Abstract
Purpose
To examine the independent roles of pre–hematopoietic cell transplantation (HCT) therapeutic exposures, transplantation-related conditioning, and comorbidities (pre- and post-HCT) in the development of late congestive heart failure (CHF) after HCT.
Methods
This was a nested case-control design. Individuals with late CHF (diagnosed ≥ 1 year after HCT) were identified from a cohort of 2,938 1+ year survivors who underwent transplantation at City of Hope National Medical Center, Duarte, CA. This cohort formed the sampling frame for selecting controls (without CHF) matched for age and year of HCT, donor source (allogeneic v autologous), and length of follow-up.
Results
Sixty patients with late CHF were identified; median age at HCT was 45.3 years (range, 16.6 to 68.6 years); median time to CHF was 3.0 years (range, 1.03 to 18.9 years); 68% received autologous HCT. Median ejection fraction was 36.9% (range, 15% to 53%). Compared with matched controls (n = 166), patients with late CHF received more cycles of pre-HCT chemotherapy (8.6 v 4.9 cycles; P < .01), had greater body mass index at HCT (28.4 v 26.2 kg/m2; P = .01), greater lifetime anthracycline exposure (285.3 v 175.6 mg/m2; P < .01), and were more likely to have multiple chronic comorbidities (30.0% v 13.9%; P < .01). Multivariable analysis revealed number of pre-HCT chemotherapy cycles (odds ratio [OR] = 1.2; P < .01), anthracycline dose ≥ 250 mg/m2 (OR = 3.2; P = .05), and two or more chronic comorbidities (OR = 4.3; P = .01) to be independently associated with late CHF.
Conclusion
Pre-HCT exposure to anthracyclines and presence of comorbidities are primarily responsible for the risk associated with late CHF after HCT. Conditioning-related therapeutic exposure does not contribute significantly to the risk. These results form the basis for identifying high-risk individuals for targeted surveillance, as well as developing preventive strategies in the form of aggressive management of comorbidities.
INTRODUCTION
Hematopoietic cell transplantation (HCT) is being used increasingly with a curative intent for patients with life-threatening hematologic malignancies.1,2 Improvements in transplantation strategies and supportive care have contributed to incremental increase in survival rates of 10% per decade after HCT.3 Patients with hematologic malignancies who survive for 2 years after allogeneic HCT now have survival rates that exceed 80% at 15 years,4,5 whereas survival rates for autologous HCT recipients approach 70% at 10 years.6 The growing population of long-term survivors has brought to the medical forefront a host of chronic and debilitating conditions attributed to toxicity from pretransplantation exposure, transplantation conditioning regimens, infections, immunodeficiency, and graft-versus-host disease (GvHD).7-10
Congestive heart failure (CHF) is a well-described sequela during the immediate post-HCT period. Mortality attributed to early CHF ranges from 1% to 9% and morbidity from 5% to 43%,11-13 and well-defined risk factors have been identified.13-15 The occurrence of CHF after the first year (late CHF) is less well studied, with the few reports describing this outcome hampered by relatively short lengths of follow-up and small sample size.16,17 Potential mediators of late CHF could include pretransplantation exposure to known cardiotoxic agents such as anthracyclines, alkylating agents, and mediastinal radiation, compounded by high-dose cyclophosphamide and total-body irradiation (TBI) at the time of HCT and presence of chronic GvHD after HCT.18-21 Furthermore, the magnitude of this risk could be moderated by the presence of comorbid conditions, and host characteristics such as age at exposure and sex of recipient.9,18,22
The relative contribution of these factors in the development of late CHF and, in particular, the impact of HCT and its associated exposures is not known. We examined the independent roles of pre-HCT exposures, transplantation-related conditioning, and comorbidities (pre- and post-HCT) in the development of late CHF.
METHODS
A nested case-control study design was used. Cases were identified from a cohort of 2,938 1+ year survivors who underwent transplantation at City of Hope National Medical Center (COH), Duarte, CA, between 1981 and 2003 for hematologic malignancies. To be eligible, patients were required to be free of cardiac disease before study entry and to have developed CHF after the first year after HCT, as defined by the American Heart Association (AHA)/American College of Cardiology (ACC) 2005 Guidelines for the Diagnosis and Management of Chronic Heart Failure in the Adult.23 Multiple controls (one to three) were selected at random from the same cohort and matched to patient cases for age at HCT (± 5 years), year of HCT (± 2 years), donor source (autologous v allogeneic), and length of follow-up. The human subjects committee at COH approved the protocol. Informed consent was obtained according to the Declaration of Helsinki.
