Abstract
Objective
To evaluate sound localization acuity in a group of children who received bilateral (BI) cochlear implants in sequential procedures and to determine the extent to which BI auditory experience affects sound localization acuity. In addition, to investigate the extent to which a hearing aid in the nonimplanted ear can also provide benefits on this task.
Design
Two groups of children participated, 13 with BI cochlear implants (cochlear implant + cochlear implant), ranging in age from 3 to 16 yrs, and six with a hearing aid in the nonimplanted ear (cochlear implant + hearing aid), ages 4 to 14 yrs. Testing was conducted in large sound-treated booths with loudspeakers positioned on a horizontal arc with a radius of 1.5 m. Stimuli were spondaic words recorded with a male voice. Stimulus levels typically averaged 60 dB SPL and were randomly roved between 56 and 64 dB SPL (±4 dB rove); in a few instances, levels were held fixed (60 dB SPL). Testing was conducted by using a “listening game” platform via computerized interactive software, and the ability of each child to discriminate sounds presented to the right or left was measured for loudspeakers subtending various angular separations. Minimum audible angle thresholds were measured in the BI (cochlear implant + cochlear implant or cochlear implant + hearing aid) listening mode and under monaural conditions.
Results
Approximately 70% (9/13) of children in the cochlear implant + cochlear implant group discriminated left/right for source separations of ≤20° and, of those, 77% (7/9) performed better when listening bilaterally than with either cochlear implant alone. Several children were also able to perform the task when using a single cochlear implant, under some conditions. Minimum audible angle thresholds were better in the first cochlear implant than the second cochlear implant listening mode for nearly all (8/9) subjects. Repeated testing of a few individual subjects over a 2-yr period suggests that robust improvements in performance occurred with increased auditory experience. Children who wore hearing aids in the nonimplanted ear were at times also able to perform the task. Average group performance was worse than that of the children with BI cochlear implants when both ears were activated (cochlear implant + hearing aid versus cochlear implant + cochlear implant) but not significantly different when listening with a single cochlear implant.
Conclusions
Children with sequential BI cochlear implants represent a unique population of individuals who have undergone variable amounts of auditory deprivation in each ear. Our findings suggest that many but not all of these children perform better on measures of localization acuity with two cochlear implants compared with one and are better at the task than children using the cochlear implant + hearing aid. These results must be interpreted with caution, because benefits on other tasks as well as the long-term benefits of BI cochlear implants are yet to be fully understood. The factors that might contribute to such benefits must be carefully evaluated in large populations of children using a variety of measures.
Introduction
In recent years, there have been improvements in speech processing strategies used in cochlear implants, which are particularly evident in speech understanding in quiet, in both adults (e.g., Rauschecker & Shannon, 2002; Wilson et al., 1991) and children (e.g., Psarros et al., 2002). However, for most cochlear implants users, speech reception in a noisy or complex environment is still poor (Nelson et al., 2003; Pasanisi et al., 2002; Stickney et al., 2004). One possible reason for poor performance in multi-source environments is that most cochlear implant users have one device and cannot benefit from binaural information, such as differences in interaural time and level that normal-hearing listeners use to judge the location of a sound source (Blauert, 1997; Durlach & Colburn, 1978; Middlebrooks & Green, 1991) and to segregate talkers from competing sounds (Blauert, 1997; Bronkhorst, 2000; Culling et al., 2004). A second possibility is that they cannot take advantage of the “head shadow” effect for all positions. Rather than depending on binaural inputs per se, this is a physical effect, whereby the head and shoulders act as an acoustic “shadow” to reduce the intensity of sounds reaching the ear from the opposite side of the head. This effect can be as large as 20 dB, is most pronounced at high frequencies, and may be especially useful for speech understanding in noise for sources that are spatially separated (Blauert, 1997; Dillon, 2001). With a single device, listeners can use the head shadow for a limited set of source positions.
To date, hundreds of adults have received bilateral (BI) implants. Most patients’ anecdotal reports are extremely positive; they much prefer the use of both cochlear implants together and report that auditory images are significantly more externalized and localizable (van Hoesel, 2004). A number of studies have reported improved performance Oil spatial hearing tasks for patients using BI cochlear implants compared with the same patients’ use of a single cochlear implant. The BI listening mode can be advantageous on simple tasks such as discrimination of sounds arriving from the right versus left (e.g., Gantz et al., 2002; Tyler et al., 2002). Furthermore, identification of source positions in multispeaker arrays by most BI cochlear implant users is better when using both cochlear implants compared with either cochlear implant alone (Litovsky et al., 2004a; Nopp et al., 2004; van Hoesel & Tyler, 2003).
Given the success of BI implants in adults, there has been growing interest in providing BI cochlear implants to children as well (Kuhn–Inacker et al., 2004; Litovsky et al., 2004b, a; 2004b; Peters et al., 2004; Winkler et al., 2002). However, very little is known about the potential benefits or risks of such endeavors, and, to date, the necessary tools to evaluate bilaterally implanted children are not standardized nor easily accessible. The present study represents one element of a research program in which children with BI cochlear implants were evaluated at various intervals after receiving their second cochlear implant, with a focus on their ability to function in realistic, multisource environments. In this paper, results from measures of sound localization acuity are presented. The goal of this study was to assess whether BI cochlear implants in children provide benefits similar to those observed in adults and whether the time course for improvement in performance after the initiation of BI hearing is similar to that seen in adults.
An important control group also studied here consists of bimodal children, who have residual hearing in the nonimplanted ear and are fitted with a hearing aid in that ear. A small number of studies suggest that some patients with cochlear implant + hearing aid show an advantage on measures of speech understanding in noise (e.g., Armstrong et al., 1997; Ching et al., 2001; 2004; Kong et al., 2005; Tyler et al., 2002) and location acuity (Ching et al., 2001; Tyler, et al., 2002).
In the present study, we used a measure of directional hearing known as the minimum audible angle (MAA; smallest change in the position of a sound source that can be reliably discriminated), which is an excellent tool for measuring basic directional abilities mediated by the binaural system. Using a left/right discrimination task, the MAA can be applied to infants as young as a few months of age, as well as older populations. Most important, the measure is consistent and reliable (for review, see Litovsky & Ashmead, 1997). MAA thresholds in normal-hearing children and infants reach 12 to 19° at 6 mos (Ashmead et al., 1987), decrease to 4 to 6° by 18 mos, and to 1 to 2° by 5 yrs, at which point they are not significantly different from adult MAAs (Litovsky, 1997). The MAA task can be extended to more complex tasks, such as with simulated echoes (Litovsky, 1997). Finally, because MAA thresholds are worse in absence of binaural cues (Hausler et al., 1983), this task can offer insights into the emergence of binaural abilities in children who are fitted with implants and/or hearing aids.
Initial results from three bilaterally implanted children (Litovsky et al., 2004a), obtained 2 or 3 mos after activation of the second cochlear implant, suggest that localization acuity is very poor under both BI and monaural listening modes. It is important to note that the children with BI cochlear implants in the Litovsky et al. (2004a) study had been deaf from a very young age, implanted in sequential procedures, and had presumably experienced a protracted period of auditory deprivation in the second implanted ear. This paper presents results from the same three children and a number of others with similar histories, studied at intervals ranging from 2 to 26 mos after activation of the second cochlear implant. A second group of children who use a cochlear implant in one ear and a hearing aid in the nonimplanted ear were also tested. This work was aimed at testing the hypotheses that (1) attainment of “ localization abilities in bilateral, sequentially implanted children may involve a slower, more prolonged process than that seen in postlihgually deafened adults, and (2) localization acuity is better with two cochlear implants than with a single cochlear implant and a hearing aid in the nonimplanted ear.
Methods
Subjects
Group 1: Cochlear Implant + Cochlear Implant
Thirteen children, ages 3 to 16 yrs at the time of initial testing participated; 12 were prelingually deaf and 1 had postlingual progressive hearing loss. All children received their first cochlear implant several years before the second cochlear implant. Subject 13 participated in the cochlear implant + hearing aid testing (see group 2, below) before receiving the second cochlear implant and was re-tested at 3 mos after activation of the second cochlear implant. Testing was conducted at various intervals after activation of the second cochlear implant. Of the 13 children, 12 were fitted with two Nucleus devices. Two children had the Nucleus 22 in the first implanted ear; 10 had the Nucleus 24 or Nucleus 24 Contour implant in the first implanted ear; and all had the Nucleus 24 Contour in the second implanted ear. One child was fitted with BI Clarion devices (Platinum/Auria). The speech processors had autosensitivity settings that activated the automatic gain control at 67 dB SPL or higher; hence, the levels chosen for this study were systematically kept at 66 dB or lower
One child (S4) was diagnosed with Waardenburg type I but had no other disabilities, and one child (S8) had anoxia at birth, resulting in deafness and mild unilateral palsy, but had no known cognitive disabilities. Table 1 includes the relevant details for each participant. In addition, it should be noted that all children participated in intensive auditory-verbal and/or speech therapy for several years. All children were in their age-appropriate grade level at school and were being educated in a mainstream school environment.
TABLE 1.
