Abstract
Glycine receptors (GlyRs) play important roles in regulating hippocampal neural network activity and spinal nociception. Here we show that, in cultured rat hippocampal (HIP) and spinal dorsal horn (SDH) neurons, 17-β-estradiol (E2) rapidly and reversibly reduced the peak amplitude of whole-cell glycine-activated currents (IGly). In outside-out membrane patches from HIP neurons devoid of nuclei, E2 similarly inhibited IGly, suggesting a non-genomic characteristic. Moreover, the E2 effect on IGly persisted in the presence of the calcium chelator BAPTA, the protein kinase inhibitor staurosporine, the classical ER (i.e. ERα and ERβ) antagonist tamoxifen, or the G-protein modulators, favoring a direct action of E2 on GlyRs. In HEK293 cells expressing various combinations of GlyR subunits, E2 only affected the IGly in cells expressing α2, α2β or α3β subunits, suggesting that either α2-containing or α3β-GlyRs mediate the E2 effect observed in neurons. Furthermore, E2 inhibited the GlyR-mediated tonic current in pyramidal neurons of HIP CA1 region, where abundant GlyR α2 subunit is expressed. We suggest that the neuronal GlyR is a novel molecular target of E2 which directly inhibits the function of GlyRs in the HIP and SDH regions. This finding may shed new light on premenstrual dysphoric disorder and the gender differences in pain sensation at the CNS level.
Background
Studies over the last several decades have demonstrated that estrogen plays an important role in not only reproduction, but also regulation of early CNS development [1] and in synaptic plasticity of the mature hippocampus [2]. The classical estrogen actions in the CNS are primarily mediated by activating nuclear estrogen receptor α and β (ERα/β), causing long-term genomic effects [3,4]. Nevertheless, it is becoming increasingly clear that estrogen can activate cytoplasmic signaling events at or near the plasma membrane [5,6], presumably through either membrane-localized classical ERs [7,8] or novel ERs [9-11]. Moreover, estradiol is reported to directly bind to and modulate certain ion channels, like the Maxi-K channels [12], indicating the existence of additional estrogen targets besides ERs. In the hippocampus, both in vivo [13] and in vitro [14-16] studies have focused on the inhibitory GABAergic machineries, and suggested that estradiol alters neuronal activity by suppressing GABAergic synaptic transmission. A recent study also indicated that estradiol inhibits human recombinant rho1 GABAC receptor [17].
Like GABAA receptors (GABAARs), the major receptor mediating central inhibition, GlyRs contribute to neuronal inhibition in hippocampus [18-20] and spinal cord [21,22]. GlyRs are pentamers and composed of α(1–4) and β subunits [21]. In hippocampal (HIP) neurons, GlyRs are thought to be primarily the homopentamer of α2 subunits that function extrasynaptically to produce tonic inhibition [21]. Tonic activation of GlyRs leads to cross-inhibition of GABAARs [23], and influences synaptic activity [18,24,25] and short-term plasticity [19]. In adult spinal dorsal horn (SDH), GlyRs are important in regulating nociception and motor function. For example, α3-containing GlyRs regulate inflammatory pain sensitization [26]. Interestingly, during the development of the spinal cord, there is a switch of GlyR subunit composition from α2 in the fetus to α1 predominance in the adult [21,27], suggesting a role of the α2 subunit in neuronal development. Indeed, two recent studies showed that GlyRs play an important role in rod photoreceptor development of the vertebrate retina [28] and regulate spinal interneuron differentiation in zebrafish [29]. On the other hand, estrogen is locally synthesized in the CNS [30] and the level of estrogen is under regulation [1,31]. A recent study showed that estradiol enhances the spontaneous synaptic release of glycine in hypoglossal motoneurones [32]. However, the estradiol effects on GlyRs remain unexplored. In this study, therefore, we examined the modulatory effects of 17-β-estradiol (E2), the most prevalent and potent form of endogenous estrogen, on native GlyRs in HIP and SDH neurons, and on recombinant GlyRs expressed in HEK293 cells. This study will add a new dimension for understanding the multifaceted estrogenic effects in the CNS.
Results
17-β-estradiol rapidly inhibits glycine-activated current (IGly) in cultured rat SDH and HIP neurons
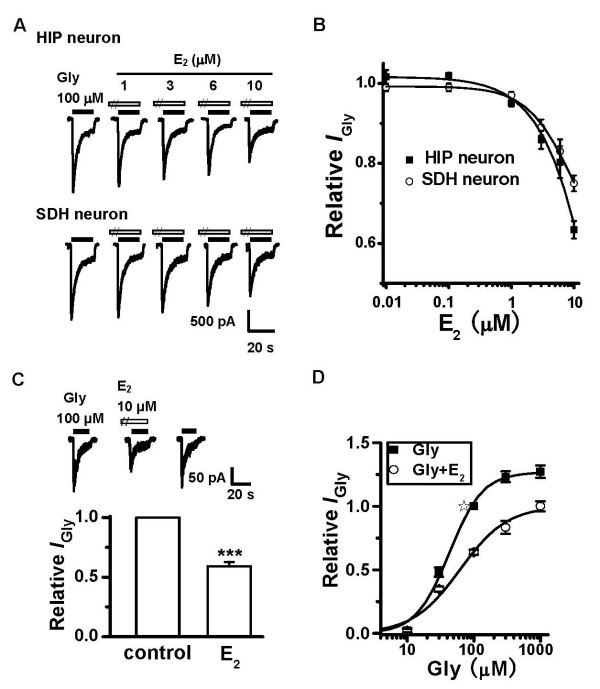
At a holding potential (VH) of -50 mV under whole-cell voltage clamp, application of glycine (100 μM) to the cultured HIP or SDH neurons elicited an inward current. The strychnine sensitivity and chloride dependence of the IGly suggests that it was mediated by GlyR-chloride channels (data not shown). After recording a stable control IGly, we pre-superfused the neurons with E2 at various concentrations for 30 s, and then recorded IGly in the presence of E2. The peak amplitude of IGly was rapidly reduced by E2 application (Figure 1A), while it was not further inhibited when the pre-perfusion time was prolonged (data not shown). In neurons derived from neonatal rats of both sexes, E2 exerted a similar inhibitory effect on IGly, therefore data from both sexes were pooled for comparison. As shown in Figure 1B, E2 concentration-dependently inhibited the peak IGly. On average, E2 at 1, 3, 6 and 10 μM significantly reduced peak IGly to 95.0 ± 0.8% (P < 0.05 compared with control, n = 4), 85.8 ± 2.2% (P < 0.01 compared with control, n = 5), 80.2 ± 4.0% (P < 0.01 compared with control, n = 5) and 63.4 ± 2.2% (P < 0.001 compared with control, n = 8, Paired Student's t-test for all) of control in HIP neurons, respectively; and in SDH neurons, peak IGly was reduced to 97.0 ± 0.9% (P < 0.05 compared with control, n = 6), 89.1 ± 2.0% (P < 0.01 compared with control, n = 7), 83.1 ± 3.0% (P < 0.01 compared with control, n = 8) and 75.3 ± 2.1% (P < 0.01 compared with control, n = 7, P < 0.01 compared with the inhibition of 10 μM E2 produced in HIP neuron) of control, respectively. The IC50 values of E2 for IGly of the HIP neuron and SDH neuron are 16.5 ± 2.7 μM and 33.2 ± 3.4 μM (P < 0.01 compared with that in HIP neuron), respectively. Therefore E2 has stronger inhibitory effect on IGly mediated by hippocampal GlyR. To assess possible contaminations of endogenous steroids derived from glial cells [30,33], we examined the E2 effect in cultures grown without blocking the proliferation of glial cells and found that E2 still inhibited IGly under such a condition (data not shown).
