Abstract
An extract from Phyllanthus engleri was identified in a bioinformatic analysis of NCI 60-cell natural product extract screening data, that selectively inhibited the growth of renal cancer cell lines. Bioassay guided fractionation yielded two new guaiane sesquiterpenes, englerins A (1) and B (2). Englerin A showed 1000-fold selectivity against 6 of 8 renal cancer cell lines with GI50 values ranging from 1-87 nM. The structures of 1 and 2 and their relative stereochemistry were established by spectroscopic methods.
Kidney cancer affects an estimated 39,000 individuals in the USA yearly, and is a major cause of morbidity and mortality in adults.1 Currently approved drugs such as bevacizumab, sunitinib and sorafenib offer benefit to patients with metastatic renal cancer but do not produce complete responses, require long term administration for continued disease control and have serious adverse side effects.1 Thus, the search for new agents which display specific activity against renal cancers is of great interest.
With this in mind an extensive study was carried out to identify natural product extracts that exhibited a preferential sensitivity towards renal tumor cells in the NCI60 cell panel when compared to the other eight organ panels in the screen (leukemia, non-small cell lung cancer, colon, CNS, melanoma, ovarian, prostate and breast). This analysis for renal panel selectivity identified an initial 34 natural product extracts (see Supporting Information). The extract of Phyllanthus engleri was chosen because of its excellent selectivity and potency, relative to the other extracts, against the renal panel.
The genus Phyllanthus is one of the largest genera of the Euphorbiaceae, containing approximately 700 species. Like most other members of the Euphorbiaceae, Phyllanthus is a source of biologically active compounds, with numerous species being widely used in various traditional medicine systems.2-4 P. engleri is found in East Africa, particularly Tanzania and Zimbabwe, and has not been subjected to chemical study in recent years. The present investigation was carried out on extracts from a collection made in Tanzania, where the roots and bark of this plant are reported to be lethal.5,6 The only previously characterized compound isolated from P. engleri is the triterpene phyllanthol,7 which has not been shown to possess biological activity. A poorly characterized toxic glycoside was also found by other early investigators.5
We report here the isolation of two new guaiane sesquiterpenes, englerins A (1) and B (2), isolated from the 1:1 CH2Cl2-MeOH extractives of the stem bark of P. engleri. Employing a 2-cell assay consisting of one sensitive (A498 or UO-31) and one resistant (SF-295) cell line, we carried out bioassay guided fractionation of the stem bark extract using an initial diol SPE separation. This identified the active components as being present in the CH2Cl2 solubles. Successive fractionation using silica gel chromatography and C18 HPLC led to the isolation of 1 as the major active compound (see supporting information).
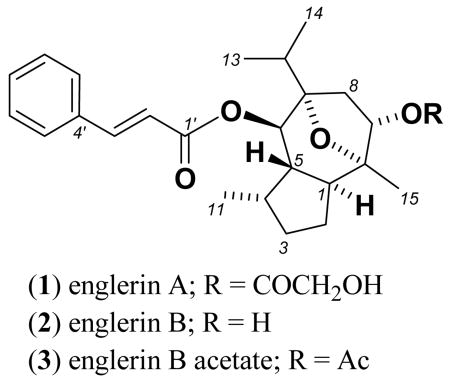
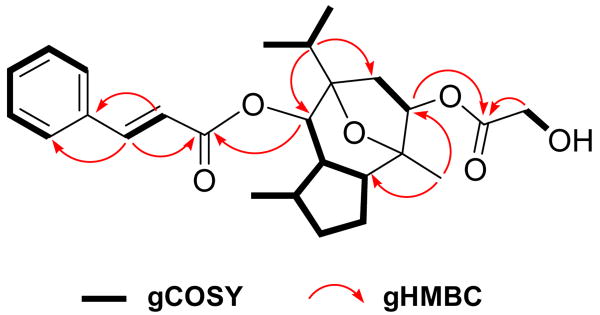
Englerin A (1) was isolated as a white solid with a pseudomolecular ion [M+H]+ consistent with a molecular formula (C26H35O6 Δ = 1.7 ppm) that required 10 double bond equivalents (DBE). COSY NMR data for 1 identified six isolated spin systems (Figure 1). The 13C NMR spectrum (Table 1) revealed ten sp2 carbons (δC 117.8–145.2) corresponding to four carbon-carbon double bonds and two carbonyl carbons. The deshielded resonances at δH 6.59 and 7.68 with a diagnostic coupling (J2′,3′ = 16.0 Hz) and δH 7.72 (2H, brd, 7.5 Hz) and δH 7.43 (3H, m) in the 1H NMR spectrum were indicative of a trans double bond, and a monosubstituted benzene ring, respectively. These signals together with the two additional deshielded sp2 carbons (δC 172.4 and 165.2), consistent with ester carbonyls, accounted for 7 DBE, requiring the remaining elements to constitute a tricyclic system. A set of HMBC correlations from the trans double bond methines to the benzene ring and C-1′ carbonyl (δC 165.2) confirmed the presence of a cinnamate side chain, while an HMBC correlation from the oxymethine signal at δH 4.97 to the C-1′ carbonyl confirmed its substitution at C-6. An additional HMBC correlation from an hydroxymethylene (δH 4.04, brs) to the C-1″ carbonyl (δC 172.4) confirmed the glycolate fragment as shown in Figure 1.