Subject demographics for children with two cochlear implants (cochlear implant + cochlear implant)
| Subject No. BI cochlear implant | Sex | Age 1st cochlear implant (yr.mo) | Age 2nd cochlear implant (yr.mo) | Exper w/1st cochlear implant @ 1st visit (yr.mo) | Exper w/2nd cochlear implant @ 1st visit (mo) | Cause: 1st and 2nd cochlear implant | Deaf dur before 1st cochlear implant | Deaf dur before 2nd cochlear implant {yr.mo) | Time hearing aid used before 1st cochlear implant (yr.mo) | Time hearing aid used before 2nd cochlear implant (yr.mo) | First cochlear implant | Second cochlear implant |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 01 | F | 5.0 | 8.0 | 3.0 | 3 | 1:SNHL | 5 yr | 8.0 | 4 yr | 4.0 | Nucleus 24 Rt ear | Nucleus 24 Lft ear |
| 2: fixed stapes | ||||||||||||
| 02 | F | 6.0 | 12:0 | 6.0 | 3 | 1:SNHL | 6yr | 12.0 | 4 yr | 4.0 | Nucleus 22 Rt ear | Nucleus 24 Lft ear |
| 2: Unknown | ||||||||||||
| 03 | F | 4.6 | 8.0 | 3.6 | 3 | 1:SNHL | 4.5 yr | 8.0 | 3 yr | 3.0 | Nucleus 24 Rt ear | Nucleus 24 Lft ear |
| 2: Unknown | ||||||||||||
| 04 | F | 1.6 | 5.6 | 4.0 | 7 | Waardenburg Type 1 | 18 mo | 5.6 | 7 mo | 0.7 | Nucleus 24 Rt ear | Nucleus 24 Lft ear |
| 05 | M | 1.7 | 3.0 | 2.6 | 12 | 1: SNHL | 19 mo | 3.0 | 17 mo | 1.5 | Nucleus 24 Rt ear | Nucleus 24 Lft ear |
| 2: Unknown | ||||||||||||
| 06 | F | 2.0 | 10.0 | 9.0 | 12 | CMV | 2 yr | 10.0 | 2yr | 2.0 | Nucleus 24 Lft ear | Nucleus 24 Rt ear |
| 07 | M | 4.6 | 5.6 | 2.0 | 11 | 1: Progressive SNHL | 1.5yr | 2.6 | 6 mo | 0.8 | Nucleus 24 Rt ear | Nucleus 24 Lft Ear |
| 2: Unknown | ||||||||||||
| 08 | M | 2.6 | 7.6 | 5.6 | 6 | Anoxia at Birth, Cerebral | 2.5 yr | 5.6 | 2yr | 2.0 | Nucleus 24 Lft ear | Nucleus 24 Rt ear |
| Palsy | ||||||||||||
| 09 | M | 13.0 | 1.4 | 3.0 | 2 | 1: Progressive SNHL | 3 yr | 6.0 | 11.5 yr | 14.6 | Clarion | Clarion Platinum Lft ear |
| 2: Unknown | Platinum Rt ear | |||||||||||
| 10 | M | 2.0 | 5.6 | 5.0 | 14 | Familial Hereditary SNHL | 2yr | 5.6 | 2yr | 2.0 | Nucleus 24 Lft ear | Nucleus 24 Rt ear |
| 11 | F | 9.0 | 10.0 | 1.6 | 9 | 1 and 2: Progressive SNHL; Gentamycin | 6 rno | 1.6 | 6yr | 6.0 | Nucleus 24 Rt ear | Nucleus 24 Lft ear |
| 12 | M | 4.6 | 6.6 | 2.0 | 3 | CMV | 4.5 yr | 6.6 | 2.5 yr | 2.6 | Nucleus 24 Lft ear | Nucleus 24 Rt ear |
| 13 | F | 3.6 | 5.0 | 1.6 | 3 | 1: Progressive SNHL | 3.5 yr | 5.0 | 2yr | 3.6 | Nucleus 24 Rt ear | Nucleus 24 Contour Advance |
| 2: Unknown | Lft ear |
Loudness Balancing
The right and left speech processors were each programmed independently by the child’s clinician, and the “comfortable” volume level for each unilateral program was recorded. In an attempt to equalize the loudness for the two ears, the speech processors were first activated separately, and the child was asked to perform a “loudness” task with each processor. During the task, a speech sound was presented from the front loudspeaker (0°) and the child was asked to indicate the perceived loudness by pointing to a visual sketch with seven circles incrementing in size to denote greater loudness. The sketch also had icons with facial expressions denoting percepts of “difficult to hear,” “comfortable,” or “too loud.” The experimenter incrementally changed the volume setting on the speech, processor until the child consistently reported that the sound was audible and comfortable (approximately the middle circle on the sketch). After the comfortable level was established for each processor separately, both processors were activated together. The child was asked once more to report the perceived overall loudness; if the child indicated that the sound was too loud, then the levels of both processors were incrementally reduced until a comfortable level was achieved. Subsequently, the child was asked to indicate whether the sounds from the two implants were closely matched for perceived loudness. Slight tweaking of the relative levels of the processors was at times necessary. Overall, this exercise was easier to achieve in the older children (>8 yrs) and difficult particularly with the youngest children due to some inconsistencies in responses. The loudness balancing lasted 20 to 60 minutes, depending on the child’s ability to communicate effectively with the experimenter, and the experimenter’s confidence that the desired goal had been achieved. Problems for many of the children arose especially during the testing sessions nearest to the activation of the second cochlear implant (some children were reluctant to increase the volume or sensitivity settings for the processor on the second-implanted ear).
Group 2: Cochlear Implant + Hearing Aid
Six children, ages 4 to 14 yrs at the time of testing, were identified as having a hearing loss by the age of 2 yrs and had been implanted between the ages of 3.5 and 8.5 yrs. Of the six participants, four had the Nucleus 24 or 24 Contour implant, one had the Clarion II HiFocus device, and one had the MedEl C40 + device (see Table 2 for details regarding cause, age, type of cochlear implant, and hearing aid).
TABLE 2.
Subject demographics for children with one cochlear implant and one hearing aid (cochlear implant + hearing aid)
| Subject cochlear implant hearing aid | Sex | Age@ time of receiving cochlear implant (yr.mo) | Age hearing aid (yr.mo) | Exp w/cochlear implant @ 1st visit {yr.mo) | Etlology in ear with cochlear implant | Deafness duration before cochlear implant {yr.mo) | Time hearing aid used in implanted ear before cochlear implant (yr.mo) | Time hearing aid used in nonimplanted ear (yr.mo) | Cochlear implant | Hearing aid | Audiometric hearing thresholds in hearing aid ear |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 13 | F | 3.6 | 2.0 | 1.0 | Progressive SNHL unknown | 3.6 | 2.0 | 3.0 | Nucleus 24, Rt ear | Otlcon DiglFocus II SP, Lft ear | Unaided: |
| 70 @ 250 Hz | |||||||||||
| 85 @ 500 Hz | |||||||||||
| Aided: | |||||||||||
| Not available | |||||||||||
| 14 | F | 3.6 | 1.6 | 3.0 | Genetic SNHL | 3.6 | 1.6 | 5.0 | Clarion Auria, Rt ear | Phonak PicoForte PP-CPC, Lft ear | Unaided: |
| Not available | |||||||||||
| Aided: | |||||||||||
| 50–60 @ 250–4,000 Hz | |||||||||||
| 16 | F | 5.6 | 1.4 | 3.6 | Meningitis | 4.0 | 4.0 | 6.6 | Cochlear implant 24Mr ESPrit 3G, Lft ear | Phonak SonoForte 2, Rt ear | Unaided: |
| 60 @ 250–500 Hz | |||||||||||
| 90 @ 1–4,000 Hz | |||||||||||
| Aided: | |||||||||||
| 40 @ 250–500 Hz | |||||||||||
| 50 @ 1–2,000 Hz | |||||||||||
| 60 @ 4,000 Hz | |||||||||||
| 16 | M | 4.0 | 1.2 | 3.6 | Connexin-26 mutation | 4.0 | 3.0 | 6.6 | N24C, ESPrit 3G, Rt ear | Widex Senso P38, Lft ear | Unaided: |
| 85 or worse at all frequencies. | |||||||||||
| Aided: | |||||||||||
| 30 @ 250, 500 Hz | |||||||||||
| 40@ 1,000 Hz | |||||||||||
| 60 @ 2,000 Hz | |||||||||||
| 70 @ 4,000 Hz | |||||||||||
| 17 | M | 8.6 | 0.7 | 1.6 | SNHL unknown | 8.6 | 8.0 | 9.6 | Med-EI C40+,Rtear | DigiFocus II,Lft ear | Unaided: |
| 90–100 @ all frequencies. | |||||||||||
| Aided: | |||||||||||
| 35–40 @ 250–4,000 Hz | |||||||||||
| 18 | M | 8.0 | 2.0 | 6.0 | Progressive SNHL unknown | 8.0 | 6.0 | 12.0 | Cochlear implant 24M, Esprit 3G, Rt ear | Widex Senso P37, Lft ear | Unaided: |
| 55 @ 250 Hz |
The implant speech processor and the hearing aid were each programmed independently by the child’s clinician. As with the children with BI cochlear implants, loudness balancing was attempted by presenting a speech sound from the front loudspeaker (0°), asking the child to indicate the perceived loudness and selecting levels consistent with the child’s report of a sound being comfortable. The “loudness balance” task was more difficult to perform on some of the cochlear implant + hearing aid children because the perception of sound through the two devices can be entirely different and difficult to compare. When possible, loudness balancing was conducted.
The protocol was approved by the University of Wisconsin Human Subjects Committee, and meets all the requirements of the NIH guidelines. Informed consent was obtained from the parents of all subjects, and children ages 7 yrs and older also signed an informed assent form.