Figure 1.
E2-induced inhibition of IGly in cultured SDH and HIP neurons. (A) Representative traces of current induced by 100 μM glycine in the presence or absence of E2 at various concentrations in cultured HIP (upper) and SDH (bottom) neurons. The neurons were pre-treated with E2 for 30 s before E2 and glycine were co-applied. (B) Summarized data illustrating the concentration dependence of E2 inhibition (n = 4–8) as shown in A. (C) E2 significantly inhibited IGly recorded from the outside-out patches (n = 5). The upper traces show the representative IGly recorded from outside-out patches in the presence and absence of E2. ***P < 0.001, Paired Student's t-test, compared with control without adding E2. (D) The concentration-response curves of IGly in the presence and absence of 10 μM E2. For each neuron recorded, the current was normalized to the peak amplitude of IGly induced by 100 μM glycine alone (✰) from the same neuron and each point represents the average value of 5–9 neurons.
The concentration-effect relationships for E2 shown in Figure 1B indicate that E2 inhibited IGly more markedly in cultured HIP neurons. In the following experiments, therefore, we used HIP neurons to study the mechanisms underlying E2 inhibition. To explore whether a genomic mechanism is responsible for E2 inhibition on IGly, we first examined the effect of E2 in large outside-out patches excised from cultured HIP neurons. The patches were exposed to rapid changes of glycine or glycine plus E2. As shown in Figure 1C, the peak amplitude of IGly was significantly inhibited by E2 (59.2 ± 3.6% of control, P < 0.001, Paired Student's t-test). Since outside-out patches contain no cellular nuclei, this result indicates that E2 inhibited IGly via a non-genomic mechanism. We next examined the concentration-response relationships of IGly in the absence or presence of E2. As shown in Figure 1D, E2 at 10 μM suppressed IGly evoked by both subsaturating and saturating concentrations of glycine. The EC50 and Hill coefficient of glycine were 42.9 ± 4.3 μM and 1.7 in the absence of E2 and 61.7 ± 8.1 μM and 1.2 in the presence of E2, respectively. This result suggests that E2 inhibits IGly in a noncompetitive manner.
No involvement of intracellular signaling pathways and classical ERs
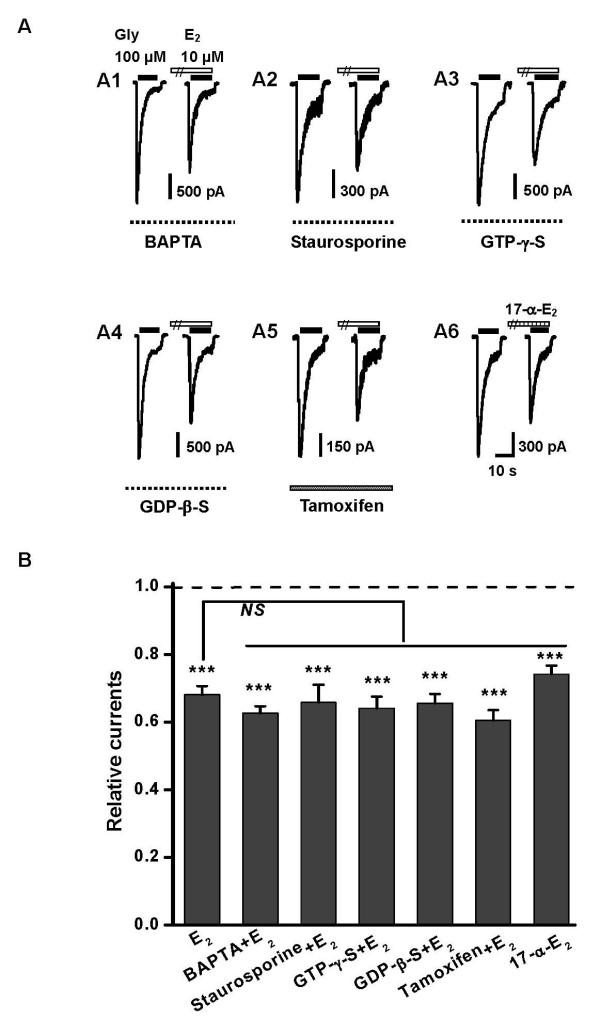
Previous studies have indicated that the acute effect of E2 occurring within a time course of milliseconds to minutes are attributed to the activation of intracellular signaling pathways mediated by presumably membrane-bound classical ERs [7,8,34,35] or novel ERs [9,10]. Additionally, E2 can modulate calcium channels and affect the intracellular calcium level [36,37]. To explore the possible involvement of any intracellular pathways in mediating E2 inhibition of IGly, following experiments were conducted. We first examined the role of the intracellular Ca2+. When neurons were loaded with 15 mM BAPTA via the recording pipette, E2 reduced the peak IGly to 62.7 ± 2.0% of the control, which was not significantly different from that obtained in the absence of BAPTA (Figure 2A1 and 2B1, P > 0.05, Unpaired Student's t-test). In order to test the role of protein phosphorylation and dephosphorylation in E2 inhibition, we loaded the neurons with staurosporine (5 μM), a nonselective protein kinase inhibitor, to disrupt the balance between phosphorylation and dephosphorylation. Likewise, the inhibitory effect of E2 on IGly was not altered (Figure 2A2 and 2C1, P > 0.05, Unpaired Student's t-test). A previous study [38] demonstrated that the GlyR is a target of the G protein βγ dimer. To examine the role of G proteins, we loaded the neurons with GTP-γ-S (500 μM) or GDP-β-S (500 μM) to activate or block the G protein, respectively. Neither of these treatments affected the inhibition induced by E2 on IGly (Figure 2A3, A4, and 2C, P > 0.05, Unpaired Student's t-test). Thus, it is unlikely that E2 exerts its inhibition on IGly through intracellular signaling pathways.
Figure 2.
E2-induced inhibition of IGly is independent of intracellular signaling pathways and classical estrogen receptors (ERs). (A) Sample traces illustrating the inhibitory effects of E2 on the peak IGly under the conditions of intracellular application 15 mM BAPTA (A1), 5 μM staurosporine (A2), 0.5 mM GTP-γ-S (A3) and 0.5 mM GTP-β-S (A4), respectively. A5, Effect of E2 on IGly after incubation of neurons with tamoxifen for 2 h. A6, Effect of 17-α-E2 on IGly. (B) Pooled data summarizing the effect of E2 on IGly under various conditions shown in A. Each column represents the average values from 4–6 neurons, ***P < 0.001, Paired Student's t-test, compared with control without adding E2 or 17-α-E2 (dashed line). NS indicates no significant difference in this and the following figures.
We further employed tamoxifen, a classical ER antagonist in the hippocampus [15], to examine whether membrane-localized classical ERs were involved in E2 inhibition of IGly. After incubation of 1 μM tamoxifen for 2 h, we examined the effects of E2on IGly in the continued presence of tamoxifen. Though, consistent with the previous study [32], the peak amplitude of IGly induced by 100 μM glycine was decreased after the treatment with tamoxifen (Figure 2A5, note the difference in the scale bar), the inhibitory effect of E2 on IGly was not affected (Figure 4A5 and 4B1, P > 0.05, Unpaired Student's t-test). Moreover, 17-α-estradiol (17-α-E2, 10 μM), the inactive stereoisomer of E2, mimicked the inhibitory effect of E2 on IGly (Figure 2A6 and 2B1, P > 0.05, Unpaired Student's t-test), suggesting that E2 inhibition on GlyR is independent of classical ERs.