Exposure Variables
For both patient cases and controls, medical records from within and outside COH were used to abstract clinical and therapeutic information regarding the pretransplantation period, transplantation conditioning, and the post-HCT period. The following data were collected: demographics, disease characteristics (diagnosis, date of diagnosis, and stage), treatment before HCT (chemotherapy: protocols/regimens, agents, and cumulative dose of anthracyclines and alkylating agents; radiation therapy: total dose, field, and dose per fraction), conditioning regimens used (chemotherapeutic agents, radiation [TBI, number of fractions, and total dose]), and immediate post-HCT complications (day 0 through 30; sepsis requiring pressor support, respiratory failure requiring mechanical ventilation, or renal failure requiring dialysis).
Therapeutic exposures were summarized for patient cases and controls. Anthracycline cardiotoxicity risk factor score16 was calculated by multiplying cumulative dose by a factor that reflects the cardiotoxic potential of each drug. Cumulative alkylating agent dose was recorded as a continuous variable; high-dose cyclophosphamide was recorded as a dichotomous variable.
Comorbidities
Comorbidities that could potentially increase the risk of CHF included the following: noncardiac (diabetes, hypertension, chronic lung disease, renal insufficiency, thyroid disease, and dyslipidemia) and cardiac (history of arrhythmias, angina, myocardial infarction, and relevant cardiac surgery).
Pre-HCT comorbidities, as well as post-HCT comorbidities that had developed after HCT but before the onset of CHF and were active at the time of event (cases) or equivalent follow-up (controls), were identified through medical record abstraction.
Outcome Variable: Late CHF
Post-HCT information was obtained as part of the long-term follow-up program at COH that actively observes patients who have undergone HCT and survived at least 1 year. Medical records maintained at COH are the primary source of data. If there are unexpected gaps in patients’ history, physicians taking care of patients outside COH are contacted to obtain the pertinent information. If the physician is not available or unable to provide recent information, the patient is called directly.
Cases of late CHF identified from the long-term follow-up program were further validated using medical record information regarding history and physical examination at the time of CHF presentation. Diagnostic echocardiogram/multiple gate acquisition scan (MUGA) reports were used to document extent of cardiac compromise. Cases needed to satisfy ACC/AHA diagnostic guidelines of demonstrating symptoms (dyspnea and fatigue) and signs (edema and rales) consistent with CHF. Variables of interest were recorded and coded according to the recommendations of the ACC/AHA (2005) Task Force on Clinical Data Standards.24
Statistical Analysis
Cases and controls were compared with respect to demographics, pre-HCT exposures, HCT-related conditioning, and comorbidities, using either χ2 and Fisher's exact tests for dichotomous or t tests for continuous variables. An α level of less than 0.05 was considered significant. Primary diagnosis was analyzed as lymphoma (non-Hodgkin's lymphoma, Hodgkin's lymphoma) versus nonlymphoma (multiple myeloma, acute lymphatic leukemia, acute myeloid leukemia, chronic myeloid leukemia, and other).
Multivariable conditional logistic regression was used to identify variables that were significantly and independently associated with late CHF after HCT (dependent variable). Variables included in the model were those significantly associated with late CHF in the univariate analysis, as well as those thought to impact clinical outcome, and included age at initial diagnosis, number of cycles of chemotherapy before HCT, and body mass index (BMI) as continuous variables and sex, primary diagnosis (lymphoma v nonlymphoma), anthracycline exposure (< 250 v ≥ 250 mg/m2), number of pre-HCT comorbidities (yes/no), and history of second HCT (yes/no) as dichotomous variables. Number of post-HCT comorbidities was entered as an ordinal variable (none v 1 v ≥ 2). Statistical analyses were performed using SAS 9.1 (SAS Institute, Cary, NC).
RESULTS
Sixty cases with late CHF and 166 matched controls were included in the analysis. All cases had at least one matched control; 58 cases (97%) had at least two controls. Median follow-up after HCT was 6.4 years (range, 1.3 to 22.1 years) for cases and 8.3 years (range, 1.5 to 25.1 years) for controls.
Patient Characteristics
Table 1 presents data on the characteristics of cases and controls. There were no significant differences between cases and controls with respect to age at primary disease diagnosis, sex, and race or ethnicity. Cases and controls were not matched for primary diagnosis. As such, non-Hodgkin's lymphoma and Hodgkin's lymphoma accounted for 53% of the cases and 44% of the controls; additionally, there was an overrepresentation of acute lymphatic leukemia among cases and of chronic myeloid leukemia among controls. All other diagnoses were equally represented. Importantly, there were no significant differences in the prevalence or nature of pre-HCT comorbidities between cases and controls (8.3% of cases with two or more comorbidities v 6.6% of controls; P = .77).
Table 1.