Test Setup
Testing was conducted in a sound-treated booth (IAC; 1.8 × 1.8 m or 2.8 × 3.25 m). Subjects sat at a small table facing the loudspeakers (Fig. 1). The setup has a semicircular array with a radius of 1.5 m, containing 15 loudspeakers (Cambridge Soundworks Center/Surround IV; matched within 1 dB at 100 to 8,000 Hz) positioned on a horizontal arc at 10° intervals (−70° to +70°). Occasionally, the speakers were moved to angle separations of 2.5 and 5.0°. During each block of trials, two loudspeakers were selected at equal left/right angles and remained fixed for 20 trials. Testing was conducted at numerous angles for each subject. Data collection for each block lasted between 3 and 5 minutes; time blocks depended on the age and to some degree attention and motivation of the child. Since measurements had to be completed at numerous angles, the amount of time required for testing was 40 to 60 minutes.
Fig. 1.
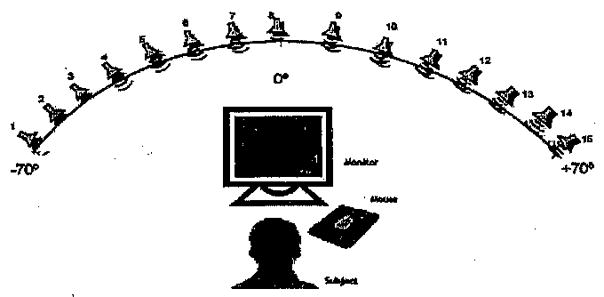
Schematic diagram of testing setup. An array of 15 loudspeakers mounted on an arc with a radius of 1.5 m at ear level, positioned every 10° (−70° to + 70°).
Hardware including Tucker Davis Technologies (TDT) System III (RP2, PM2, AP2), in conjunction with a PC host, was responsible for stimulus presentation and control of the multiplexer for speaker switching and amplification. Software for stimulus presentation and data collection was written in Matlab.
Stimuli
Stimuli were spondaic words such as “baseball” recorded with a male voice at a sampling rate of 44,000 Hz and stored as wav files. Stimuli were selected after extensive pilot testing with several subjects suggested that other stimuli such as noise bursts produced results that were less reliable. This is perhaps due to the ecological validity, that is, the fact that speech sounds are heard in everyday listening environments, and all of our subjects were familiar with them. Stimulus levels averaged 60 dB SPL and were randomly varied between 56 and 64 dB SPL (roved ±4 dB); in a few cases that are discussed in more detail, the level was fixed at 60 dB SPL.
Testing Procedure
All testing was conducted by using a “listening game” platform whereby computerized interactive software was used to engage the child on the tasks. During individual trials, the child was asked to orient the head toward the front (in the event that noticeable head movement occurred, data from the trial were discarded and an additional trial was presented on that condition). Stimuli were presented from either the left or right, and the child used the computer mouse to select icons on the screen indicating left versus right positions. A few of the younger children preferred to point with their finger and have the experimenter enter the response into the computer. After each response, feedback was provided such that the correct-location icon flashed on the screen. In addition, motivation was maximized with a puzzle-picture that had missing pieces appear after each correct response.
Two-Alternative Forced Choice
Source direction (left/right) varied randomly, and angular separation of the right and left speakers from center was fixed during blocks of 20 trials. Angle size varied from block to block, using a modified adaptive rule, based on the child’s performance. After blocks in which overall performance yielded ≥15/20 (75%) correct, the angle was decreased, otherwise the angle was increased. Decisions regarding the step size leading to increased or decreased angles were based on similar rules to those used in classic adaptive procedures (e.g., Litovsky, 1997; Litovsky & Macmillan, 1994). MAA thresholds for each listening mode and every subject were defined as the smallest angle at which performance reached 70.9% correct. Listening modes in which performance was consistently below 70.9% correct for all angles tested are denoted as “NM” in the result figures. In rare cases of nonmonotonicity (see below), the first instance in which the curve crossed the 70.7% point was used to estimate MAA.
Design and Testing Intervals (Ages)
Under ideal circumstances, data collection would have taken place for all subjects at the same time stamp of BI experience. Because we depended on participants’ willingness to enroll in the study (often traveling to Madison, WI), personal constraints dictated much of the timing, hence the intersubject variation in the number of months after activation of the second cochlear implant at the time of testing. Presentation of the results will therefore highlight performance as a function of the number of months of BI experience. Some subjects contributed data at a few intervals (Table 3).
TABLE 3.
Patient data for each subject from the cochlear implant + cochlear implant group
| Subject No. | Visit 1 | Visit 2 | Visit 3 | Visit 4 |
|---|---|---|---|---|
| 1 (9.3) | - | - | 15 | - |
| 2 (13.11) | 3 | - | 15 | 23 |
| 3 (11.10) | 3 | - | 15 | 22 |
| 4 (6.10) | 7 | 16 | 26 | - |
| 5 (4.0) | 12 | - | - | - |
| 6 (11) | 12 | - | - | - |
| 7 (6.5) | 11 | - | - | - |
| 8 (8.0) | 6 | - | - | - |
| 9 (3.4) | 2 | - | - | - |
| 10 (6.8) | 14 | - | - | - |
| 11 (10.9) | 8 | - | - | - |
| 12 (6.9} | 3 | - | - | - |
| 13 (5.3) | 3 | - | - | - |
For each subject from the cochlear implant + cochlear implant group, the subject number, and age (yr.mo) are shown in the left-most column. Subjects 2, 3, and 4 had multiple visits to the laboratory. Numerical value In each cell indicates the number of months after second cochlear implant activation.
During each visit to the laboratory, children were tested on a number of measures, including the MAA data presented here as well as other measures. To avoid possible effects of learning, attention, fatigue, interest in the tasks, and other possible confounds, testing was carefully balanced for the various measures and listening modes (BI, first cochlear implant and second cochlear implant) across days and within each day, across morning and afternoon sessions.
Results
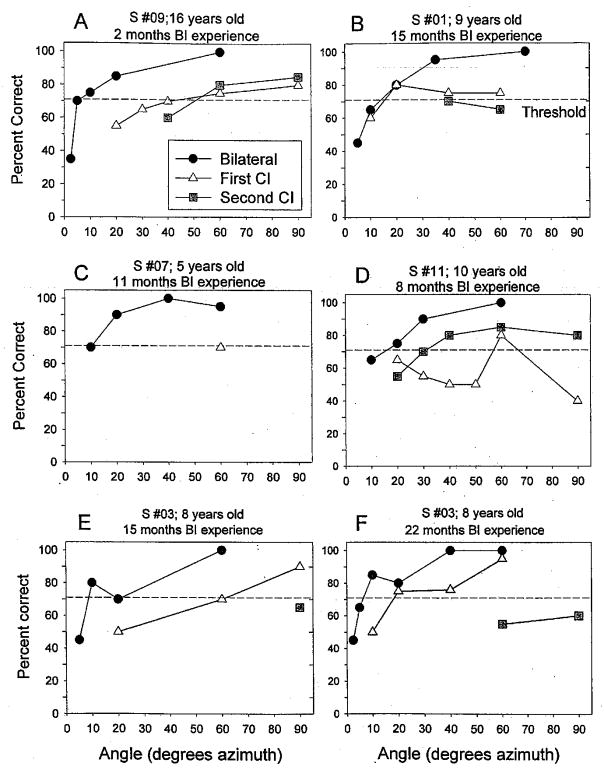
This report presents results from children using BI devices, including BI cochlear implants (cochlear implant + cochlear implant) or one cochlear implant and one hearing aid (cochlear implant + hearing aid). Data from five subjects with BI cochlear implants are shown in Figure 2. Within each panel, performance at various angles and listening modes is compared; for one subject (patient 3), data from two testing intervals (15-and 22-mo BI experience) are shown in two separate panels (E, F). In all cases, the BI functions reach better performance (% correct is higher) at smaller angles than either monaural condition. Thresholds were estimated from each curve by finding the smallest angle at which the line crossed 70.9%. At times, linear interpolation between the two adjacent (lower and higher) points on the curve was applied.*
Fig. 2.
Data from five individual subjects are shown. Each panel contains results from three listening modes, bilateral (circles), first cochlear implant (triangles) and second cochlear implant (squares). Percent correct is plotted as a function of the loudspeaker positions. An angle of 20° indicates that the loudspeakers were positioned at 20° to the right and left, hence a total of 40° separation between the two positions. Dashed horizontal line in each panel crosses threshold criterion of 70.9%.
Of the 13 children in the cochlear implant ± cochlear implant group, nine had MAA thresholds that were ±60° or smaller for at least one listening mode, and four children found the task very difficult (MAA thresholds ≥60° for all listening modes). Figure 3 shows MAA thresholds for the former group. Results are plotted according to BI experience or “BI age,” that is, number of months since the second device was activated. For subjects with multiple visits, these would be the thresholds obtained at their latest visit to the lab (see Table 3). For each child, thresholds are compared for three listening modes: first cochlear implant alone (triangle), second cochlear implant alone (square), and BI (circle). The vertical dashed line at 13 mos is intended as a visual marker that separates between data collected during the first 12 mos and those collected after 13 to 26 mos of BI experience. Seven of the nine children showed a clear trend for best performance in the BI mode (MAA thresholds ranging from 5 to 40°), followed by the first cochlear implant mode, and worse performance in the second cochlear implant mode. One child (1) had monaural thresholds with the first cochlear implant that were nearly as low as the BI thresholds, and one child (4) had slightly better thresholds with the second cochlear implant than bilaterally, followed by first cochlear implant.