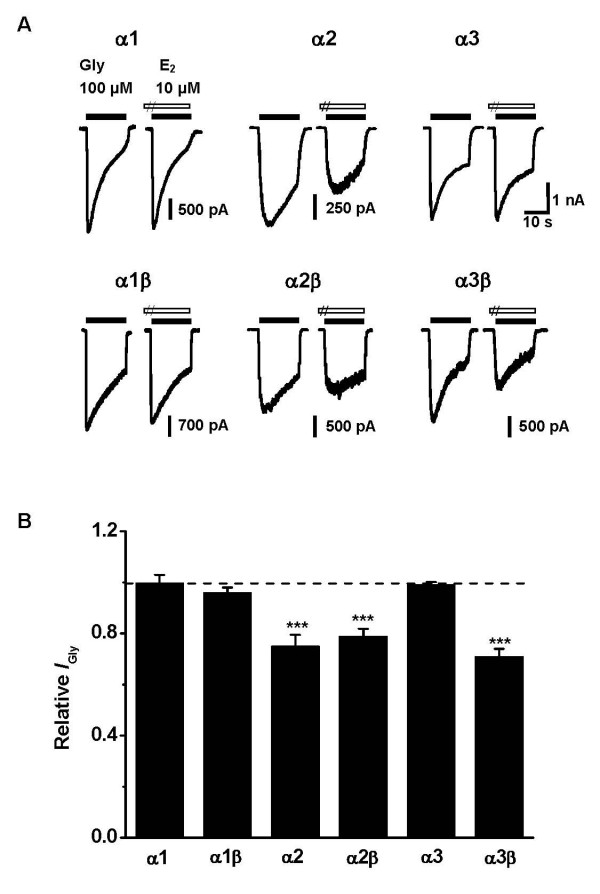
Figure 4.
Inhibitory effect of E2 on recombinant GlyRs. (A) Sample traces demonstrating the effects of 10 μM E2 on various homomeric and heteromeric GlyRs. (B) Summary of results from all experiments similar to those shown in A. E2 selectively inhibited the peak amplitude of IGly mediated by α2-containing GlyRs and α3β heteromeric GlyR. Each column represents the average value of 6–11 neurons. ***P < 0.001, Paired Student's t-test, compared with the control without E2 treatment (dashed line).
Regulatory sites for E2 and pregnanolone on GlyRs are separate
A previous study showed that another neurosteroid, pregnanolone (PGN) directly inhibited IGly in a competitive manner [39]. We were interested to know whether E2 and PGN share a common binding site on GlyRs. If the sites are separate, the inhibitory effects should be additive when E2 and PGN were co-applied. As shown in Figure 3A, PGN at 1 μM and 10 μM significantly inhibited the peak amplitude of IGly by 25.1 ± 7.6% and 49.0 ± 5.9% of control, respectively. In the presence of 10 μM E2, additional inhibition of IGly was observed by PGN at both 1 μM (Figure 3B, 51.3 ± 5.3% of control), and 10 μM (Figure 3B, 70.4 ± 7.3% of control). These data suggest that distinct binding sites may mediate the inhibition of E2 and PGN on IGly.
Figure 3.
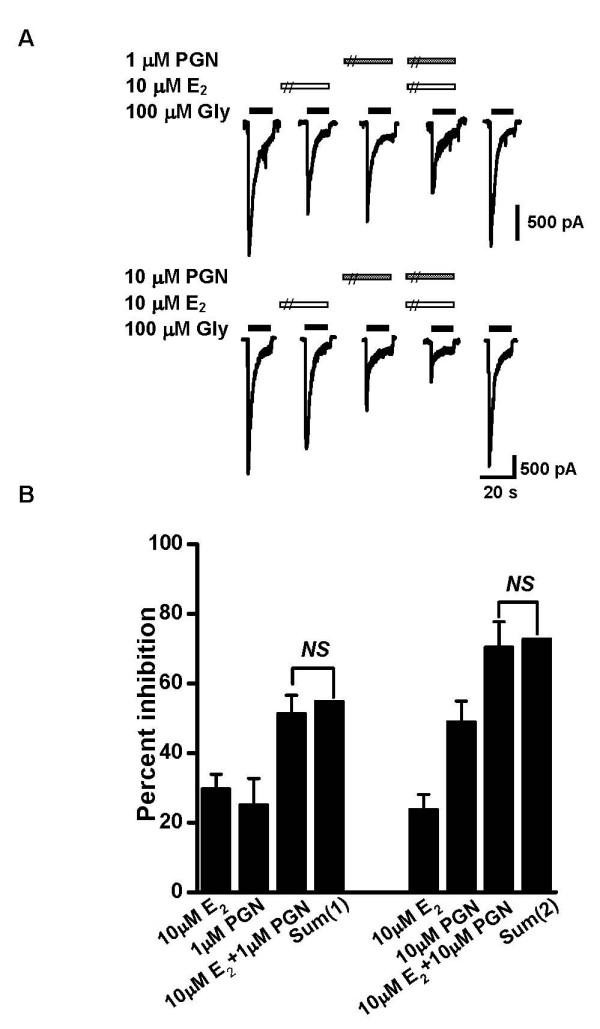
Interactions of E2 and PGN on IGly. (A) Sample traces illustrating the additive effect of E2 and PGN on IGly. (B) Summary of results from all experiments similar to those shown in A (n = 4–5). Sum (1) is the expected linear summation of the inhibition induced by 10 μM E2 and 1 μM PGN; Sum (2) is the expected linear summation of the inhibition induced by 10 μM E2 and 10 μM PGN. P > 0.05, Unpaired Student's t-test.
Subunit selectivity of E2 inhibition on GlyRs
To determine which GlyR subunits are responsible for the E2-induced inhibition, we investigated the E2 effects on IGly in HEK293 cells expressing various recombinant GlyRs (Figure 4A). In GlyR subunit-untransfected cells, no glycine responses were observed (data not shown). As shown in Figure 4, E2 selectively inhibited the peak IGly mediated by homomeric α2-GlyRs to 75.1 ± 4.3% of control (P < 0.001, n = 6, Paired Student's t-test), but had no significant effect on homomeric α1- and α3-GlyRs (99.8 ± 3.1% of control and 99.0 ± 1.2% of control for α1- and α3-GlyRs, respectively, P > 0.05, n = 10, Paired Student's t-test). To further examine whether co-expression of β subunit affected the E2 inhibition, we tested the inhibitory effect of E2 on heteromric GlyRs. We found that E2 inhibited the peak IGly mediated by α2β – and α3β-GlyRs to 78.9 ± 2.9% (P < 0.001, n = 9, Paired Student's t-test) and 71.0 ± 3.0% of control (P < 0.001, n = 11, Paired Student's t-test), respectively. However, IGly mediated by α1β-GlyRs was not significantly affected. Thus, it is likely that either α2- or α2β – or α3β-GlyRs mediate the E2 effect in SDH and HIP neurons.
The developmental changes of E2 inhibition of GlyRs in spinal cord neurons
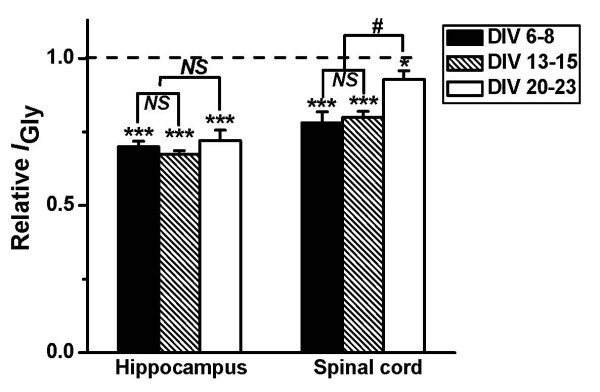
The GlyR subunit expression is developmentally regulated in some areas of CNS such as spinal cord. To test whether E2 effects are different in different times in culture, we investigate the effects of E2 on GlyR in spinal cord and HIP neurons from different days in culture. We first recorded IGly in cultured HIP neurons after different days of in vitro (DIV) differentiation. As shown in Figure 5, the inhibitory extent of E2 on IGly did not change with time in vitro (P > 0.05, comparing DIV6–8, 13–15 and 20–23, n = 5–7, one-way ANOVA). We next examined the effects of E2 on IGly induced by 100 μM glycine of spinal cord neurons in cultures after DIV6–8, 13–15 and 20–23. Interestingly, E2 significantly inhibited IGly of the neurons at DIV6–8 and 13–15, but the inhibitory effects declined in the neurons at DIV20–23 (P = 0.012, comparing between DIV20–23 and DIV6–8; P = 0.023, comparing between DIV20–23 and DIV13–15. n = 6–8, one-way ANOVA).