Characteristics of Patient Population and Pre-HCT Exposure
| Characteristic | Cases* (n = 60)
|
Controls* (n = 166)
|
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age at initial diagnosis, years | |||||
| Mean | 43.0 | 43.8 | |||
| SD | 13.7 | 13.3 | .70 | ||
| Female sex | 30 | 50.0 | 73 | 44.0 | .42 |
| Ethnicity/race | |||||
| Non-Hispanic white | 37 | 61.7 | 112 | 67.5 | |
| Others | 23 | 38.3 | 54 | 32.5 | .52 |
| Diagnosis† | |||||
| Lymphoma | 32 | 53.3 | 73 | 44.0 | |
| Non-Hodgkin's lymphoma | 25 | 41.7 | 50 | 30.1 | |
| Hodgkin's lymphoma | 7 | 11.7 | 23 | 13.9 | |
| Nonlymphoma | 28 | 46.7 | 93 | 56.0 | .21 |
| Multiple myeloma | 9 | 15.0 | 25 | 15.1 | |
| Acute lymphatic leukemia | 9 | 15.0 | 8 | 4.8 | |
| Acute myeloid leukemia | 8 | 13.3 | 31 | 18.7 | |
| Chronic myeloid leukemia | 2 | 3.3 | 18 | 10.8 | |
| Other | 0 | 0 | 11 | 6.6 | |
| Pre-HCT comorbidity | |||||
| Hypertension | 5 | 8.3 | 14 | 8.4 | 1.00 |
| Renal insufficiency | 4 | 6.7 | 4 | 2.4 | .26 |
| Chronic lung disease | 6 | 10.0 | 7 | 4.2 | .10 |
| Thyroid, hyper/hypo | 5 | 8.3 | 11 | 6.6 | .66 |
| Diabetes | 2 | 3.3 | 4 | 2.4 | .70 |
| Dyslipidemia | 1 | 1.7 | 6 | 3.6 | .40 |
| ≥ 2 comorbidities | 5 | 8.3 | 11 | 6.6 | .77 |
| Pre-HCT courses of chemotherapy | |||||
| Mean | 8.6 | 4.9 | |||
| SD | 4.9 | 4.1 | < .01 | ||
| Anthracycline, mg/m2 | |||||
| Pre-HCT | < .01 | ||||
| Mean | 261.4 | 171.8 | |||
| SD | 134.4 | 125.8 | |||
| Lifetime anthracycline | < .01 | ||||
| Mean | 285.3 | 175.6 | |||
| SD | 144.0 | 128.2 | |||
| Cyclophosphamide, g/m2 | |||||
| Mean | 2.3 | 1.6 | .10 | ||
| SD | 3.0 | 2.4 | |||
| Cisplatin, mg/m2 | |||||
| Mean | 58.3 | 43.7 | .35 | ||
| SD | 104.6 | 101.7 | |||
| Melphalan, mg/m2 | |||||
| Mean | 10.1 | 3.9 | .30 | ||
| SD | 42.3 | 22.9 | |||
| Mediastinal radiation | 6 | 10.0 | 14 | 8.4 | .71 |
Abbreviations: HCT, hematopoietic cell transplantation; SD, standard deviation.
Group matching criteria included age at HCT (± 5 years), type of HCT (autologous v allogeneic), year of HCT (± 2 years), and duration of follow-up.
Analyzed as lymphoma (non-Hodgkin's lymphoma, Hodgkin's lymphoma) versus nonlymphoma (multiple myeloma, acute lymphatic leukemia, acute myeloid leukemia, chronic myeloid leukemia, and other).
Pre-HCT Treatment Exposures
The mean time from diagnosis to HCT was comparable between cases and controls (2.6 v 1.7 years; P = .1). However, cases received a significantly greater number of courses of pre-HCT chemotherapy (8.6 v 4.9 courses; P < .01). Lifetime anthracycline exposure was also significantly higher among cases as compared with controls (285.3 v 175.6 mg/m2; P < .01). The proportion of patients exposed to mediastinal radiation did not differ between cases and controls.
HCT Conditioning
Etoposide, high-dose cyclophosphamide, and fractionated TBI were the most commonly used agents. As shown in Table 2, there were no significant differences in types of conditioning-related exposures between cases and controls. Eight cases (13.3%) and 12 controls (7.2%) underwent allogeneic transplantation after an autologous transplantation, with a median time to second transplantation of 2.7 versus 1.6 years, respectively.
Table 2.