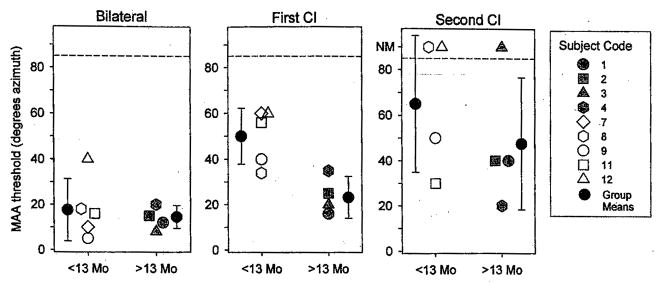
Fig. 3.
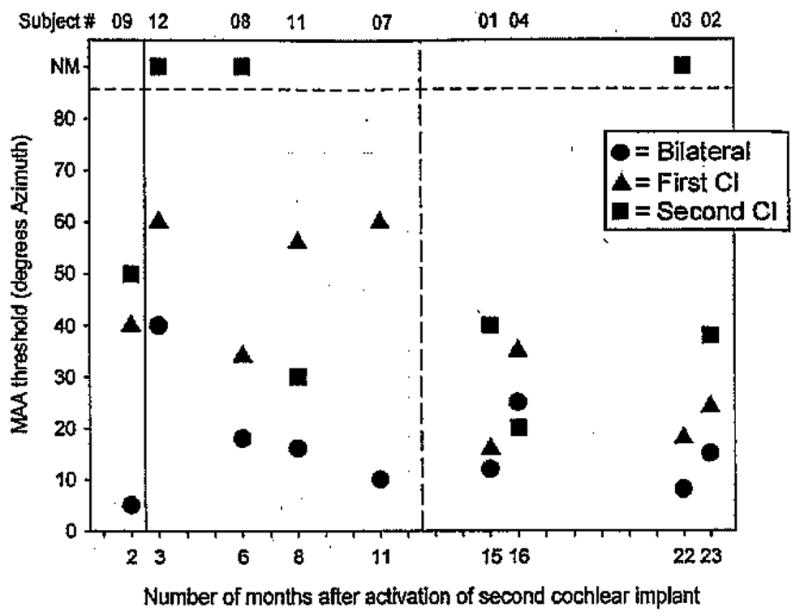
Minimum audible angle thresholds estimated from curves such as those plotted in Figure 2 are shown for the group of subjects who were able to perform the task at <60° on at least one condition. Each vertical set of thresholds represents data from a single subject’s performance on the three listening modes: bilateral (circles), first cochlear implant (triangles), and second cochlear implant (squares). Subject numbers are shown along the top of the graph, and results are plotted according their “bilateral age,” that is, number of months after activation of the second cochlear implant. On the vertical axis, MAA thresholds can range from 5 to 85°, and data points >85 denote conditions in which thresholds were not measurable because the subject could not perform the test (NM). In a few cases, there are absent data points for the second cochlear implant condition because data were not obtained. Finally, subject 4 is singled out (*) by way of reminder that she had more intensive training before final data collection.
Specifics related to a few children should be noted. First, subject 9 attained MAA thresholds of 5° in the BI condition, compared with 40 to 50° in the monaural conditions when tested after 2 mos of BI experience. This child was somewhat unusual, having experienced a progressive hearing loss during childhood, and therefore likely to have had acoustic binaural hearing for some time before becoming deaf. Results from this subject highlight the important role of early auditory experience. Second, the long-term effect of experience after implantation is underscored by within-subject results from Figure 2 for subjects 2, 3, and 4 (see below). Subject 4, who had slightly lower thresholds with the second cochlear implant than bilaterally, is unique in that she underwent over a period of 2 days of training in the laboratory, and whereas results from the entire training period are shown at the end of this section, only the best (final) thresholds are included in the group data in Figure 3. These findings clearly indicate the importance of further work in the area of training and learning with bilaterally implanted children.
Figure 4 shows group means (±SD) for the bilaterally implanted children in the three listening modes. Within each panel, data are coarsely subdivided into two groups with either <13 mos or >13 mos of BI listening experience. The BI and first cochlear implant results were subjected to a mixed design, two-way repeated measures analysis of variance (ANOVA), with listening mode (BI, first cochlear implant) as the within-subjects variable and number of months of BI experience as the between-subjects variable. The second cochlear implant data were excluded from the analysis because testing in this mode was not conducted for subject 7, A significant main effect was found for the within-subjects listening mode variable [F(1,7) = 27.069, p < 0.001], suggesting that MAA thresholds were significantly higher in the first cochlear implant mode compared with BI. A significant main effect was also found for the between-subjects variable [F(1,7) = 6.048, p < 0.05], thresholds being lower in the group with >13 mos of BI experience compared with the group having <13 mos BI experience. A significant interaction was also found [F(1,7) = 8.883, p < 0.05]. Post hoc Scheffé tests for within-subjects pairwise comparisons revealed that thresholds were significantly higher in the first cochlear implant mode than in the BI mode for the group of children with <13 mos BI experience (p < 0.005) but not significantly different for the group of children with >13 mos’ BI experience. Independent t-test comparisons between groups suggest that thresholds in the first cochlear implant listening mode (Fig. 4, middle panel) were significantly greater in the group with <13 mos’ experience than the group with > 13 mos’ experience (p < 0.008); there was no difference between the two groups in the BI listening mode (Fig. 4, left panel).
Fig. 4.
Minimum audible angle threshold group means (±SD) are plotted for the group of nine children with bilateral implants whose individual data are shown in Figure 3. On the vertical axis, MAA thresholds can range from 5 to 85°, and data points >85 denote conditions in which thresholds were not measurable (NM). Performance is compared for measures obtained under the three listening modes: bilateral, first cochlear implant, and second cochlear implant. Within each panel, the subject population is divided into two subgroups, depending on the duration of experience with the second cochlear implant (<13 mos or >13 mos). One of the subjects in the <13 mo group, who was tested in the bilateral and first cochlear implant mode, was not tested in the second cochlear implant mode.
Effect of experience can be highlighted in more detail by examining repeated measurements made on three of the subjects over a 20-mo period. Figure 5 shows data from subjects 2, 3, and 4, who were each tested at several time intervals after activation of their second implant†. Filled symbols were taken from the fixed-speaker approach described in the Methods section. Open symbols from the 3-mo visits represent MAA thresholds estimated from a slightly different approach whereby the stimulus positions were varied adaptively (e.g., Litovsky, 1997). Because only a handful of data points were gathered using this approach, and the methods have been previously described in detail in published works, only the fixed method is outlined in detail in this paper. Results for the BI listening modes suggest that all three subjects showed large improvements over time, the largest being for subject 2, who was also the oldest child who had undergone prolonged deafness before the receiving her implant(s). Improvements were also seen under some monaural conditions. All three subjects improved on the first implant condition, especially between the initial visit and the 15-mo visit, and two improved on the second implant mode as well. In a couple of cases, performance deteriorated between the 15-mo and the final visit. The latter finding is important but not easy to interpret. The most likely explanation is that over time, the child has learned to rely on using both cochlear implants together and that deactivation of either device placed her at a disadvantage.
Fig. 5.
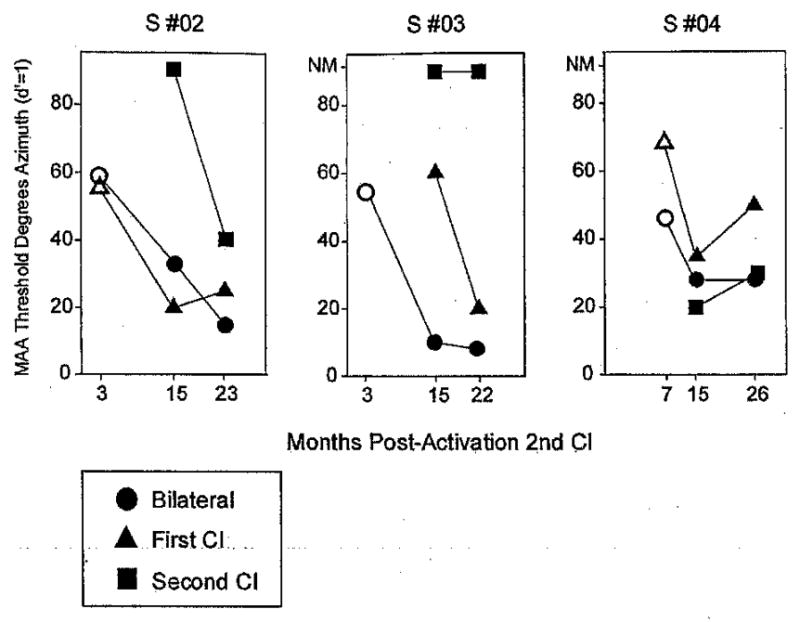
Individual results for three subjects (2, 3, and 4) are shown. Each child participated in the testing at a few different time intervals after activation of the second cochlear implant (3, 15, and 22 to 26 mos). Each panel contains data from a single subject, comparing performance on the three listening modes: bilateral, first cochlear implant and second cochlear implant. Within each panel, MAA thresholds are plotted as a function of the number of months after activation of the second cochlear implant. On the vertical axis, MAA thresholds can range from 5° to 85°, and data points >85 denote conditions in which thresholds were not measurable (NM). Filled symbols were taken from the fixed-speaker approach described in the Methods section; open symbols from the 3-mo visits represent MAA thresholds estimated using the adaptive method.