Figure 5.
Developmental regulation of E2 inhibition on IGly. Hippocampal and spinal cord neurons in culture were used for electrophysiological recording following 6–8, 13–15 and 20–23 days of in vitro (DIV) differentiation to examine the developmental dependence of E2 inhibition on IGly. Each column represents the average value of 5–8 neurons. * P < 0.05 and *** P < 0.001 (Student's paired t test, n = 8), compared to control without adding E2; # P < 0.05 (one-way ANOVA, n = 8), comparing DIV 20–23 with either DIV 6–8 or DIV 13–15.
Inhibitory effect of E2 on GlyR-mediated tonic current in HIP slices
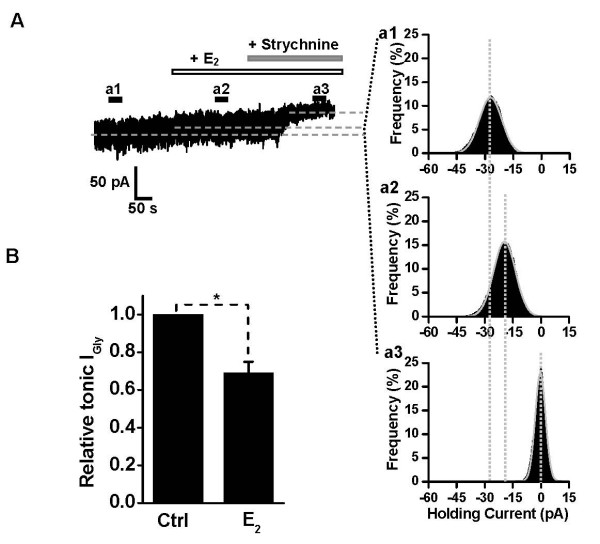
In the developing and mature hippocampus, α2 subunit represents the primary component of the functional GlyRs [21]. This unique property enables us to examine the action of E2 on GlyRs from pyramidal neurons in CA1 region of HIP slices. Glycine concentration in cerebrospinal fluid has been estimated to be in the micromolar range [40]. In order to magnify the current, we recorded the GlyR-mediated tonic current by adding 20 μM glycine to ACSF in addition to the presence of GlyT1 inhibitor sarcosine (0.5 mM) [25]. At the same time, TTX (0.3 μM) was added in the bath to reduce random baseline current fluctuations, and 10 μM bicuculline, 10 μM DL-2-amino-5-phosphovaleric acid (APV) and 3 μM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) were added to block GABAA and ionotropic glutamate receptors, respectively. After recording a control period in the cocktail solution, E2 at 10 μM was superfused (Figure 6A). As shown in Figure 6B, the GlyR-mediated tonic current was significantly reduced to 69.3 ± 5.8% of control by E2 perfusion (P = 0.043, n = 8, Wilcoxon matched-pairs signed-ranks test), a level compatible with that obtained in the cultured neurons (Figure 1B) or HEK 293 cells expressing recombinant α2- and α2β-GlyRs (Figure 4B).
Figure 6.
Inhibitory effect of E2 on GlyR-mediated tonic current in HIP slices. (A) Left, Whole-cell voltage-clamp recording showing the current in the presence of 0.3 μM TTX, 10 μM bicuculline, 3 μM CNQX, 10 μM APV, 20 μM glycine and 0.5 mM sarcosine. Application of strychnine (2 μM) decreased the membrane current noise and revealed the tonically activated GlyR current. In the presence of E2 (10 μM), the amplitude of GlyR-mediated tonic current was significantly reduced. Right (a1–a3), Gaussian fit to all-point histograms of 30 s traces at the time point a1, a2 and a3 (A, inset). The differences among the Gaussian means are marked by the dotted lines. (B) The normalized GlyR-mediated tonic current in the absence or presence of E2 (n = 8). *P < 0.05, Paired Student's t-test, compared with the control without E2 treatment.
Discussion
Here, we present evidence that in cultured HIP and SDH neurons, E2 directly inhibited GlyRs which are likely composed of α2 or α3 subunit. Furthermore, the GlyR-mediated tonic current in HIP slices was reduced by E2. Thus, in addition to the well-known signaling pathways of E2 via ERs, the GlyR may be an additional central target of E2. Since GlyRs are important regulators of spinal nociception [26,41,42], and affect HIP network activity [19,20], our findings may shed new light on the gender differences in pain sensation at the CNS level.
Mechanisms underlying E2 inhibition of GlyRs
Estrogen exerts effects in the CNS primarily by activating ERs [3]. In this study, however, several lines of evidence suggest that E2 inhibited IGly through a direct interaction with plasma membrane GlyRs. First, the effects of E2 are most likely non-genomic as the inhibition occurred within seconds following E2 application and persisted in large outside-out patches devoid of nuclei. Second, it is unlikely that E2 physically disrupts the plasma membrane via a nonspecific way as E2 only affected α2 subunit-containing and heteromeric α3β-GlyRs. Moreover, when E2 was applied, the membrane capacitance remained unchanged, indicating that the membrane structure was unaltered (see Materials and Methods). Third, E2 inhibitory effect persisted in the presence of tamoxifen, the antagonist of classical ERs [43], or 17-α-E2, the inactive stereoisomer of E2 [44]. In addition, E2 inhibited the recombinant GlyRs expressed in HEK293 cells which are devoid of classical ERs [45]. Finally, in the presence of the calcium chelator, the protein kinase inhibitor or G-protein modulators, the E2-induced inhibition remained unchanged. These results strongly support the notion that E2 inhibits GlyRs independent of any intracellular signaling pathways activated by either classical ERs or novel ERs [9-11].
Our data suggest that E2 inhibited IGly in a noncompetitive manner because E2 reduced IGly independent of glycine concentrations, and the effect can be additive with that of PGN, a competitive steroid inhibitor of GlyR without changing the membrane viscosity [39,46]. Two general mechanisms may underlie the noncompetitive inhibition of E2 on GlyR, open-channel blocker or allosteric modulation. Nevertheless, E2 is not charged at physiological pH and inhibited IGly independent of membrane potentials (P.J. and T.L.X., unpublished data), which makes it unlikely that E2 acts as an open-channel blocker. Thus, we propose that E2 directly binds to and allosterically inhibits GlyRs.
Functional implications
Generally, the physiological concentration of estradiol in plasma is in the nanomolar range [6]. In the present study, the concentrations at which E2 exerted significant effects on GlyRs were much higher than its physiological level. However, in the CNS of both male and female rats, estrogen can be de novo synthesized and accumulated locally [30,47], leading to E2 concentration in the cerebrospinal fluid far surpass those measured in plasma. For example, a previous study has demonstrated that the concentration of estradiol in the hippocampus of male rats is six-fold higher than that in plasma [48]. More interestingly, a recent study indicates that forebrain estradiol levels in zebra finches are also acutely increased during social interactions [49]. In addition, certain cofactors, like sexual hormone-binding proteins, can make E2 to achieve an effect even at low concentrations [50]. Therefore, it is reasonable to speculate that, under certain physiological or pathological condition, locally elevated endogenous estrogen may have an impact on GlyRs through the process revealed in this study.