Figure 1.
Some key 2D correlations for englerin A (1)
Table 1.
NMR (DMSO-d6) data for englerins A (1) and B (2)
| no. | δH (m, J (Hz)) | δC | COSY | HMBC (1H-13C) | δH (m, J (Hz)) | δC |
|---|---|---|---|---|---|---|
| (1) | (2) | |||||
| 1 | 1.66 (m) | 47.1 | H-5, H-2b | C-15, C-10, C-9, C-6, C-5, C-4, C-2 | 1.60 (m) | 47.3 |
| 2a | 1.62 (m) | 24.2 | H-3b, H-2b, H-1 | C-4, C-1 | 1.60 (m) | 24.4 |
| 2b | 1.23 (m) | H-3a, H-3b, H-2a, H-1 | C-15, C-10, C-9, C-3, C-1 | 1.12 (m) | ||
| 3a | 1.93 (m) | 30.6 | H-4, H-3b, H-2b | C-11, C-4, C-1 | 1.87 (m) | 30.7 |
| 3b | 1.16 (m) | H-3a, H-2a | C-11, C-5, C-4, C-2 | 1.12 (m) | ||
| 4 | 2.04 (m) | 30.7 | H-11, H-5, H-3a | C-11, C-3, C-2, C-1 | 1.99 (m) | 30.6 |
| 5 | 1.62 (m) | 46.0 | H-6, H-4, H-1 | C-11, C-10, C-6, C-4, C-2, C-1 | 1.50 (m) | 45.8 |
| 6 | 4.97 (d, 10.0) | 72.6 | H-5 | C-12, C-8, C-7, C-5, C-1′ | 4.91 (d, 10.0) | 71.1 |
| 7 | 84.8 | 84.1 | ||||
| 8a | 2.64 (dd, 14.5, 8.0) | 39.5 | H-9, H-8b | C-12, C-10, C-6, C-5 | 2.49 (dd, 13.5, 7.5) | 42.5 |
| 8b | 1.73 (dd, 14.5, 2.5) | H-9, H-8a | C-12, C-9, C-7, C-6 | 1.57 (m) | ||
| 9 | 5.15 (dd, 8.0, 2.5) | 74.4 | H-8a, H-8b | C-10, C-7, C-6, C-1 | 3.92 (brs) | 70.8 |
| 10 | 84.4 | 85.0 | ||||
| 11 | 0.85 (d, 7.5) | 16.7 | H-4 | C-5, C-3 | 0.82 (d, 7.0) | 16.8 |
| 12 | 1.79 (m) | 32.7 | H-14, H-13 | C-14, C-13, C-8, C-7, C-6 | 1.75 (m) | 32.9 |
| 13 | 0.94 (d, 7.0) | 17.3 | H-12 | C-14, C-12, C-7 | 0.92 (d, 7.0) | 17.4 |
| 14 | 0.88 (d, 7.0) | 18.1 | H-12 | C-13, C-12, C-7 | 0.86 (d, 7.0) | 18.3 |
| 15 | 1.10 (s) | 18.8 | C-10, C-9, C-2, C-1 | 1.11 (s) | 19.4 | |
| 1′ | 165.2 | 165.3 | ||||
| 2′ | 6.59 (d, 16.0) | 117.8 | H-3′ | C-9′/ C-5′, C-6, C-4′, C-3′, C-1′ | 6.57 (d, 16.0) | 118.0 |
| 3′ | 7.68 (d, 16.0) | 145.2 | H-2′ | C-9′/ C-5′, C-4′, C-2′, C-1′ | 7.66 (d, 16.0) | 145.0 |
| 4′ | 133.9 | 134.0 | ||||
| 5′ | 7.72 (brd, 7.5) | 128.5 | H-6′ | C-9′, C-7′, C-4′, C-3′ | 7.71 (m) | 128.5 |
| 6′ | 7.43 (m) | 129.0 | H-5′ | C-8′, C-5′, C-4′, C-3′ | 7.42 (brdd, 3.5, 3.0) | 129.0 |
| 7′ | 7.43 (m) | 130.7 | a | C-8′/C-6′, C-9′/C-5′, | 7.42 (brdd, 3.5, 3.0) | 130.6 |
| 8′ | 7.43 (m) | 129.0 | H-9 | C-9′, C-6′, C-4′, C-3′ | 7.42 (brdd, 3.5, 3.0) | 129.0 |
| 9′ | 7.72 (brd, 7.5) | 128.5 | H-8′ | C-7′, C-5′, C-4′, C-3′ | 7.71 (m) | 128.5 |
| 1″ | 172.4 | |||||
| 2″ | 4.04 (brs) | 59.8 | C-1″ | |||
| OH | 5.36 (brs) | 4.66 (brd, 4.0) | ||||
overlapping signals prevented unambiguous assignments.