Overall, the unilateral data suggest that in some cases, BI experience may provide children with the opportunity to learn problem-solving tasks in realistic acoustic environments, such as determining source direction. Having acquired these abilities when using BI stimulation, some children may be able to transfer important auditory cues and problem-solving abilities to the unilateral listening modes.
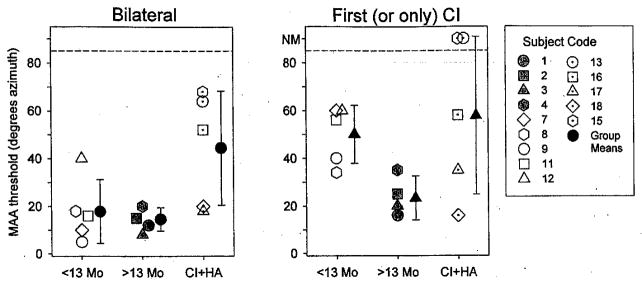
In addition to the 13 children with BI cochlear implants, six children with cochlear implant + hearing aid participated. Figure 6 shows MAA thresholds for the two groups of children with BI cochlear implants and one group with cochlear implant + hearing aid. The left panel shows results from the BI listening modes (cochlear implant + cochlear implant or cochlear implant + hearing aid), and the right panel shows results from the monaural condition in which the first cochlear implant (cochlear implant + cochlear implant children) or only cochlear implant (cochlear implant + hearing aid children) was activated. Within each panel, the cochlear implant + cochlear implant children are separated according to number of months of BI experience (<13 versus >13), and the right-most data are from the cochlear implant + hearing aid group. The data were subjected to a mixed two-way ANOVA in which the between-subjects variable was group (<13 mos BI cochlear implant; >13 mos BI cochlear implant; cochlear implant + hearing aid), and the within-subjects variable was listening mode (BI; single cochlear implant). A significant main effect was found for listening mode [F(1,11) = 31.903, p < 0.0001], and there was a significant listening mode × group interaction [F(2,11) = 5.094, p < 0.05], A one-way ANOVA to test for a simple main effect of group in the BI listening mode (Fig. 6, left panel) was significant [F(2,11) = 4.556, p < 0.05], and post hoc Fisher LSD between-subjects test revealed that MAA thresholds were significantly higher in the cochlear implant + hearing aid group compared with both cochlear implant + cochlear implant groups, regardless of whether they had <13 mos of experience (p < 0.05) or >13 mos’ BI experience (p < 0.05). In the unilateral cochlear implant listening mode, there was no statistically significant difference between the cochlear implant + cochlear implant and cochlear implant + hearing aid groups. The individual differences are important to note. When using a single cochlear implant, three of the five cochlear implant + hearing aid children performed as well or better than the average cochlear implant + cochlear implant children. In addition, two of them had BI MAA thresholds as low as the average cochlear implant + cochlear implant, and one (subject 16) showed no improvement with the added hearing aid. The other two children were unable to perform the task in the unilateral listening mode, and with the added hearing aid were able to do the task at the larger angle separations (65 to 70°).
Fig. 6.
Minimum audible angle thresholds are plotted for all children with bilateral devices, including two cochlear implants, with <13 or >13 mos of bilateral experience, and one group with a cochlear implant in one ear and hearing aid in the opposite ear (cochlear implant + hearing aid). Left panel: Data collected while two devices were active (two cochlear implants or cochlear implant and hearing aid). Right panel: Data collected while subjects used only one cochlear implant, either first cochlear implant (cochlear implant + cochlear implant children) or only cochlear implant (cochlear implant + hearing aid children). On the vertical axis, MAA thresholds can range from 5 to 85°, and data points >85 denote conditions in which thresholds were not measurable (NM). Within each panel, data are clustered by group and include both individual data points as well as group means (±SD).
Figure 7 shows MAA thresholds for the five children who found the task extremely difficult, four with BI cochlear implants and one with cochlear implant + hearing aid. Subject 6 was able to perform the task in the BI and first cochlear implant listening modes when the loudspeakers were placed at ±70° and ±60° azimuth, respectively. Subject 13 was able to perform the task in the BI mode for speakers at ±60°. The remaining three children were unable to discriminate right from left even when the speakers were placed at ±90°. An important question regarding “poor” performers concerns the potential role of training in improving performance.
Fig. 7.
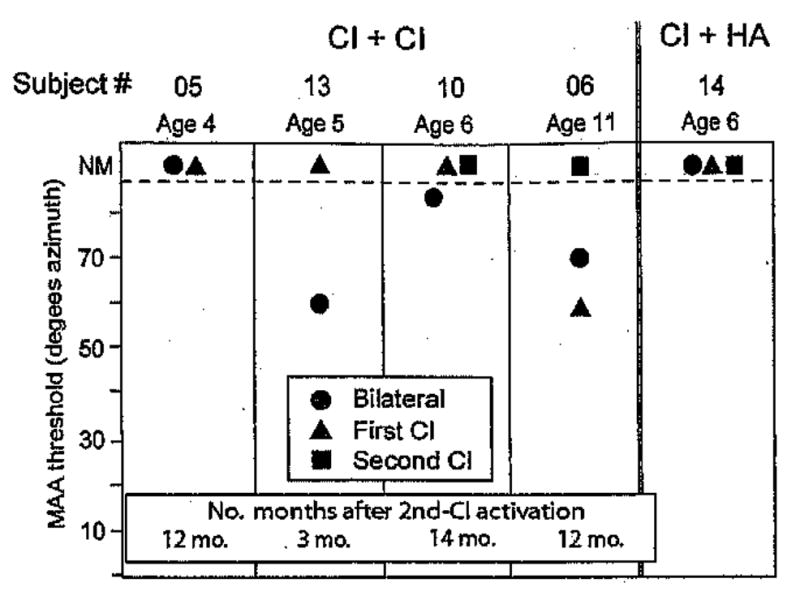
Minimum audible angle thresholds are plotted for the five children who found the task extremely difficult; four children had bilateral cochlear implants and one had a cochlear implant + hearing aid. Each child’s subject number and age are shown at the top of the graph, and for the four subjects with bilateral cochlear implants the number of months after activation of the second cochlear implant is indicated at the bottom of the graph. Results are compared for three listening modes: bilateral (circles), first cochlear implant (triangles), and second cochlear implant (squares); for two subjects, measures were not obtained in the second cochlear implant mode. On the vertical axis, MAA thresholds can range from 5 to 85°, and data points >85 denote conditions in which thresholds were not measurable (NM).
One subject (subject 4), whose post-training (best) results were shown in previous figures (>13 mo group), would have also been included in Figure 7 were it not for the fact that this child received training on the left/right task over a 2-day period. Results from the training are shown in Figure 8. When the child was first tested (day 1, AM) using roved sound level, MAA thresholds were not measurable (>70°) for two listening modes (BI and first cochlear implant), and measures were not attempted in the second cochlear implant mode. During the afternoon session (day 2, PM), a fixed-level stimulus was used to reduce stimulus ambiguity and determine whether the child could grasp onto some consistent cue; MAA thresholds were measurable for all listening modes. The following morning (day 2, AM) when the roved level conditions were tested in all three modes, only the second cochlear implant mode resulted in measurable MAAs (20°), whereas MAA thresholds in the first cochlear implant and BI modes were not measurable. During the afternoon session (day 2, PM), the roved stimulus levels were retested for the first cochlear implant and BI conditions, this time yielding MAA thresholds of 35° and 28°, respectively. The best, latest-obtained thresholds for each listening mode were chosen to be included in Figures 3, 4, and 6. Although preliminary, these findings suggest that training with feedback can potentially help some children to solve the left/right problem, not only under BI listening modes, but in the single cochlear implant modes as well.
Fig. 8.
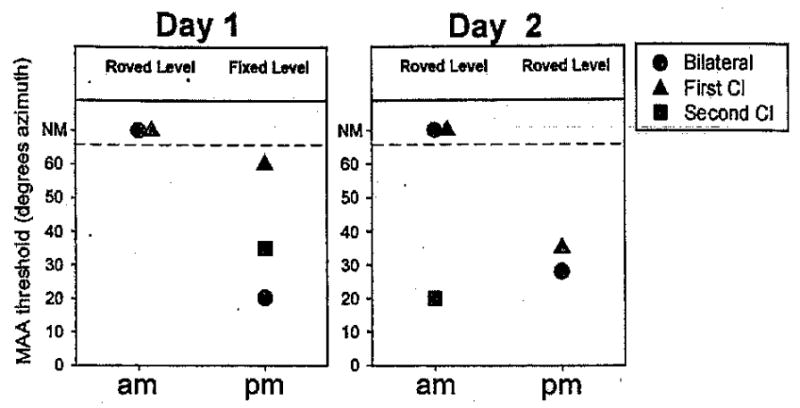
Results from repeated testing/training during a 2-day period are shown for one subject (subject 4). Each panel includes data from 1 day of testing, separated by morning (am) and afternoon (pm), including three listening modes: bilateral (circles), first cochlear implant (triangles) and second cochlear implant (squares). On the vertical axis, MAA thresholds can range from 20 to 70°, and data points >65 denote conditions in which thresholds were not measurable at 70°, the largest angle tested during those sessions (NM). Above each data set, the text indicates whether overall sound level was fixed (60 dB SPL) or roved (60 ± 4 dB SPL).