Conditioning and Post-HCT Outcomes
| Variable | Cases* (n = 60)
|
Controls* (n = 166)
|
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age at HCT, years | |||||
| Mean | 45.7 | 45.5 | |||
| SD | 13.7 | 13.0 | |||
| Donor source, autologous transplant | 41 | 68.3 | 114 | 68.7 | |
| Time from diagnosis to HCT, years | |||||
| Mean | 2.6 | 1.7 | .10 | ||
| SD | 4.0 | 2.6 | |||
| Body mass index at HCT, kg/m2 | |||||
| Mean | 28.4 | 26.2 | .01 | ||
| SD | 7.0 | 5.2 | |||
| Conditioning regimen | |||||
| Cyclophosphamide | 45 | 75.0 | 125 | 75.3 | .96 |
| Etoposide | 42 | 70.0 | 111 | 66.9 | .66 |
| Total-body irradiation | 39 | 65.0 | 109 | 65.7 | .93 |
| Melphalan | 13 | 21.7 | 29 | 17.5 | .47 |
| Carmustine | 12 | 20.0 | 21 | 12.7 | .17 |
| Busulfan | 8 | 13.3 | 30 | 18.1 | .40 |
| Post-HCT comorbidity by type | |||||
| Hypertension | 24 | 40.0 | 24 | 14.5 | < .01 |
| Renal Insufficiency | 14 | 23.3 | 11 | 6.6 | < .01 |
| Chronic lung disease | 12 | 20.0 | 13 | 7.8 | .01 |
| Diabetes | 12 | 20.0 | 9 | 5.4 | < .01 |
| Thyroid, hyper/hypo | 6 | 10.0 | 18 | 10.8 | .86 |
| Dyslipidemia | 4 | 6.7 | 17 | 10.2 | .41 |
| Post-HCT comorbidity by number | |||||
| No comorbidity | 21 | 35.0 | 104 | 62.6 | |
| 1 comorbidity | 21 | 35.0 | 39 | 23.5 | < .01 |
| ≥ 2 comorbidities | 18 | 30.0 | 23 | 13.9 | |
Abbreviations: HCT, hematopoietic cell transplantation; SD, standard deviation.
Group matching criteria included age at HCT (± 5 years), type of HCT (autologous v allogeneic), year of HCT (± 2 years), and duration of follow-up.
Comorbidities
Median BMI at HCT was significantly greater among cases than among controls (28.4 v 26.2, respectively; P = .01). As seen in Table 2, cases were significantly more likely to have hypertension (40.0% v 14.5%; P < .01), chronic renal insufficiency (23.3% v 6.6%; P < .01), chronic lung disease (20.0% v 7.8%; P = .01), and diabetes (20.0% v 5.4%; P < .01) as compared with controls with equivalent follow-up. Cases were also significantly more likely to have multiple comorbidities (30.0% v 13.9%; P < .01) compared with controls. Among allogeneic HCT recipients, no statistically significant differences were observed regarding acute GvHD, history of ever having chronic GvHD, or presence of active GvHD (data not shown).
Clinical Presentation of Late CHF
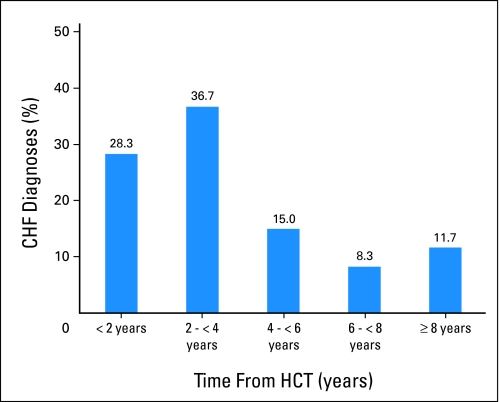
Figure 1 illustrates the proportion of CHF cases diagnosed as a function of years from HCT. Median time to late CHF was 3.0 years (range, 1.03 to 13.9 years), and median age at presentation was 51.2 years (range, 22.5 to 71.0 years). None of the cases had clinical evidence of transient cardiac dysfunction during the first year after HCT. Echocardiogram or MUGA reports were available in 50 cases, whereas a diagnostic history and physical examination performed by a cardiologist was available in 54 cases. For the remaining six cases, diagnosis of CHF relied on physician report.
Fig 1.
Distribution of congestive heart failure diagnoses over time after hematopoietic cell transplantation (HCT).
Table 3 lists the clinical features of late CHF. Dyspnea on exertion was the most common presenting symptom (96.3%), followed by fatigue (90.7%), orthopnea (55.6%), and weight gain (48.6%). Fifty (92.6%) of the 54 cases reported at least three symptoms associated with CHF. Physical examination revealed extremity edema in 77.8% and decreased breath sounds or rales in 64.8% and 44.4% of the cases, respectively. Among the cases who had chest radiographs at the time of presentation (72% of all cases), pulmonary edema (83.7%) and cardiomegaly (69.8%) were the most frequently reported abnormalities.
Table 3.
Clinical Presentation at CHF Diagnosis
| Characteristic | No. | % |
|---|---|---|
| Ejection fraction, %* | ||
| ≤ 25 | 9 | 18.0 |
| 26-40 | 23 | 46.0 |
| 41-49 | 15 | 30.0 |
| ≥ 50 | 3 | 6.0 |
| Reported symptoms† | ||
| Dyspnea with exertion | 52 | 96.3 |
| Dyspnea at rest | 21 | 38.9 |
| Orthopnea | 30 | 55.6 |
| Paroxysmal nocturnal dyspnea | 12 | 24.1 |
| Weight gain | 26 | 48.1 |
| Fatigue | 49 | 90.7 |
| ≥ 3 of above | 50 | 92.6 |
| Physical examination† | ||
| Lung findings | ||
| Clear | 11 | 20.3 |
| Rales | 24 | 44.4 |
| Decreased breath sounds | 35 | 64.8 |
| Rhonchi | 7 | 13.0 |
| Wheezing | 7 | 13.0 |
| Extremity edema | 42 | 77.8 |
| Hepatomegaly | 2 | 3.7 |
Abbreviation: CHF, congestive heart failure.