In HEK293 cells expressing recombinant GlyRs, we found that E2 significantly inhibited α2-GlyRs. Accumulative evidence demonstrates that the α2-GlyRs distribute throughout the developing CNS and in the mature hippocampus [21,51,52]. In CA1 region of HIP slices, we observed that GlyR-mediated tonic currents, which are likely mediated by α2-GlyRs [51-53], were reduced to a level compatible with that measured in cultured neurons or HEK 293 cells expressing recombinant GlyRs. The tonic activation of the α2 subunit-containing GlyRs is functionally important in the development of the cortex [54], the retina [28] and the spinal cord [29]. Furthermore, previous studies have shown that GlyR subunit composition of spinal cord neurons changes during development from α2 to α1 predominance both in cultures and in in vivo conditions [27,55]. Because of this switch of GlyR subunit composition in the spinal cord during development, a previous study [56] suggested that the selective inhibition of progesterone on α2-GlyRs endows an important role of progesterone in the developing spinal cord. Since the levels of estrogen fluctuate in both males and females during brain development [31], E2-induced inhibition of GlyRs may contribute to the modulation of estrogen on the early CNS development. It remains unexplored whether GlyRs also participate in regulation of neuronal excitability, through E2-induced disinhibition in the mature hippocampus [14].
Under certain disease conditions, for example, premenstrual dysphoric disorder (PMDD), anxiety is enhanced when neuronal excitability is disturbed during the ovarian cycle [57]. A recent study reported that the periodic alterations of the GABAAR-mediated tonic inhibition, which resulted from the fluctuation of hormones in the hippocampus during estrous cycle, play a crucial role in altered seizure susceptibility and anxiety during PMDD [58]. Similar to the tonic GABAergic inhibition, GlyRs-mediated tonic currents modulate neuronal excitation and network function [18,19,24,25]. Our present data showed that E2 can inhibit more than 30% of the tonic IGly in hippocampal slices. Therefore, the significant inhibition of IGly produced by E2 may have strong effect on the neuronal excitability, suggesting an alternative cellular mechanism by which periodic sex hormone fluctuations affect CNS neuronal excitation and cause mood disorders. Considering the ubiquitous distribution profiles of GABAARs in the CNS and the hypnotic side-effect of GABA-mimic drugs, the present finding suggests new directions for treatment of PMDD by using GlyR-enhancing drugs and/or glycine reuptake inhibitors [25].
Besides, sex-related difference in pain perception has been revealed by previous studies which indicate that noxious stimuli are perceived as more painful by women than by men [13,43]. Although sex hormones have been shown to be involved in this difference, the underlying mechanisms are still unknown. Functional α3β-GlyRs exist in inhibitory synapse of SDH neurons, which is the first site for integration, relay and modulation of nociceptive information from nociceptor [59]. Moreover, previous studies have suggested that α3β-GlyRs in the SDH neurons regulate inflammatory pain sensitization [26,41]. In this study, we noted that E2 significantly inhibited IGly in cultured SDH neurons and the current mediated by α3β-GlyRs expressed in HEK293 cells, providing important insights into the mechanisms underlying gender differences in pain sensation at the CNS level.
Finally, several studies indicate that the expression of high affinity GlyR subunit α2192L and α3185L produced by RNA editing [60] was increased in the brain after experimentally induced brain lesion of rat [61] and in temporal lobe epilepsy patient with a severe course of disease [62]. According to the selectivity of E2 inhibition on α2 and α3-containing GlyR, E2 thus may play a role through modulating these high affinity GlyRs in some pathological situations such as temporal lobe epilepsy and brain damage.
Conclusion
We demonstrated that the neuronal GlyR is a novel molecular target of E2 which directly inhibits the function of GlyRs in the HIP and SDH regions. Through their impact on GlyRs in the CNS, sex hormones may regulate neuronal excitability and contribute to premenstrual dysphoric disorder and sex-related pain sensation under both physiological and pathological conditions.
Methods
The care and use of animals in these experiments followed the guidelines of the Institutional Animals Care and Use Committee of the Institute of Neuroscience, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences. All efforts were made to minimize the number of animals used and their suffering.
Cell culture
Cultures of HIP neurons were prepared as previously described [63] with some modifications. Briefly, hippocampus from neonatal (< 24-hour-old) Sprague-Dawley rats with identified sex were dissociated in Ca2+-free saline with sucrose (20 mM) and hippocampal neurons were isolated using a standard enzyme treatment protocol. Cultures of SDH neurons were prepared from embryonic day 15 (E15) Sprague-Dawley rats as previously described [39]. The neurons were plated (1–5 × 105 cell/ml) on poly-L-lysine (Sigma, USA) coated cover glasses and grown in Dulbecco's modified Eagle's medium (DMEM, Gibco, USA) with L-glutamine plus 10% fetal bovine serum (Gibco). The neurons were allowed to attach the cover glasses for 24 h, after which the medium was changed to neuron-basal medium (1.5 ml, Gibco) with 2% B27 (Gibco) and replaced every 3–4 days. Treatment with 5-fluoro-5'-deoxyuridine (20 μg/ml, Sigma, St. Louis, MO) on the fourth day after plating was used to block cell division of non-neuronal cells, which helped to stabilize the cell population. The cultures were maintained at 37°C in a 5% CO2 humidified atmosphere. Cells were used for electrophysiological recordings 7–23 days after plating.
Slice preparation
Experiments were performed on 400 μm transverse hippocampal slices from 14- to 17-day-old Sprague-Dawley rats. After decapitation, the brain was removed and placed in oxygenated (95% O2/5% CO2) artificial cerebrospinal fluid (ACSF) at 4°C. Slices were cut from the dorsal hippocampus with a vibratome (Leika VT 1000S) and maintained at room temperature (23–25°C) in a holding chamber filled with oxygenated ACSF. After an equilibration period of at least 2 h, a single slice was transferred to the recording chamber, where it was continuously perfused with oxygenated ACSF (23–25°C) at a flow rate of 2.5–3 ml/min.
Expression of recombinant GlyRs
All constructs were expressed in HEK293 cells as previously reported [39]. In brief, HEK293 cells were cultured at 37°C in a humidified atmosphere of 5% CO2 and 95% air. Cells were maintained in DMEM supplemented with 2 mM L-glutamine, 10% fetal bovine serum, and 100 units/ml penicillin/streptomycin (all from Invitrogen). Transient transfection of HEK293 cells was carried out by using the Lipofectamine 2000 reagent (Invitrogen) according to the supplied protocol. Co-transfection with a green fluorescent protein expression vector, pEGFP-N1, was used to enable identification of transfected cells for patch clamping in some experiments. When co-transfecting the GlyR α and β subunits, their respective cDNAs were combined in a ratio of 1:2 to ensure the formation of functional heterooligomers. Taking advantage of the insensitivity of αβ heteromeric GlyR to picrotoxin [64], we further tested the picrotoxin sensitivity to ensure the formation of heterooligomers. After exposure to transfection solution for 6 h, cells were washed twice using the culture medium and used for electrophysiological recordings over following 16–48 h.