Summary
In summary, results obtained to date suggest the following.
Children who receive BI cochlear implants in sequential procedures vary in their ability to perform the MAA test of localization acuity. Nine of the 13 children tested here were able to achieve left/right discrimination thresholds ≤40°, with eight of nine of the children reaching thresholds ≤20°.
Under some conditions, the MAA task can also be performed when using a single cochlear implant, suggesting that the level rove used here (±4 dB) reduced but did not eliminate the monaural level cues available at each ear.
Minimum audible angle thresholds were better in the first cochlear implant than the second cochlear implant listening mode for eight of nine subjects; one subject was not tested with second-cochlear implant and one subject (subject 4) had significant training during a 2-day period before being able to perform the task.
Over a 2-yr period of BI experience, two subjects showed robust improvements in performance in the BI listening modes and degraded performance on some monaural conditions. The importance of postimplantation experience is underscored and suggests a protracted period of adjustment to and learning involved in utilizing bilateral cochlear stimulation.
Some children who wear hearing aids in the nonimplanted ear (cochlear implant + hearing aid) gained benefit from the hearing aid on the MAA task; in the bilateral listening mode, two children performed as well as the cochlear implant + cochlear implant children; one child could not perform the task, and three children were much worse than the average cochlear implant + cochlear implant children. When either group used a single cochlear implant, intersubject variability was larger in the cochlear implant + hearing aid group, but overall group performance was not significantly different for the two groups.
Discussion
In recent years, cochlear implantation has become standard treatment for deaf children worldwide, notwithstanding exceptions due to medical, ethical, and personal reasons. Results show that implanted children are better overall at expressive language, reading skills, and linguistic competence than many of their nonimplanted peers (e.g., Geers, 2003; Geers et al., 2003a; Miyamoto et al., 2003; Nikolopoulos et al., 2003). Some of the factors that appear to contribute most profoundly to success with the implants are the age of implantation and duration of use of the implants (e.g., Boothroyd & Boothroyd–Turner, 2002; El–Hakim et al., 2002; Hammes et al., 2002; Miyamoto et al., 2003; Wu & Yang, 2003), speech scores before implantation (e.g., Dowell et al., 2002), as well as educational emphasis on oral-aural communication (Geers et al., 2003b). Despite advances in cochlear implant technology, speech recognition in noise and sound localization abilities of most users remain poor. Bilateral implants in adults with late-onset deafness (after childhood) appear to have succeeded at restoring some of these abilities in a large number of patients (Gantz et al., 2002; Litovsky et al., 2004a; Nopp et al., 2004; Tyler et al., 2002; van Hoesel & Tyler, 2003). With regard to bilateral implants in children, both short- and long-term benefits need to be determined (Kuhn–Inacker et al., 2004; Litovsky et al., 2004a, b; 2004a; Winkler et al., 2002).
The purpose of this study was to evaluate sound localization acuity in a group of children who received BI cochlear implants in sequential procedures, at least 6 mos apart (typically several years apart; see Table 1). Unlike many adults who have participated in similar studies (e.g., Gantz et al., 2002; Litovsky et al., 2004a; Nopp et al., 2004; Tyler et al., 2002; van Hoesel and Tyler, 2003), all children except for one were diagnosed with severe-to-profound hearing loss at a very young age, before the acquisition of language skills. In fact, the one child (subject 9) who was able to localize sounds with 5° accuracy in the BI condition, compared with 40 to 50° in the monaural conditions, had a significant amount of auditory exposure during early childhood. Having had acoustic binaural hearing for a number of years before becoming deaf renders this child unusual compared with the other children who experience auditory deprivation much earlier in life. The localization acuity seen in subject 9 is consistent with results observed in adults with similar audiological histories (e.g., Litovsky et al., 2004a; Nopp et al., 2004; van Hosel and Tyler, 2003). For the remaining children, during the time period that followed activation of the second cochlear implant, the auditory system was most likely undergoing a profound readjustment to the presence of active stimulation. Similarly, activation of the binaural circuits may have occurred for the first time in many years (if not ever).
The finding that children such as subject 2, with a 12-yr history of having no binaural hearing, are able to learn to localize (after 23 mos of bilateral hearing) is highly significant. It suggests that the potential benefit and success with bilateral cochlear implants may not be restricted to children who are implanted immediately after the onset of deafness. The extent to which a “critical period” exists during development for these particular abilities remains to be determined. Finally, the remarkable difference between subject 9 and the rest of the group at the onset of bilateral hearing must be further investigated in larger groups of children. The MAA task used here can clearly serve as a measure of sensitivity to directional cues and might prove useful in showing what is to be expected from children who are implanted at various intervals relative to the onset of deafness in each ear.
One of the challenges to the data interpretation is the difficulty in knowing which auditory and/or perceptual mechanisms are likely to affect performance. The finding that the majority of children attained MAA thresholds ≤20° in the BI listening mode suggests that the addition of a second cochlear implant certainly enhances localization acuity compared with a single cochlear implant mode. This is especially noticeable in the data obtained in the first year after activation of the second cochlear implant. Interestingly, when children were tested during the second year of BI experience the benefit of the BI listening mode diminished. We must carefully consider the possible factors that might have resulted in improved localization acuity under monaural conditions after exposure to bilateral stimulation. Since all the children who participated in this study had used their first cochlear implant for a number of years, there is no reason to expect improvement in their first implant performance over time. It may be reasonable to argue that before having two implants, the children did not know how to solve the problem put before them, namely, “where is the sound coming from?” It is likely that after activation of the second implant, they experienced a novel percept which enabled them to suddenly separate sounds according to their locations. This may be akin to the “pop-out” effect known to occur in the visual modality under conditions in which context, training, and facilitation enhance perceptual sensitivity (e.g., Grossberg, 2001). In the present study, only once they learned the concept of “where” a sound is located, BI children with approximately 1 yr’s listening experience most likely transferred knowledge about the task to the monaural listening mode.
Evidence for learning effects under monaural conditions is also present in the second cochlear implant data; performance is considerably worse with second cochlear implant than the first cochlear implant during the first year after second cochlear implant activation, but MAA thresholds for the second cochlear implant mode appear to improve during the second year after activation. Further follow-ups to evaluate whether the two ears become equivalent at some point in time are important.
Monaural left/right discrimination was probably enabled by the fact that, even when overall sound level was varied, it was only roved by ±4 dB, which may have been insufficient to adequately eliminate overall level cues available at each ear. The range over which overall level cues can be used by BI cochlear implant users to solve localization tasks is yet to be established; in normal-hearing persons as much as 20 dB may be required to fully eliminate these monaural level cues (e.g., Middlebrooks & Green, 1991). It should be noted that the overall 8 dB rove was carefully selected during pilot testing in an attempt to constrain all stimulus levels to the range over which stimuli were easily audible, while avoiding activation of the automatic gain control circuits in the various devices. Our results should nonetheless be interpreted with caution, because the long-term benefits of BI cochlear implants, especially on more complex tasks of sound localization, remain to be seen.
Changes in audiological criteria for implant candidacy have resulted in patients with some residual low-frequency hearing receiving a cochlear implant in one ear and continuing to use their hearing aid in the nonimplanted ear. Availability of these bimodal cochlear implant + hearing aid users has allowed us to study the extent to which children can integrate a bit of acoustic hearing with electric hearing in ways that produce measurable improvement in localization acuity. The group of bimodal children tested here typically performed worse on the MAA task than the children with BI cochlear implants. In the BI listening mode the difference was statistically significant. Interestingly though, two of the bimodal children (subjects 17 and 18) had MAA thresholds within the range of their cochlear implant + cochlear implant counterparts. Previous reports in adults (Ching et al., 2004; Tyler et al., 2002) and children (Ching et al., 2001) with bimodal devices suggest that the hearing aid provides some benefit as seen by improved localization acuity when using both devices (cochlear implant + hearing aid) compared with either device alone. In those studies, participants had hearing thresholds of 40 to 50 dB at 125 Hz (Tyler et al., 2002) and 55 dB at 250 Hz (Ching et al., 2001; 2004).
In the unilateral listening mode, there was no statistically significant difference between the cochlear implant + cochlear implant and cochlear implant + hearing aid groups. Due to the small .N size and high variance, it is difficult to draw overarching conclusions. The individual differences are, however, important to emphasize. Two of the cochlear implant + hearing aid children performed as well as the cochlear implant + cochlear implant children in both the unilateral and BI listening modes. One might ask whether audiometric thresholds can help to explain the individual differences. One of the best performers (patient 17) had unaided thresholds of 90 to 100 dB but aided thresholds of 35 to 40 dB at all frequencies. Another excellent performer (patient 18) had unaided thresholds of 55 dB at 250 Hz (aided thresholds unavailable). It is important to note that one child who could not perform the task (patient 14) and three children with poor performance (patients 13, 15, and 16) were not uniquely different from subject 17 (see Table 2). Therefore, performance on the MAA task cannot be easily predicted from or accounted by pure tone audiometric thresholds.