Echocardiogram or multiple gate acquisition scan reports from the time of CHF diagnosis were available for 50 cases.
Based on history and physical examination performed by a cardiologist at diagnosis (n = 54). For the remaining six cases, diagnosis of CHF was based on direct communication with their primary care physician.
Median left ventricular ejection fraction (EF) was 36.9% (range, 15% to 53%), and all assessable cases (n = 50) had greater than 10% reduction of EF from their pre-HCT baseline. Thirty-two cases (64%) were diagnosed with moderate to severe heart failure, characterized as having an EF of ≤ 40%. Forty-seven cases (78.3%) had stage C heart failure (structural heart disease with symptoms of CHF) and the remaining 13 cases (21.7%) had stage D disease (refractory CHF requiring specialized interventions), as defined by the ACC/AHA.
Of the 60 patients with late CHF, 40 patients have died (overall survival, 49.8% at 2 years after CHF diagnosis). The most common causes of mortality were relapse/progression of primary disease (37.5%), infection (20%), and renal failure (10%). Heart failure was the primary cause of death in three cases (7.5%).
Risk Factors for Late CHF
Multivariable conditional logistic regression revealed that cumulative anthracycline dose ≥ 250 mg/m2, pre-HCT cycles of chemotherapy, and having two or more comorbidities after HCT were significantly and independently associated with late CHF (Table 4). Limiting comorbidities in the model to hypertension, renal insufficiency, and chronic lung disease resulted in a three-fold increased risk of CHF in the presence of one comorbidity (odds ratio [OR] = 3.3; 95% CI, 1.14 to 9.41) and a 10-fold increased risk of CHF in the presence of two or more comorbidities (OR = 10.9; 95% CI, 2.81 to 42.27). Female patients were two times more likely to develop late CHF after HCT, an association that approached statistical significance (OR = 2.2; P = .07).
Table 4.
Multivariable Analysis of Risk Factors Associated With Late CHF
| Risk Factor | Odds Ratio | 95% CI | P |
|---|---|---|---|
| Sex | |||
| Male | 1.0 | ||
| Female | 2.2 | 0.93 to 5.20 | .07 |
| Age at diagnosis | 1.1 | 0.96 to 1.18 | .20 |
| Diagnosis* | |||
| Nonlymphoma | 1.0 | ||
| Lymphoma | 1.9 | 0.62 to 5.80 | .26 |
| Anthracycline, mg/m2 | |||
| < 250 | 1.0 | ||
| ≥ 250 | 3.2 | 1.02 to 10.22 | .05 |
| Pre-HCT risk factors | |||
| < 2 comorbidities | 1.0 | ||
| ≥ 2 comorbidities | 0.9 | 0.20 to 4.02 | .90 |
| Pre-HCT cycles of chemotherapy | 1.2 | 1.08 to 1.38 | < .01 |
| BMI at HCT | 1.1 | 0.97 to 1.14 | .21 |
| Second HCT | |||
| No | 1.0 | ||
| Yes | 3.2 | 0.45 to 22.34 | .25 |
| Post-HCT risk factors | |||
| No comorbidity | 1.0 | ||
| 1 comorbidity | 2.5 | 0.97 to 6.26 | .06 |
| ≥ 2 comorbidities | 4.3 | 1.37 to 13.67 | .01 |
Abbreviations: CHF, congestive heart failure; HCT, hematopoietic cell transplantation; BMI, body mass index.
Analyzed as lymphoma (non-Hodgkin's lymphoma, Hodgkin's lymphoma) versus nonlymphoma (multiple myeloma, acute lymphatic leukemia, acute myeloid leukemia, chronic myeloid leukemia, and other).
Separate regression models were created for autologous and allogeneic HCT recipients to identify risk factors that may be unique to these populations. Excluded from both analyses were those individuals who underwent a second transplantation (eight cases and 12 controls). Among autologous HCT recipients (33 cases and 105 controls), female sex (OR = 4.6; P = .05), cumulative anthracycline dose (OR = 33.1; P = .02), pre-HCT cycles of chemotherapy (OR = 1.3; P = .02), and number of post-HCT comorbidities (OR = 77.8; P = .04) were significantly and independently associated with late CHF.