Two of the three remaining COSY spin systems accounted for an isopropyl group, and a methylene (H-8, δHa 2.64 and δHb 1.73) attached to an oxymethine (H-9, δH 5.15 and δC 74.4), respectively. The remaining COSY spin system involved three adjacent sp3 methines connected via two methylenes to form a five membered (H-1 to H-5) ring (Figure 1). Additional COSY correlations suggested the methyl substitution at H-4 and extended H-5 to the H-6 oxymethine (δH 4.97 and δC 72.6). Strong HMBC correlations from a methyl singlet to C-1 and C-9 and likewise, from H-12 to C-6 and C-8 via two quarternery carbons (δC 84.4 and 84.8) defined the guaiane sesquiterpene core8 incorporating the cinnamate and glycolate substituents as shown. The above data accounted for all but one oxygen atom and included nine of the 10 DBEs, necessitating the C-7 and C-10 connection through the unassigned oxygen atom to complete the planar structure of englerin A (1).
The 1H and 13C NMR data (Table 1) for englerin B (2) were very similar to those of 1 with common structure fragments confirmed by a sequence of diagnostic 2D NMR correlations. The key differences were observed in the upfield shift of the 1H NMR resonance for H-9 (δH 3.92, Δ = 1.2 ppm) and in the absence of a methylene singlet around ∼4.00 ppm with lack of a corresponding resonance in the 13C NMR spectrum for one carbonyl resonance. HRMS analysis of compound 2 revealed a pseudomolecular ion [M+H]+ consistent with a molecular formula (C24H33O4 Δ = 1.5 ppm) requiring 9 DBE. These data supported englerin B lacking glycolate substitution at C-9 (δH 4.66). Acetylation of englerin B (2) with pyridine/acetic anhydride, followed by subsequent NMR analysis of 3 revealed a methyl singlet (δH 2.05) and the expected downfield shift for H-9 (δH 5.09) in the 1H NMR spectrum, further supporting a free hydroxy group at this position in the parent molecule (2).
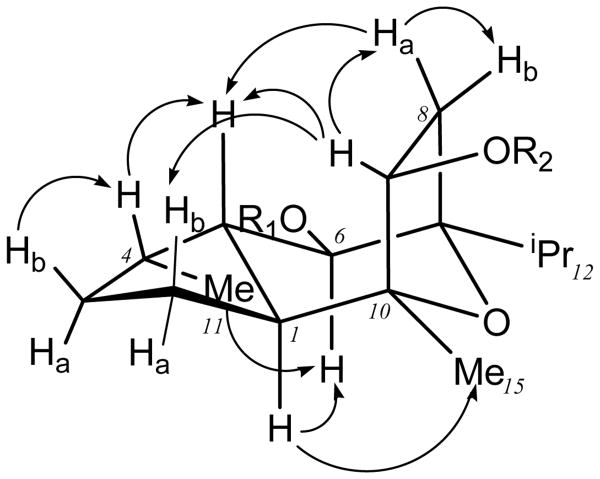
The vicinal coupling data along with selective 1D NOESY experiments for englerin A (1) allowed the relative stereochemistry to be assigned as follows. The coupling constant for H-6 (J5,6 = 10.0 Hz) was indicative of a pseudodiaxial relationship between H-5 and H-6. Selective 1D NOESY experiments revealed strong correlations from H-4, H-8a and H-9 to H-5, suggesting that they were on the same face of the molecule (Figure 2). The absence of any NOESY correlations from H-6 to H-4, H-5, H-8a and H-9 in three different solvents for both compounds 1 and 2 supported an anti relationship between H-5 and H-6 as proposed. However, the resolution of H-5, H-1 and H-2a was insufficient to unambiguously assign NOESY correlations from H-6. Fortuitously, a strong NOESY correlation from H-9 to H-2b suggested that these protons were on the same face. The overlapping 1H NMR signals for H-1 and H-2a notwithstanding, a strong correlation from H-6 to the multiplet at δH 1.62-1.67 was in favor of H-6/H-2a/H-1 (Table 1) being in an α orientation. Therefore with H-5 eliminated from this sequence (see above) the correlation observed was preferably for H-2a/H-1. An energy minimized 3D model clearly favored a 1D NOESY correlation from H-6 to H-1 (2.7 Å) in preference to H-2a (4.9 Å) thereby supporting a trans ring junction with H-1 and H-5 both in pseudoaxial orientations.