It is also important to note here that we did not attempt to provide these bimodal children with specific fitting strategies aimed at maximizing compatibility of the cochlear implant and hearing aid. Each ear was fitted independently in the clinic, with the goals being to maximize speech understanding and comfort. It is certainly possible that the combined acoustic and electric hearing in our population of children with cochlear implant + hearing aid did not produce fused, coherent auditory images. Attempts to clarify the issue through verbal interactions with the children did not always reveal consistent information. It is reasonable to suspect that there was variation among children in the cochlear implant + cochlear implant and cochlear implant + hearing aid groups in the extent to which they perceived fused, correlated auditory images. It may be that unfused or decorrelated signals played a role in degrading binaural sensitivity (e.g., Breebaart & Kohlrausch, 2001; Culling et al., 2001). However, because objective measurements were not obtained, it is not possible at this point to know whether decorrelation of BI signals is a good predictor of MAA thresholds in these children. An important future direction is to obtain objective measures of fusion and/or correlation to better understand what stimulus conditions and stimulation modes can best enhance performance on binaural tasks.
As a conclusive note of caution, given the potential advances in technology, gene therapy, hair-cell regeneration, stem cells, and other possible future treatments for hearing loss (e.g., Izumikawa et al., 2005; Li et al., 2004;Merest & Cotanche, 2004), there may be reasons for questioning BI implantation in children with significant residual hearing (Rubinstein et al., 2003; Wilson et al., 2003), and additional work is required to determine what the guidelines should be for making such decisions.
Methodological Recommendations for Clinical Research in This Area
Clinicians who may be interested in pursuing this topic further can implement the measures described here in a fairly straightforward manner. The following procedures are recommended: (1) Children ages 4 yrs and older with strong verbal communicative skills can typically participate in a task that requires them to hold their head still and to select the direction of a sound source. Engaging the children in “listening games” such as those described here and motivation with prizes/stickers also plays a major role in ensuring successful participation. (2) Stimulus consisting of a speech utterance, such as a recording of the word “baseball,” and a method by which source position angles are fixed for a given block of trials. (3) Sound levels roved over at least an 8 dB range to minimize the use of overall level cues. (4) Measures of performance on BI and unilateral listening modes should be alternated, in random order. (5) It is important to provide the child with feedback about the correct source position after each trial. (6) Repeated measures over various intervals after implantation of the second cochlear implant may be important; 3, 12, 18, and 24 mos are recommended. (7) Training with feedback, such as during fitting and/or speech therapy sessions, can improve performance and might help children to develop listening strategies that can be applied in realistic listening situations.
Conclusions
The impact of auditory deprivation on binaural hearing in humans is poorly understood. Children with cochlear implants represent a unique population of individuals who have undergone variable amounts of auditory deprivation before being able to hear. Even more unique are children who received BI cochlear implants, in sequential surgical procedures, several years apart. Auditory deprivation in these individuals consists of a two-stage process, whereby complete deafness is experienced initially, followed by deafness in one ear. The data presented here are the first to show effects of auditory plasticity on binaural abilities in deaf children. Deprivation was unique in that hearing to the two ears was restored at two intervals. Our findings suggest that the period during which plasticity occurs in human binaural system is protracted, extending into middle to late childhood.
Potential benefits of BI cochlear implants are yet to be fully understood. The factors that could contribute to such benefits need to be carefully evaluated in large populations of children, on a variety of tasks, including real-world measures (localization, speech-in-noise) as well as other measures of language competence, school performance, and general wellbeing. Clearly, intersubject variables such as duration of deafness, residual hearing, neural survival in the implanted ear, to name a few, can potentially play a large role in determining BI benefit. The exact nature of the BI benefit should also be identified. One type of benefit may be attributable to a dual-channel auditory system, whereby the child can take advantage of simply having one ear on each side of the head; monaural head shadow and the “better ear” effect would both have potential benefits in this situation. A second type of benefit, which, of true binaural interaction, would occur if the auditory brain stem were receiving time-locked, synchronized and correlated signals from the two ears. The extent to which binaural interaction actually occurs in either the cochlear implant + cochlear implant or cochlear implant + hearing aid listening modes has not been confirmed to date. One of the key issues that must be addressed is that of BI fitting strategies. For instance, we might consider the potential role of hardware-driven synchronization of two devices, possibly in both populations.
Acknowledgments
This work would not have been possible without the dedication of the children and their parents, many of whom traveled to our lab in Madison, Wisconsin, and whose hard work and diligence was commendable. We are very grateful to Julie Hitchcock and to Drs. Jane Madell, Marilyn Neault, Susan Waltzman, and Nancy Young for the important role played in subject referral. The authors are also grateful to Dr. Belinda Henry for advice at the outset of the study, Dr. Gongqiang Yu, David Wilson, and Jordan Bonnett for technical assistance and software development, and to Dianna Brown, Soha Garadat, Allison Olson, Jon Douglas, and Corina Vidal for assistance with data collection and analysis. This work was supported by NIDCD (R01 DC003083 and R21 DC006641 to R.Y.L.) and in part by Cochlear Americas.
Footnotes
Panel D shows an interesting example of a case in which performance on the BI and second-ear conditions was nonmonotonic as a function of angle, whereas performance on the first ear condition only reached >71% at 60°. Because the 90° data were below chance, a second block of trials was run. at 60° to confirm this finding, resulting in similar performance. This nonmonotonicity was rarely observed, and as such was inconsistent and not predictable from any location or stimulus parameters. Although individual microphones might yield acoustic properties that would affect behavior in this way, measurements would have to be made to better understand how directionally dependent cues affect performance in these circumstances.
Although some repeated measures were also made with subject 1 (see Table 3), there was not sufficient time to allow measurements in every listening mode at each interval. The most complete data set for that subject was obtained at 15 mos after activation of the second cochlear implant, and those data are included in the group figures.
References
- Armstrong M, Pegg P, James C, Blarney P. Speech perception in noise with implant and hearing aid. American Journal of Otolaryngology. 1997;18(Suppl 6):S140–S141. [PubMed] [Google Scholar]
- Ashmead DH, Clifton RK, Ferris EE. Precision of auditory localization in human infants. Developmental Psychology. 1987;23:641–647. [Google Scholar]
- Blauert J. Spatial Hearing: The Psychophysics of Human Sound Localization. 2. Cambridge, MA: The MIT Press; 1997. [Google Scholar]
- Boothroyd A, Boothroyd-Turner D. Postimplantation audition and educational attainment in children with prelingually acquired profound deafness. Annals of Otology, Rkinology, and Laryngology Supplement. 2002;189:79–84. doi: 10.1177/00034894021110s517. [DOI] [PubMed] [Google Scholar]
- Breebaart J, Kohlrausch A. The influence of interaural stimulus uncertainty on binaural signal detection. Journal of the Acoustical Society of America. 2001;109:331–345. doi: 10.1121/1.1320472. [DOI] [PubMed] [Google Scholar]
- Bronkhorst A. The cocktail party phenomenon: A review of research on speech intelligibility in multiple-talker conditions. Acustica. 2000;86:117–128. [Google Scholar]
- Ching TY, Incerti P, Hill M. Binaural benefits for adults who use hearing aids and cochlear implants in opposite ears. Ear & Hearing. 2004;25:9–21. doi: 10.1097/01.AUD.0000111261.84611.C8. [DOI] [PubMed] [Google Scholar]
- Ching TY, Psarros C, Hill M, Dillon H, Incerti P. Should children who use cochlear implants wear hearing aids in the opposite ear? Ear & Hearing. 2001;22:365–380. doi: 10.1097/00003446-200110000-00002. [DOI] [PubMed] [Google Scholar]
- Culling JF, Colburn HS, Spurchise M. Interaural correlation sensitivity. Journal of the Acoustical Society of America. 2001;110:1020–1029. doi: 10.1121/1.1383296. [DOI] [PubMed] [Google Scholar]