For allogeneic HCT recipients (19 cases and 49 controls), documented history of having chronic GvHD was also included. Although a primary diagnosis of lymphoma, number of pre-HCT cycles of chemotherapy, and post-HCT comorbidities were identified to be significantly associated with late CHF, the small number of patients in this group precluded stable estimates (data not shown). Of note, no association was identified between chronic GvHD and late CHF.
DISCUSSION
The overall goal of this study was to understand the impact of HCT on the development of late CHF, over and above that of the well-known risks associated with conventional chemotherapy. Previous studies have limited the evaluation of post-HCT CHF to the first year after HCT, often focusing on conditioning-related exposures and immediate complications such as sepsis or multiorgan failure.11-13,15 It is increasingly recognized, however, that variables that lead to early CHF are different from those associated with late CHF. Our study was the first to incorporate pre-HCT therapeutic exposures, conditioning, and post-HCT exposures and comorbidities in the characterization of late CHF in a large cohort of HCT survivors. Using a nested case-control design, we identified number of pre-HCT chemotherapy cycles, cumulative anthracycline dose ≥ 250 mg/m2, and presence of post-HCT comorbidities to be significantly and independently associated with late CHF. Specifically, conditioning exposures such as high-dose chemotherapy, TBI, or other early transplantation-related complications, including GvHD, did not significantly increase the risk of late CHF.
Anthracyclines are the most commonly implicated agents for therapy-related cardiomyopathy.25 The incidence of anthracycline-related CHF in non-HCT populations varies from 4% at a cumulative dose of 500 to 550 mg/m2 to more than 36% in patients receiving 600 mg/m2.26 The incidence increases with follow-up, and the damage is often irreversible.18,27,28 The role of anthracyclines in the development of late CHF among HCT recipients is not clear, because most studies have evaluated them exclusively in the context of CHF within the first year after HCT. Our finding that cumulative dose of greater than 250 mg/m2 was associated with late CHF is the lowest threshold for anthracycline dose to be associated with CHF, supporting growing evidence that clinical cardiac dysfunction can occur at much lower doses than previously believed.18,29 The cardiotoxic effect of anthracyclines was higher for autologous HCT recipients, with a cumulative dose of ≥ 250 mg/m2 being associated with a 30-fold increased risk of late CHF.
Although anthracycline exposure was the primary variable of interest, number of chemotherapy cycles was included as a variable to serve as a surrogate for intensity of therapy. Our report that the number of chemotherapy cycles before HCT is significantly and independently associated with increased risk of late CHF after HCT is consistent with previous studies that have found direct correlation between the two variables.12,17 The case patients had received nearly twice as many cycles of chemotherapy compared with controls, and the difference remained significant despite adjustment for baseline demographics, diagnosis, time from diagnosis to HCT, and the cumulative anthracycline dose. This study demonstrates that, as anticipated, lifetime anthracycline exposure is associated with late CHF; in addition, there is an independent association of late CHF with the intensity of chemotherapeutic exposures.
It is increasingly recognized that HCT survivors are at greater risk for developing comorbidities.7,9,31 However, little is known about the role of these conditions in exacerbating cardiac dysfunction. Comorbidities selected for the current study were those identified by the ACC/AHA as ones that contributed most to the risk of heart failure.24 More importantly, control of each of these diseases has been associated with reduction in risk of late CHF in the general population.24 After HCT, case patients were significantly more likely to have been diagnosed with hypertension, renal insufficiency, chronic lung disease, and diabetes when compared with controls. We found a four-fold increased risk of late CHF for those with multiple post-HCT comorbidities, despite adjustment for patient demographics, pre-HCT comorbidities, and BMI.
Stratifying the analysis by donor source (allogeneic and autologous) revealed that among autologous transplant recipients, women had a greater than four-fold increased risk of late CHF. This is consistent with previous reports in autologous HCT recipients.6,32 A similar observation has been reported in long-term survivors of childhood cancer, where female sex and cumulative anthracycline dose were the most consistent predictors of late CHF.32,33 The mechanism for sex-specific association with CHF is not clear. Differences in body fat composition between men and women could alter the pharmacokinetics and pharmacodynamics of the drug, because anthracyclines do not reach a high concentration in adipose tissue.33,34 If women have a higher percentage of body fat for the same body-surface area, equivalent doses of the drug could lead to greater concentrations in nonadipose tissues such as the heart and lead to more cardiotoxicity than their male counterparts.35,36
Any retrospective review of medical records is limited by the amount of information available for review. Lack of consistency with which data were recorded in medical charts by health care staff could have influenced the validity of the information collected. To prevent recorder bias, pre-HCT treatment information obtained from the institutional screening history and physical examination was confirmed with the treatment summary submitted by the referring primary oncologist.
