based chemotherapy. Ann Oncol 8:41-47, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Shahidi M, Norman AR, Dearnaley DP, et al: Late recurrence in 1263 men with testicular germ cell tumors: Multivariate analysis of risk factors and implications for management. Cancer 95:520-530, 2002 [DOI] [PubMed] [Google Scholar]
- 4.George DW, Foster RS, Hromas RA, et al: Update on late relapse of germ cell tumor: A clinical and molecular analysis. J Clin Oncol 21:113-122, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Dieckmann KP, Albers P, Classen J, et al: Late relapse of testicular germ cell neoplasms: A descriptive analysis of 122 cases. J Urol 173:824-829, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Oldenburg J, Alfsen GC, Waehre H, et al: Late recurrences of germ cell malignancies: A population-based experience over three decades. Br J Cancer 94:820-827, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ronnen EA, Kondagunta GV, Bacik J, et al: Incidence of late-relapse germ cell tumor and outcome to salvage chemotherapy. J Clin Oncol 23:6999-7004, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Oldenburg J, Martin JM, Fossa SD: Late relapses of germ cell malignancies: Incidence, management, and prognosis. J Clin Oncol 24:5503-5511, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Sobin LH, Wittekind C: International Union against Cancer: TNM Classification of Malignant Tumours. New York, NY, Wiley-Liss, 2002
- 10.Eggener SE, Carver BS, Sharp DS, et al: Incidence of disease outside modified retroperitoneal lymph node dissection templates in clinical stage I or IIA nonseminomatous germ cell testicular cancer. J Urol 177:937-942; discussion 942-943, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Donohue JP, Thornhill JA, Foster RS, et al: Primary retroperitoneal lymph node dissection in clinical stage A non-seminomatous germ cell testis cancer: Review of the Indiana University experience 1965-1989. Br J Urol 71:326-335, 1993 [DOI] [PubMed] [Google Scholar]
- 12.Stephenson AJ, Bosl GJ, Motzer RJ, et al: Retroperitoneal lymph node dissection for nonseminomatous germ cell testicular cancer: Impact of patient selection factors on outcome. J Clin Oncol 23:2781-2788, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Carver BS, Shayegan B, Eggener S, et al: Incidence of metastatic nonseminomatous germ cell tumor outside the boundaries of a modified postchemotherapy retroperitoneal lymph node dissection. J Clin Oncol 25:4365-4369, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Lutke Holzik MF, Hoekstra HJ, Mulder NH, et al: Non-germ cell malignancy in residual or recurrent mass after chemotherapy for nonseminomatous testicular germ cell tumor. Ann Surg Oncol 10:131-135, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Carver BS, Bianco FJ Jr, Shayegan B, et al: Predicting teratoma in the retroperitoneum in men undergoing post-chemotherapy retroperitoneal lymph node dissection. J Urol 176:100-103; discussion 103-104, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Kondagunta GV, Bacik J, Donadio A, et al: Combination of paclitaxel, ifosfamide, and cisplatin is an effective second-line therapy for patients with relapsed testicular germ cell tumors. J Clin Oncol 23:6549-6555, 2005 [DOI] [PubMed] [Google Scholar]