Solutions and drugs
The standard external solution for cultured neurons recording contained (mM): 150 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 10 N-hydroxyethylpiperazine-NV-2-ethanesulphonic acid (HEPES), 10 Glucose (pH 7.4; osmolarity 310–320 mOsm/l). The patch pipette solution for cultured neurons recording was (mM): 120 KCl, 30 NaCl, 1 MgCl2, 0.5 CaCl2, 5 EGTA, 2 Mg-ATP, 10 HEPES. The internal solution was adjusted to pH 7.2 with Tris-base. The ACSF for slices incubation and recording was composed of (mM): 126 NaCl, 2.5 KCl, 2 MgSO4, 1.25 NaH2PO4, 26 NaHCO3, 2 CaCl2 and 10 D-glucose, aerated with 95% O2 and 5% CO2 at a final pH of 7.4. The osmolarity of the ACSF was 289–295 mOsm/l. The ionic composition of the internal solution for slice recording was (mM): 140 CsCl, 1 MgCl2, 10 HEPES, 0.1 EGTA, 4 NaCl, 2 MgATP and 0.3 NaGTP, adjusted to pH 7.2. Drugs used in the present experiments were purchased from Sigma. Steroidal agents were initially dissolved as concentrated stock solutions in dimethylsulphoxide (DMSO) and subsequently diluted to the desired concentration in standard external solution. The final concentration of DMSO employed in the experiments was always ≤ 0.1%, which had no detectable effect on IGly in vehicle control experiments. Other drugs were first dissolved in ion-free water and then diluted to the final concentrations in the standard external solution or ACSF just prior to use. Unless otherwise indicated, drugs were applied using a rapid application technique termed the 'Y-tube' method throughout the experiments. This system allows a complete exchange of external solution surrounding a neuron within 20 ms [65].
Electrophysiological recordings and data analysis
Conventional whole-cell patch-clamp recording was performed under voltage-clamp conditions. Patch pipettes were pulled from glass capillaries with an outer diameter of 1.5 mm on a two-stage puller (PP-830, Narishige, Tokyo, Japan). The resistance between the recording electrode filled with pipette solution and the reference electrode was 4–6 MΩ. Membrane currents were measured using a patch-clamp amplifier (Axon 200B, Axon Instruments, Foster City, CA, USA), sampled and analyzed using a Digidata 1320A interface and a personal computer with Clampex and Clampfit software (Version 9.0.1, Axon Instruments). In most experiments, 70–90% series resistance was compensated. To make outside-out patch, after obtaining the whole-cell recording configuration, the patch was excised by carefully withdrawing the patch pipette from the cell [66]. Additionally, in some experiments that drugs were applied in pipette, the current was measured at least 5 minutes after whole-cell configuration was established to ensure cell dialysis [67]. Unless otherwise indicated, the membrane potential was held at -50 mV throughout the experiment. All experiments were carried out at room temperature (22–25°C).
Membrane capacitance measurements
Whole-cell voltage clamp was used to step membrane potential from -70 to +20 mV. A cesium based pipette solution was used to block voltage-gated potassium channels. The capacitance transient was recorded in HEK293 cell expressing α2-GlyRs in the absence or presence of 10 μM E2. According to the calculation method of capacitance provided by the previous study [46], the capacitance of HEK293 cell expressing α2-GlyRs was estimated to be 33.3 ± 2.2 pF and 33.2 ± 2.4 pF in the absence or presence of E2, respectively. The membrane capacitance was not changed by E2, indicating that the alteration of plasma membrane capacitance was not involved in E2 inhibition of GlyRs.
Data analysis
Clampfit software was used for data analysis. The continuous theoretical curves for concentration-response relationship of glycine in the presence or absence of steroids were drawn according to a modified Michaelis-Menten equation by the method of least-squares (the Newton-Raphson method) after normalizing the amplitude of the response:
| I = Imax Ch/(Ch + EC50 h) |
where I is the normalized value of the current, Imax the maximal response, C the drug concentration, EC50 the concentration which induced the half-maximal response and h the apparent Hill coefficient. The curve for the effect of E2 on IGly was fitted using the following equation:
| I = Imax (IC50)h/(Ch + IC50 h) |
where IC50 represents the concentration that induced the half-maximal inhibitory effect.
For analysis of the GlyR-mediated tonic currents in HIP slices, all-point histograms of 30 s epochs at period a1, a2 and a3 was used to measure the baseline current in different condition of drug application (see Figure 6A), and a Gaussian distribution was fitted to the histogram at period a1, a2 and a3. The GlyR-mediated tonic current was revealed by strychnine application. Therefore, the difference between the means of the fitted Gaussians at period a1 and a3 represents the GlyR-mediated tonic current in the absence of E2. Similarly, the difference between period a2 and a3 represents the GlyR-mediated tonic current in the presence of E2.
The statistical comparisons were carried out by using Student's t-test for two groups' comparison, and one-way analysis of variance (ANOVA) for multiple comparisons. All data were reported as the mean ± standard error (S.E.M.). P and n represent the value of significance and the number of neurons, respectively. Statistically significant differences were assumed as P < 0.05.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
PJ and YK carried out all electrophysiological experiments and wrote the manuscript. XBZ participated in the electrophysiological experiment in slices and revised the manuscript. WW participated in cell culture. TLX and CFL conceived of the study, and participated in its design and coordination. All authors read and approved the final manuscript.
Contributor Information
Peng Jiang, Email: ppjiang@mail.ustc.edu.cn.
Yan Kong, Email: kong0919@163.com.
Xiao-Bing Zhang, Email: xbzhang@ion.ac.cn.
Wei Wang, Email: wang2002@mail.ustc.edu.cn.
Chun-Feng Liu, Email: liucf20@hotmail.com.
Tian-Le Xu, Email: tlxu@ion.ac.cn.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 30621062), the National Basic Research Program of China (No. 2006CB500803) and the Knowledge Innovation Project from the Chinese Academy of Sciences (KSCX2-YW-R-35). We thank Drs. Neng Gong and James Celentano for discussion.