The definition of clinical heart failure is based as much on clinical signs and symptoms as it is on demonstration of reduced cardiac function on echocardiogram or MUGA. The recent position statement by the ACC/AHA has described heart failure as a syndrome, characterized by specific symptoms (dyspnea and fatigue) in the medical history and signs (edema and rales) on physical examination.23 All cases had clinical evidence of heart failure (stage C or D), and we were able to obtain corresponding echocardiograms or MUGA on most patients to demonstrate reduced cardiac function from baseline. Our cases did not include those individuals who may have had diminished cardiac function but were asymptomatic. However, the focus of this study was on understanding the impact of HCT-related exposures and events on the development of late clinical heart failure, and as such, asymptomatic CHF would not fit this definition.
In summary, we found that pre-HCT exposure to anthracyclines and the presence of post-HCT chronic comorbidities to be associated with an increased risk of late CHF after HCT. High-dose chemotherapy or TBI used for conditioning did not contribute significantly to the risk. These data form the basis of developing predictive models for identifying those at risk for targeted surveillance, as well as developing preventive strategies in the form of aggressive management of comorbidities.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Saro H. Armenian, Can-Lan Sun, Smita Bhatia
Administrative support: Stephen J. Forman, Smita Bhatia
Provision of study materials or patients: Saro H. Armenian, Liton Francisco, Seira Kurian, Jon Sharp, Smita Bhatia
Collection and assembly of data: Saro H. Armenian, Liton Francisco, F. Lennie Wong, Jon Sharp, Smita Bhatia
Data analysis and interpretation: Saro H. Armenian, Can-Lan Sun, Julia Steinberger, Richard Sposto, Smita Bhatia
Manuscript writing: Saro H. Armenian, Can-Lan Sun, Julia Steinberger, Seira Kurian, F. Lennie Wong, Richard Sposto, Smita Bhatia
Final approval of manuscript: Saro H. Armenian, Can-Lan Sun, Liton Francisco, Julia Steinberger, Seira Kurian, F. Lennie Wong, Jon Sharp, Richard Sposto, Stephen J. Forman, Smita Bhatia
published online ahead of print at www.jco.org on September 22, 2008
Supported by National Institutes of Health Grants No. R01 CA078938 (S.B.) and P01 CA30206 (S.J.F.), Lymphoma/Leukemia Society Scholar Award for Clinical Research Grant No. 2191-02 (S.B.), and Childrens Hospital Los Angeles Research Career Development Fellowship (S.H.A.).
Presented in part at the 44th Annual Meeting of the American Society of Clinical Oncology (Clinical Science Symposium: Treatment-Related Cardiovascular Disease in Survivors of Pediatric and Adult Cancers), May 30-June 3, 2008, Chicago, IL.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Passweg JR, Rowlings PA, Armitage JO, et al: Report from the International Bone Marrow Transplant Registry and Autologous Blood and Marrow Transplant Registry–North America. Clin Transpl 117-127, 1995 [PubMed]
- 2.Horowitz MM, Rowlings PA: An update from the International Bone Marrow Transplant Registry and the Autologous Blood and Marrow Transplant Registry on current activity in hematopoietic stem cell transplantation. Curr Opin Hematol 4:395-400, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Wingard JR, Vogelsang GB, Deeg HJ: Stem cell transplantation: Supportive care and long-term complications. Hematology Am Soc Hematol Educ Program 422-444, 2002 [DOI] [PubMed]
- 4.Socié G, Stone JV, Wingard JR, et al: Long-term survival and late deaths after allogeneic bone marrow transplantation: Late Effects Working Committee of the International Bone Marrow Transplant Registry. N Engl J Med 341:14-21, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Bhatia S, Francisco L, Carter A, et al: Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: Report from the BMT survivor study. Blood 110:3784-3792, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatia S, Robison LL, Francisco L, et al: Late mortality in survivors of autologous hematopoietic-cell transplantation: Report from the Bone Marrow Transplant Survivor Study. Blood 105:4215-4222, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Syrjala KL, Langer SL, Abrams JR, et al: Late effects of hematopoietic cell transplantation among 10-year adult survivors compared with case-matched controls. J Clin Oncol 23:6596-6606, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Bhatia S, Ramsay NK, Steinbuch M, et al: Malignant neoplasms following bone marrow transplantation. Blood 87:3633-3639, 1996 [PubMed] [Google Scholar]
- 9.Baker KS, Ness KK, Steinberger J, et al: Diabetes, hypertension, and cardiovascular events in survivors of hematopoietic cell transplantation: A report from the bone marrow transplantation survivor study. Blood 109:1765-1772, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Socié G, Salooja N, Cohen A, et al: Nonmalignant late effects after allogeneic stem cell transplantation. Blood 101:3373-3385, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Hertenstein B, Stefanic M, Schmeiser T, et al: Cardiac toxicity of bone marrow transplantation: Predictive value of cardiologic evaluation before transplant. J Clin Oncol 12:998-1004, 1994 [DOI] [PubMed] [Google Scholar]