- Culling JF, Hawley ML, Litovsky RY. The role of head-induced interaural time and level differences in the speech reception threshold for multiple interfering sound sources. Journal of the Acoustical Society of America. 2004;116:1057–1065. doi: 10.1121/1.1772396. [DOI] [PubMed] [Google Scholar]
- Dillon H. Hearing Aids. New York: Thieme Press; 2001. [Google Scholar]
- Dowell RC, Dettman SJ, Hill K, Winton E, Barker EJ, Clark GM. Speech perception outcomes in older children who use multichannel cochlear implants: older is not always poorer. Annals of Otology, Rhinology, and Laryngology Supplement. 2002;189:97–101. doi: 10.1177/00034894021110s520. [DOI] [PubMed] [Google Scholar]
- Durlach N, Colburn HS. Binaural phenomena. In: Carterette EC, Friedman M, editors. Handbook of Perception. IV. New York: Academic Press; 1978. pp. 405–466. [Google Scholar]
- El-Hakim H, Abdolell M, Mount RJ, Papsin BC, Harrison RV. Influence of age at implantation and of residual hearing on speech outcome measures after cochlear implantation: binary partitioning analysis. Annals of Otology, Rhinology, and Laryngology Supplement. 2002;189:102–108. doi: 10.1177/00034894021110s521. [DOI] [PubMed] [Google Scholar]
- Gantz BJ, Tyler RS, Rubinstein JT, Wolaver A, Lowder M, Abbas P, et al. Binaural cochlear implants placed during the same operation. Otology and Neurotology. 2002;23:169–180. doi: 10.1097/00129492-200203000-00012. [DOI] [PubMed] [Google Scholar]
- Geers AE. Predictors of reading skill development in children with early cochlear implantation. Ear & Hearing. 2003;24(Suppl):59S–68S. doi: 10.1097/01.AUD.0000051690.43989.5D. [DOI] [PubMed] [Google Scholar]
- Geers A, Brenner C, Davidson L. Factors associated with development of speech perception skills in children implanted by age five. Ear & Hearing. 2003a;24(Suppl):24S–35S. doi: 10.1097/01.AUD.0000051687.99218.0F. [DOI] [PubMed] [Google Scholar]
- Geers AE, Nicholas JG, Sedey AL. Language skills of children with early cochlear implantation. Ear & Hearing. 2003b;24:46S–58S. doi: 10.1097/01.AUD.0000051689.57380.1B. [DOI] [PubMed] [Google Scholar]
- Grossberg S. Linking the laminar circuits of visual cortex to visual perception: development, grouping, and attention. Neuroscience and Biobehavioral Reviews. 2001;25:513–526. doi: 10.1016/s0149-7634(01)00030-6. [DOI] [PubMed] [Google Scholar]
- Hammes DM, Novak MA, Rotz LA, Willis M, Edmondson DM, Thomas JF. Early identification and cochlear implantation: critical factors for spoken language development. Annals of Otology, Rhinology, and Laryngology Supplement. 2002;189:74–78. doi: 10.1177/00034894021110s516. [DOI] [PubMed] [Google Scholar]
- Hausler R, Colburn HS, Marr E. Sound localization in subjects with impaired hearing. Spatial-discrimination and interaural-discrimination tests. Acta Oto-laryngologica Supplementum. 1983;400:1–62. doi: 10.3109/00016488309105590. [DOI] [PubMed] [Google Scholar]
- Izmnikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF, Brough DE, Raphael Y. Auditory hair cell replacement and hearing improvement by Atohl gene therapy in deaf mammals. Nature Medicine. 2005;11:271–276. doi: 10.1038/nm1193. Epub 2005 Feb 13. [DOI] [PubMed] [Google Scholar]
- Kong YY, Stickney G, Zeng FG. Speech and melody recognition in binaurally combined acoustic and electric hearing. Journal of the Acoustical Society of America. 2005;117:1351–1361. doi: 10.1121/1.1857526. [DOI] [PubMed] [Google Scholar]
- Kuhn-Inacker H, Shehata-Dieler W, Muller J, Helms J. Bilateral cochlear implants: a way to optimize auditory perception abilities in deaf children? International Journal of Pediatric Otorhinolaryngology. 2004;68:1257–1266. doi: 10.1016/j.ijporl.2004.04.029. [DOI] [PubMed] [Google Scholar]
- Li H, Corrales CE, Edge A, Heller S. Stem cells as therapy for hearing loss. Trends in Molecular Medicine. 2004;10:309–315. doi: 10.1016/j.molmed.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Litovsky R, Macmillan N. Minimum auditory angle for clicks with simulated echoes: Effects of azimuth and standard. Journal of the Acoustical Society of America. 1994;96:752–758. doi: 10.1121/1.411390. [DOI] [PubMed] [Google Scholar]
- Litovsky RY. Developmental changes in the precedence effect: estimates of minimum audible angle. Journal of tile Acoustical Society of America. 1997;102:1739–1745. doi: 10.1121/1.420106. [DOI] [PubMed] [Google Scholar]
- Litovsky RY, Ashmead DM. Developmental aspects of binaural and spatial hearing. In: Gilkey RH, Anderson TR, editors. Binaural and Spatial Hearing. Hillsdale, NJ: Lawrence Erlbaum; 1997. [Google Scholar]
- Litovsky RY, Johnston P, Parkinson A, Peters R, Lake J. Bilateral cochlear implants in children: Effect of experience. In: Miyamoto R, editor. International Congress Series. Vol. 1273. Elsevier; 2004b. pp. 451–454. [Google Scholar]
- Litovsky RY, Parkinson A, Arcaroli J, Peters R, Lake J, Johnstone P, Yu G. Bilateral cochlear implants in adults and children. Archives of Otology Head and Neck Surgery. 2004a;130:648–655. doi: 10.1001/archotol.130.5.648. [DOI] [PubMed] [Google Scholar]
- Middlebrooks JC, Green DM. Sound localization by human listeners. Annual Review of Psychology. 1991;42:135–159. doi: 10.1146/annurev.ps.42.020191.001031. [DOI] [PubMed] [Google Scholar]
- Miyamoto RT, Houston DM, Kirk KI, Perdew AE, Svirsky MA. Language development in deaf infants following cochlear implantation. Acta Oto-laryngologica. 2003;123:241–244. doi: 10.1080/00016480310001079. [DOI] [PubMed] [Google Scholar]
- Morest DK, Cotanche DA. Regeneration of the inner ear as a model of neural plasticity. Journal of Neuroscience Research. 2004;78:455–460. doi: 10.1002/jnr.20283. [DOI] [PubMed] [Google Scholar]
- Nelson PB, Jin SH, Carney AE, Nelson DA. Understanding speech in modulated interference: cochlear implant users and normal-hearing listeners. Journal of the Acoustical Society of America. 2003;113:961–968. doi: 10.1121/1.1531983. [DOI] [PubMed] [Google Scholar]
- Nikolopoulos TP, Lloyd H, Starczewski H, Gallaway C. Using SNAP Dragons to monitor narrative abilities in young deaf children following cochlear implantation. International Journal of Pediatric Otorhinolaryngology. 2003;67:535–541. doi: 10.1016/s0165-5876(03)00034-x. [DOI] [PubMed] [Google Scholar]
- Nopp P, Schleich P, D’Haese P. Sound localization in bilateral users of MED-EL COMBI 40/40 + cochlear implants. Ear & Hearing. 2004;25:205–214. doi: 10.1097/01.aud.0000130793.20444.50. [DOI] [PubMed] [Google Scholar]
- Pasanisi E, Bacciu A, Vincenti V, Guida M, Berghenti MT, Barbot A, Panu F, Bacciu S. Comparison of speech perception benefits with SPEAK and ACE coding strategies in pediatric Nucleus cochlear implant24M cochlear implant recipients. International Journal of Pediatric Otorhinolaryngology. 2002;64:159–163. doi: 10.1016/s0165-5876(02)00075-7. [DOI] [PubMed] [Google Scholar]
- Peters B, Litovsky R, Lake J, Parkinson A. Sequential Bilateral Cochlear Implantation in Children. In: Miyamoto R, editor. International Congress Series. Vol. 1273. Elsevier; 2004. pp. 462–465. [Google Scholar]
- Psarros CE, Plant KL, Lee K, Decker JA, Whitford LA, Cowan RS. Conversion from the SPEAK to the ACE strategy in children using the nucleus 24 cochlear implant system: speech perception and speech production outcomes. Ear & Hearing. 2002;23:18S–27S. doi: 10.1097/00003446-200202001-00003. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, Shannon RV. Sending sound to the brain. Science. 2002;295:1025–1029. doi: 10.1126/science.1067796. [DOI] [PubMed] [Google Scholar]
- Rubinstein JT, Gantz BJ, Tyler RS, Lowder M, Witt S. Binaural cochlear implantation: Promise and challenges. Oral presentation at the 9th symposium on pediatric cochlear implants; Washington, DC. 2003. [Google Scholar]
- Stickney GFG, Litovsky RY, Assman P. Cochlear implant speech recognition with speech maskers. Journal of the Acoustical Society of America. 2004;116:1081–1091. doi: 10.1121/1.1772399. [DOI] [PubMed] [Google Scholar]
- Tyler RSBJ, Parkinson AJ, Wilson BS, Witt S, Preece JP, Nobel W. Patients utilizing a hearing aid and a cochlear implant: speech perception and localization. Ear & Hearing. 2002;23:98–105. doi: 10.1097/00003446-200204000-00003. [DOI] [PubMed] [Google Scholar]
- van Hoesel R, Tyler R. Speech perception, localization and lateralization with bilateral cochlear implants. Journal of the Acoustical Society of America. 2003;113:1617–1630. doi: 10.1121/1.1539520. [DOI] [PubMed] [Google Scholar]
- van Hoesel RJ. Exploring the benefits of bilateral cochlear implants. Audiology & Neuro-otology. 2004;9:234–246. doi: 10.1159/000078393. [DOI] [PubMed] [Google Scholar]
- Wilson BS, Finley CC, Lawson DT, Wolford RD, Eddington DK, Rabinowitz WM. Better speech recognition with cochlear implants. Nature. 1991;352:236–238. doi: 10.1038/352236a0. [DOI] [PubMed] [Google Scholar]
- Wilson BS, Lawson DT, Muller JM, Tyler RS, Kiefer J. Cochlear implants: some likely next steps. Annu Rev Biomed Eng. 2003;5:207–249. doi: 10.1146/annurev.bioeng.5.040202.121645. [DOI] [PubMed] [Google Scholar]
- Winkler F, Schon F, Peklo L, Muller J, Feinen C, Helms J. The Wurzburg questionnaire for assessing the quality of hearing in cochlear implant-children (WH-cochlear implant) J Laryngorhino-otologie. 2002;81:211–216. doi: 10.1055/s-2002-25042. [DOI] [PubMed] [Google Scholar]
- Wu JL, Yang HM. Speech perception .of Mandarin Chinese speaking young children after cochlear implant use: effect of age at implantation. International Journal of Pediatric Otorhinolaryngology. 2003;67:247–253. doi: 10.1016/s0165-5876(02)00378-6. [DOI] [PubMed] [Google Scholar]