Figure 2.
Some key selective 1D NOESY correlations observed for englerin A (1)
Although the chemical shift for H-5 in DMSO-d6 (Table 1) and methanol-d4 (Table S1) partly overlapped with H-1 and H-2a, the H-5 resonance was resolved (δH 1.60, ddd, J = 10.0, 7.0, 3.0 Hz) in deuterated methylene chloride. This provided a means to observe unambiguous correlations in the 1D NOESY experiments. Further, the vicinal coupling (J1,5 = 7.0 Hz) was consistent with the proposed pseudo diaxial relationship between H-5 and H-1 protons. Similar patterns of 1D NOESY correlations were also observed for englerin B (2) in both deutererated DMSO and methylene chloride. Hence, we propose the relative stereochemistry for the englerins as depicted.
Englerin A demonstrated excellent selectivity for the renal cancer cell line panel, with 5 of 8 renal lines having GI50 values under 20 nM (Table 2, Figure 3), while for most other cell lines the GI50 values ranged from 10-20 μM (see Supporting Information). The low activity and selectivity of the structural analog englerin B (2) (see supporting information), suggests that substitution at the C-9 position by the glycolate ester may be important for the observed potency and selectivity. It is well known that glycolic acid (GA), an important metabolite of ethylene glycol, causes acute renal toxicity in mammals.9 Hydroxy acid containing natural products are relatively rare; some of the known GA containing metabolites include pleuromutilin,10 saframycin R11 and an ecdysteroid from a Caribbean sponge.12 The NCI60 cell data13 for pleuromutilin indicated no significant cytotoxicity for this compound while saframycin R, although quite potent, did not show renal selectivity in the NCI60 cell panel.13 Therefore, it appears that glycolate substitution alone cannot account for the renal selectivity of 1. We have not yet systematically determined which ester substitutions will enhance or reduce englerin activity. Interestingly, in a 2-cell assay englerin B acetate (3) showed an approximate 400 fold selectivity against the renal cell line (A498); 60-cell testing is pending to confirm this result. Encouraged by this observation, it is our intention to prepare a series of englerin analogs with different ester substitution at both C-6 and C-9 for biological evaluation.
Table 2.
Renal cancer cell growth inhibition data (mean GI50 in μM) for englerin A (1), compared to average values for taxol.
| renal cell line | 1 | taxol |
|---|---|---|
| 786-0 | <0.01 | 0.034 |
| A498 | <0.01 | 0.10 |
| ACHN | <0.01 | 0.65 |
| CAKI-1 | 15.5 | 0.35 |
| RXF-393 | 0.011 | 0.041 |
| SN12C | 0.087 | 0.018 |
| TK-10 | 15.5 | 0.11 |
| UO-31 | <0.01 | 0.45 |
Figure 3.