References
- Beyer C. Estrogen and the developing mammalian brain. Anat Embryol (Berl) 1999;199:379–390. doi: 10.1007/s004290050236. [DOI] [PubMed] [Google Scholar]
- Prange-Kiel J, Rune GM. Direct and indirect effects of estrogen on rat hippocampus. Neuroscience. 2006;138:765–772. doi: 10.1016/j.neuroscience.2005.05.061. [DOI] [PubMed] [Google Scholar]
- Maggi A, Ciana P, Belcredito S, Vegeto E. Estrogens in the nervous system: mechanisms and nonreproductive functions. Annu Rev Physiol. 2004;66:291–313. doi: 10.1146/annurev.physiol.66.032802.154945. [DOI] [PubMed] [Google Scholar]
- Nadal A, Diaz M, Valverde MA. The estrogen trinity: membrane, cytosolic, and nuclear effects. News Physiol Sci. 2001;16:251–255. doi: 10.1152/physiologyonline.2001.16.6.251. [DOI] [PubMed] [Google Scholar]
- Collins P, Webb C. Estrogen hits the surface. Nat Med. 1999;5:1130–1131. doi: 10.1038/13453. [DOI] [PubMed] [Google Scholar]
- Edwards DP. Regulation of signal transduction pathways by estrogen and progesterone. Annu Rev Physiol. 2005;67:335–376. doi: 10.1146/annurev.physiol.67.040403.120151. [DOI] [PubMed] [Google Scholar]
- Levin ER. Cellular functions of plasma membrane estrogen receptors. Steroids. 2002;67:471–475. doi: 10.1016/S0039-128X(01)00179-9. [DOI] [PubMed] [Google Scholar]
- Abraham IM, Todman MG, Korach KS, Herbison AE. Critical in vivo roles for classical estrogen receptors in rapid estrogen actions on intracellular signaling in mouse brain. Endocrinology. 2004;145:3055–3061. doi: 10.1210/en.2003-1676. [DOI] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Ronnekleiv OK, Kelly MJ. Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J Neurosci. 2003;23:9529–9540. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, Connolly ES Jr, Nethrapalli IS, Tinnikov AA. ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J Neurosci. 2002;22:8391–8401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal A, Ropero AB, Fuentes E, Soria B. The plasma membrane estrogen receptor: nuclear or unclear? Trends Pharmacol Sci. 2001;22:597–599. doi: 10.1016/S0165-6147(00)01846-0. [DOI] [PubMed] [Google Scholar]
- Valverde MA, Rojas P, Amigo J, Cosmelli D, Orio P, Bahamonde MI, Mann GE, Vergara C, Latorre R. Acute activation of Maxi-K channels (hSlo) by estradiol binding to the beta subunit. Science. 1999;285:1929–1931. doi: 10.1126/science.285.5435.1929. [DOI] [PubMed] [Google Scholar]
- Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, Fillingim RB, Gold MS, Holdcroft A, Lautenbacher S, Mayer EA. et al. Studying sex and gender differences in pain and analgesia: a consensus report. Pain. 2007;132(Suppl 1):S26–45. doi: 10.1016/j.pain.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudick CN, Woolley CS. Estrogen regulates functional inhibition of hippocampal CA1 pyramidal cells in the adult female rat. J Neurosci. 2001;21:6532–6543. doi: 10.1523/JNEUROSCI.21-17-06532.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudick CN, Gibbs RB, Woolley CS. A role for the basal forebrain cholinergic system in estrogen-induced disinhibition of hippocampal pyramidal cells. J Neurosci. 2003;23:4479–4490. doi: 10.1523/JNEUROSCI.23-11-04479.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledoux VA, Woolley CS. Evidence that disinhibition is associated with a decrease in number of vesicles available for release at inhibitory synapses. J Neurosci. 2005;25:971–976. doi: 10.1523/JNEUROSCI.3489-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Jin X, Covey DF, Steinbach JH. Neuroactive steroids and human recombinant rho1 GABAC receptors. J Pharmacol Exp Ther. 2007;323:236–247. doi: 10.1124/jpet.107.127365. [DOI] [PubMed] [Google Scholar]
- Song W, Chattipakorn SC, McMahon LL. Glycine-gated chloride channels depress synaptic transmission in rat hippocampus. J Neurophysiol. 2006;95:2366–2379. doi: 10.1152/jn.00386.2005. [DOI] [PubMed] [Google Scholar]
- Zhang LH, Xu L, Xu TL. Glycine receptor activation regulates short-term plasticity in CA1 area of hippocampal slices of rats. Biochem Biophys Res Commun. 2006;344:721–726. doi: 10.1016/j.bbrc.2006.03.198. [DOI] [PubMed] [Google Scholar]
- Zhang LH, Gong N, Fei D, Xu L, Xu TL. Glycine Uptake Regulates Hippocampal Network Activity via Glycine Receptor-Mediated Tonic Inhibition. Neuropsychopharmacology. 2008;33(3):701–711. doi: 10.1038/sj.npp.1301449. [DOI] [PubMed] [Google Scholar]
- Lynch JW. Molecular structure and function of the glycine receptor chloride channel. Physiol Rev. 2004;84:1051–1095. doi: 10.1152/physrev.00042.2003. [DOI] [PubMed] [Google Scholar]
- Graham BA, Schofield PR, Sah P, Callister RJ. Altered inhibitory synaptic transmission in superficial dorsal horn neurones in spastic and oscillator mice. J Physiol. 2003;551:905–916. doi: 10.1113/jphysiol.2003.049064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Xu TL. State-dependent cross-inhibition between anionic GABA(A) and glycine ionotropic receptors in rat hippocampal CA1 neurons. Neuroreport. 2002;13:223–226. doi: 10.1097/00001756-200202110-00010. [DOI] [PubMed] [Google Scholar]
- Chattipakorn SC, McMahon LL. Strychnine-sensitive glycine receptors depress hyperexcitability in rat dentate gyrus. J Neurophysiol. 2003;89:1339–1342. doi: 10.1152/jn.00908.2002. [DOI] [PubMed] [Google Scholar]
- Zhang LH, Gong N, Fei D, Xu L, Xu TL. Glycine uptake regulates hippocampal network activity via glycine receptor-mediated tonic inhibition. Neuropsychopharmacology. 2008;33:701–711. doi: 10.1038/sj.npp.1301449. [DOI] [PubMed] [Google Scholar]
- Harvey RJ, Depner UB, Wassle H, Ahmadi S, Heindl C, Reinold H, Smart TG, Harvey K, Schutz B, Abo-Salem OM. et al. GlyR alpha3: an essential target for spinal PGE2-mediated inflammatory pain sensitization. Science. 2004;304:884–887. doi: 10.1126/science.1094925. [DOI] [PubMed] [Google Scholar]
- Aguayo LG, van Zundert B, Tapia JC, Carrasco MA, Alvarez FJ. Changes on the properties of glycine receptors during neuronal development. Brain Res Brain Res Rev. 2004;47:33–45. doi: 10.1016/j.brainresrev.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Young TL, Cepko CL. A role for ligand-gated ion channels in rod photoreceptor development. Neuron. 2004;41:867–879. doi: 10.1016/S0896-6273(04)00141-2. [DOI] [PubMed] [Google Scholar]
- McDearmid JR, Liao M, Drapeau P. Glycine receptors regulate interneuron differentiation during spinal network development. Proc Natl Acad Sci USA. 2006;103:9679–9684. doi: 10.1073/pnas.0504871103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rune GM, Frotscher M. Neurosteroid synthesis in the hippocampus: role in synaptic plasticity. Neuroscience. 2005;136:833–842. doi: 10.1016/j.neuroscience.2005.03.056. [DOI] [PubMed] [Google Scholar]
- Amateau SK, Alt JJ, Stamps CL, McCarthy MM. Brain estradiol content in newborn rats: sex differences, regional heterogeneity, and possible de novo synthesis by the female telencephalon. Endocrinology. 2004;145:2906–2917. doi: 10.1210/en.2003-1363. [DOI] [PubMed] [Google Scholar]
- Chesnoy-Marchais D, Meillerais A. Oestradiol rapidly enhances spontaneous glycinergic synaptic inhibition of hypoglossal motoneurones. J Neuroendocrinol. 2008;20:233–244. doi: 10.1111/j.1365-2826.2007.01635.x. [DOI] [PubMed] [Google Scholar]
- Hu R, Cai WQ, Wu XG, Yang Z. Astrocyte-derived estrogen enhances synapse formation and synaptic transmission between cultured neonatal rat cortical neurons. Neuroscience. 2007;144:1229–1240. doi: 10.1016/j.neuroscience.2006.09.056. [DOI] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERalpha and ERbeta expressed in Chinese hamster ovary cells. Mol Endocrinol. 1999;13:307–319. doi: 10.1210/me.13.2.307. [DOI] [PubMed] [Google Scholar]