- 12.Fujimaki K, Maruta A, Yoshida M, et al: Severe cardiac toxicity in hematological stem cell transplantation: Predictive value of reduced left ventricular ejection fraction. Bone Marrow Transplant 27:307-310, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Murdych T, Weisdorf DJ: Serious cardiac complications during bone marrow transplantation at the University of Minnesota, 1977-1997. Bone Marrow Transplant 28:283-287, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Braverman AC, Antin JH, Plappert MT, et al: Cyclophosphamide cardiotoxicity in bone marrow transplantation: A prospective evaluation of new dosing regimens. J Clin Oncol 9:1215-1223, 1991 [DOI] [PubMed] [Google Scholar]
- 15.Sakata-Yanagimoto M, Kanda Y, Nakagawa M, et al: Predictors for severe cardiac complications after hematopoietic stem cell transplantation. Bone Marrow Transplant 33:1043-1047, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Lehmann S, Isberg B, Ljungman P, et al: Cardiac systolic function before and after hematopoietic stem cell transplantation. Bone Marrow Transplant 26:187-192, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Tang WH, Thomas S, Kalaycio M, et al: Clinical outcomes of patients with impaired left ventricular ejection fraction undergoing autologous bone marrow transplantation: Can we safely transplant patients with impaired ejection fraction? Bone Marrow Transplant 34:603-607, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Lipshultz SE, Lipsitz SR, Sallan SE, et al: Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol 23:2629-2636, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Gharib MI, Burnett AK: Chemotherapy-induced cardiotoxicity: Current practice and prospects of prophylaxis. Eur J Heart Fail 4:235-242, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Adams MJ, Lipsitz SR, Colan SD, et al: Cardiovascular status in long-term survivors of Hodgkin's disease treated with chest radiotherapy. J Clin Oncol 22:3139-3148, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Rackley C, Schultz KR, Goldman FD, et al: Cardiac manifestations of graft-versus-host disease. Biol Blood Marrow Transplant 11:773-780, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Chiodi S, Spinelli S, Ravera G, et al: Quality of life in 244 recipients of allogeneic bone marrow transplantation. Br J Haematol 110:614-619, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Hunt SA, Abraham WT, Chin MH, et al: ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure)—Developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: Endorsed by the Heart Rhythm Society. Circulation 112:e154-e235, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Radford MJ, Arnold JM, Bennett SJ, et al: ACC/AHA key data elements and definitions for measuring the clinical management and outcomes of patients with chronic heart failure: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Heart Failure Clinical Data Standards)—Developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: Endorsed by the Heart Failure Society of America. Circulation 112:1888-1916, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Ng R, Better N, Green MD: Anticancer agents and cardiotoxicity. Semin Oncol 33:2-14, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Singal PK, Iliskovic N: Doxorubicin-induced cardiomyopathy. N Engl J Med 339:900-905, 1998 [DOI] [PubMed] [Google Scholar]
- 27.van Dalen EC, van der Pal HJ, Kok WE, et al: Clinical heart failure in a cohort of children treated with anthracyclines: A long-term follow-up study. Eur J Cancer 42:3191-3198, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Uderzo C, Pillon M, Corti P, et al: Impact of cumulative anthracycline dose, preparative regimen and chronic graft-versus-host disease on pulmonary and cardiac function in children 5 years after allogeneic hematopoietic stem cell transplantation: A prospective evaluation on behalf of the EBMT Pediatric Diseases and Late Effects Working Parties. Bone Marrow Transplant 39:667-675, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Hudson MM, Rai SN, Nunez C, et al: Noninvasive evaluation of late anthracycline cardiac toxicity in childhood cancer survivors. J Clin Oncol 25:3635-3643, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Reference deleted
- 31.Deeg HJ, Friedman D, Bohr VA, et al: Transplantation and aging. Biol Blood Marrow Transplant 12:893-898, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Majhail NS, Ness KK, Burns LJ, et al: Late effects in survivors of Hodgkin and non-Hodgkin lymphoma treated with autologous hematopoietic cell transplantation: A report from the bone marrow transplant survivor study. Biol Blood Marrow Transplant 13:1153-1159, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lipshultz SE, Lipsitz SR, Mone SM, et al: Female sex and drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N Engl J Med 332:1738-1743, 1995 [DOI] [PubMed] [Google Scholar]
- 34.Lee YT, Chan KK, Harris PA: Tissue disposition of doxorubicin in experimental animals. Med Pediatr Oncol 10:259-267, 1982 [DOI] [PubMed] [Google Scholar]
- 35.Rodvold KA, Rushing DA, Tewksbury DA: Doxorubicin clearance in the obese. J Clin Oncol 6:1321-1327, 1988 [DOI] [PubMed] [Google Scholar]
- 36.Piazza E, Natale N, Trabattoni A, et al: Plasma and tissue distribution of adriamycin in patients with pelvic cancer. Tumori 67:533-537, 1981 [DOI] [PubMed] [Google Scholar]