- Clarke CH, Norfleet AM, Clarke MS, Watson CS, Cunningham KA, Thomas ML. Perimembrane localization of the estrogen receptor alpha protein in neuronal processes of cultured hippocampal neurons. Neuroendocrinology. 2000;71:34–42. doi: 10.1159/000054518. [DOI] [PubMed] [Google Scholar]
- Lee DY, Chai YG, Lee EB, Kim KW, Nah SY, Oh TH, Rhim H. 17Beta-estradiol inhibits high-voltage-activated calcium channel currents in rat sensory neurons via a non-genomic mechanism. Life Sci. 2002;70:2047–2059. doi: 10.1016/S0024-3205(01)01534-X. [DOI] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Jamali K, Xue C, Kelly MJ, Ronnekleiv OK. Estrogen upregulates T-type calcium channels in the hypothalamus and pituitary. J Neurosci. 2006;26:11072–11082. doi: 10.1523/JNEUROSCI.3229-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yevenes GE, Peoples RW, Tapia JC, Parodi J, Soto X, Olate J, Aguayo LG. Modulation of glycine-activated ion channel function by G-protein betagamma subunits. Nat Neurosci. 2003;6:819–824. doi: 10.1038/nn1095. [DOI] [PubMed] [Google Scholar]
- Jiang P, Yang CX, Wang YT, Xu TL. Mechanisms of modulation of pregnanolone on glycinergic response in cultured spinal dorsal horn neurons of rat. Neuroscience. 2006;141:2041–2050. doi: 10.1016/j.neuroscience.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Westergren I, Nystrom B, Hamberger A, Nordborg C, Johansson BB. Concentrations of amino acids in extracellular fluid after opening of the blood-brain barrier by intracarotid infusion of protamine sulfate. J Neurochem. 1994;62:159–165. doi: 10.1046/j.1471-4159.1994.62010159.x. [DOI] [PubMed] [Google Scholar]
- Ahmadi S, Lippross S, Neuhuber WL, Zeilhofer HU. PGE(2) selectively blocks inhibitory glycinergic neurotransmission onto rat superficial dorsal horn neurons. Nat Neurosci. 2002;5:34–40. doi: 10.1038/nn778. [DOI] [PubMed] [Google Scholar]
- Zeilhofer HU. The glycinergic control of spinal pain processing. Cell Mol Life Sci. 2005;62:2027–2035. doi: 10.1007/s00018-005-5107-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesenfeld-Hallin Z. Sex differences in pain perception. Gend Med. 2005;2:137–145. doi: 10.1016/S1550-8579(05)80042-7. [DOI] [PubMed] [Google Scholar]
- Korenman SG. Comparative binding affinity of estrogens and its relation to estrogenic potency. Steroids. 1969;13:163–177. doi: 10.1016/0039-128X(69)90004-X. [DOI] [PubMed] [Google Scholar]
- Langosch D, Thomas L, Betz H. Conserved quaternary structure of ligand-gated ion channels: the postsynaptic glycine receptor is a pentamer. Proc Natl Acad Sci USA. 1988;85:7394–7398. doi: 10.1073/pnas.85.19.7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennerick S, Lamberta M, Shu HJ, Hogins J, Wang C, Covey DF, Eisenman LN, Zorumski CF. Effects on membrane capacitance of steroids with antagonist properties at GABAA receptors. Biophys J. 2008;95:176–185. doi: 10.1529/biophysj.107.124768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassart-Schiess E, Baulieu EE. Neurosteroids: recent findings. Brain Res Brain Res Rev. 2001;37:133–140. doi: 10.1016/S0165-0173(01)00113-8. [DOI] [PubMed] [Google Scholar]
- Hojo Y, Hattori TA, Enami T, Furukawa A, Suzuki K, Ishii HT, Mukai H, Morrison JH, Janssen WG, Kominami S. et al. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017alpha and P450 aromatase localized in neurons. Proc Natl Acad Sci USA. 2004;101:865–870. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Maidment NT, Schlinger BA. Forebrain steroid levels fluctuate rapidly during social interactions. Nat Neurosci. 2008;11:1327–1334. doi: 10.1038/nn.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhla AM, Khan MS, Romas NP, Rosner W. Estradiol causes the rapid accumulation of cAMP in human prostate. Proc Natl Acad Sci USA. 1994;91:5402–5405. doi: 10.1073/pnas.91.12.5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattipakorn SC, McMahon LL. Pharmacological characterization of glycine-gated chloride currents recorded in rat hippocampal slices. J Neurophysiol. 2002;87:1515–1525. doi: 10.1152/jn.00365.2001. [DOI] [PubMed] [Google Scholar]
- Thio LL, Shanmugam A, Isenberg K, Yamada K. Benzodiazepines block alpha2-containing inhibitory glycine receptors in embryonic mouse hippocampal neurons. J Neurophysiol. 2003;90:89–99. doi: 10.1152/jn.00612.2002. [DOI] [PubMed] [Google Scholar]
- Wang DS, Mangin JM, Moonen G, Rigo JM, Legendre P. Mechanisms for picrotoxin block of alpha2 homomeric glycine receptors. J Biol Chem. 2006;281:3841–3855. doi: 10.1074/jbc.M511022200. [DOI] [PubMed] [Google Scholar]
- Flint AC, Liu X, Kriegstein AR. Nonsynaptic glycine receptor activation during early neocortical development. Neuron. 1998;20:43–53. doi: 10.1016/S0896-6273(00)80433-X. [DOI] [PubMed] [Google Scholar]
- Bechade C, Colin I, Kirsch J, Betz H, Triller A. Expression of glycine receptor alpha subunits and gephyrin in cultured spinal neurons. Eur J Neurosci. 1996;8:429–435. doi: 10.1111/j.1460-9568.1996.tb01226.x. [DOI] [PubMed] [Google Scholar]
- Laube B, Maksay G, Schemm R, Betz H. Modulation of glycine receptor function: a novel approach for therapeutic intervention at inhibitory synapses? Trends Pharmacol Sci. 2002;23:519–527. doi: 10.1016/S0165-6147(02)02138-7. [DOI] [PubMed] [Google Scholar]
- Backstrom T, Andersson A, Andree L, Birzniece V, Bixo M, Bjorn I, Haage D, Isaksson M, Johansson IM, Lindblad C. et al. Pathogenesis in menstrual cycle-linked CNS disorders. Ann N Y Acad Sci. 2003;1007:42–53. doi: 10.1196/annals.1286.005. [DOI] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- Handwerker HO, Kobal G. Psychophysiology of experimentally induced pain. Physiol Rev. 1993;73:639–671. doi: 10.1152/physrev.1993.73.3.639. [DOI] [PubMed] [Google Scholar]
- Nakae A, Tanaka T, Miyake K, Hase M, Mashimo T. Comparing methods of detection and quantitation of RNA editing of rat glycine receptor alpha3. Int J Biol Sci. 2008;4:397–405. doi: 10.7150/ijbs.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier JC, Henneberger C, Melnick I, Racca C, Harvey RJ, Heinemann U, Schmieden V, Grantyn R. RNA editing produces glycine receptor alpha3(P185L), resulting in high agonist potency. Nat Neurosci. 2005;8:736–744. doi: 10.1038/nn1467. [DOI] [PubMed] [Google Scholar]
- Eichler SA, Kirischuk S, Juttner R, Legendre P, Lehmann TN, Gloveli T, Grantyn R, Meier JC. Glycinergic Tonic Inhibition of Hippocampal Neurons with Depolarising GABAergic Transmission Elicits Histopathological Signs of Temporal Lobe Epilepsy. J Cell Mol Med. 2008. [DOI] [PMC free article] [PubMed]
- Gao J, Duan B, Wang DG, Deng XH, Zhang GY, Xu L, Xu TL. Coupling between NMDA receptor and acid-sensing ion channel contributes to ischemic neuronal death. Neuron. 2005;48:635–646. doi: 10.1016/j.neuron.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Pribilla I, Takagi T, Langosch D, Bormann J, Betz H. The atypical M2 segment of the beta subunit confers picrotoxinin resistance to inhibitory glycine receptor channels. EMBO J. 1992;11:4305–4311. doi: 10.1002/j.1460-2075.1992.tb05529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase K, Randic M, Shirasaki T, Nakagawa T, Akaike N. Serotonin suppresses N-methyl-D-aspartate responses in acutely isolated spinal dorsal horn neurons of the rat. Brain Res. 1990;525:84–91. doi: 10.1016/0006-8993(90)91323-9. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Li Y, Wu LJ, Legendre P, Xu TL. Asymmetric cross-inhibition between GABAA and glycine receptors in rat spinal dorsal horn neurons. J Biol Chem. 2003;278:38637–38645. doi: 10.1074/jbc.M303735200. [DOI] [PubMed] [Google Scholar]