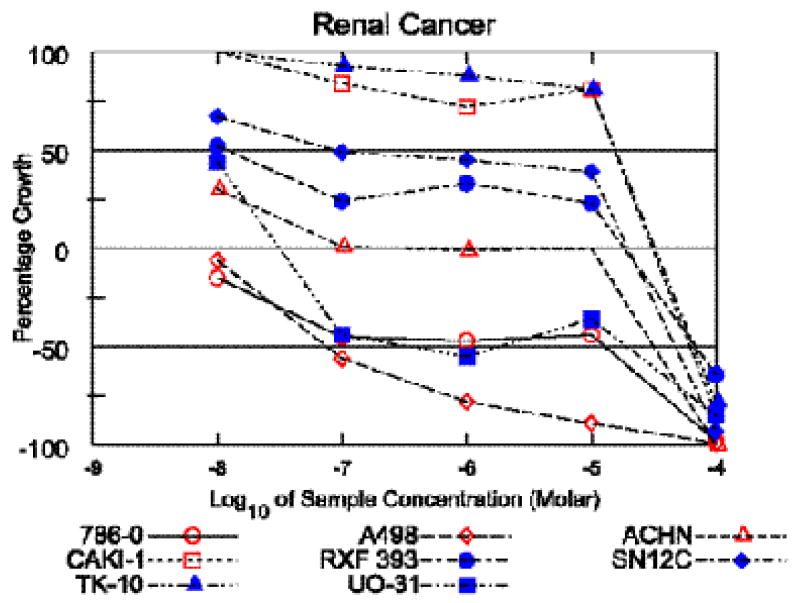
Dose reponse curves for cytotoxic activity of englerin A (1) against the renal cancer cell lines in the NCI 60-cell panel.
A COMPARE analysis of the englerin 60 cell data against the NCI standard agents did not suggest a known mechanism of action. Preliminary mouse toxicity studies with 1 identified a maximum tolerated dose of 5 mg/kg i.p. in mice. Mouse xenograft studies of 1 are underway.
Supplementary Material
Full details on the subpanel sensitivity selection of P. engleri, plant collection, isolation and spectral data, 2 cell assay methodology and NCI60 cell data for 1 and 2. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
The authors wish to thank the Missouri Botanical Garden collectors (Roy Gereau, James Lovett, C.O. Kyalawa, and Z.H. Mbwambo) for the plant collection, Thomas McCloud (Natural Products Support Group, NCI-Frederick) for plant extraction, Melinda Hollingshead (Biological Testing Branch, DTP) for mouse toxicity studies, and Sergey Tarasov and Marzena Dyba (Biophysics Resource Core, Structural Biophysics Laboratory, CCR) for assistance with high resolution mass spectrometry. This Research was supported jointly by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research and by the Developmental Therapeutics Program in the Division of Cancer Treatment and Diagnosis of the National Cancer Institute.
References
- 1.Atkins BA, Ernstoff MS, Figlin RA, Flaherty KT, George DJ, Kaelin WG, Kwon ED, Libermann TA, Linehan WM, McDermott DF, Ochoa AC, Pantuck AJ, Rini BI, Rosen MA, Sosman JA, Sukhatme VP, Vieweg JW, Wood CG, King L. Clin Cancer Res. 2007;13(suppl 2):667s. doi: 10.1158/1078-0432.CCR-06-2231. [DOI] [PubMed] [Google Scholar]
- 2.Calixto JB, Santos ARS, Filbo VC, Yunes RA. Med Res Rev. 1998;18:225. doi: 10.1002/(sici)1098-1128(199807)18:4<225::aid-med2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 3.Bagalkotkar G, Saad MSS, Stanslas J. J Pharm Pharmacol. 2006;58:1559. doi: 10.1211/jpp.58.12.0001. [DOI] [PubMed] [Google Scholar]
- 4.(a) Webster GL, Blumberg BS. J Ethnopharmacol. 1990;30:233. doi: 10.1016/0378-8741(90)90105-3. [DOI] [PubMed] [Google Scholar]; (b) Webster GL, Blumberg BS. J Ethnopharmacol. 1991;34:97. doi: 10.1016/0378-8741(91)90029-d. [DOI] [PubMed] [Google Scholar]; (c) Webster GL, Blumberg BS. J Ethnopharmacol. 1992;36:103. doi: 10.1016/0378-8741(92)90009-g. [DOI] [PubMed] [Google Scholar]; (d) Webster GL, Blumberg BS. J Ethnopharmacol. 1995;45:1. doi: 10.1016/0378-8741(94)01189-7. [DOI] [PubMed] [Google Scholar]
- 5.Breyer-Brandwijk MG. J Pharm Pharmacol. 1934;7:167. [Google Scholar]
- 6.Verdcourt B, Trump EC. Common Poisonous Plants of East Africa. Collins; London, UK: 1969. pp. 58–59. [Google Scholar]
- 7.Alberman KB, Kipping FB. J Chem Soc. 1951:2296. [Google Scholar]
- 8.(a) Ferreira MJP, Oliveira FC, Rodrigues GV, Emerenciano VP. Internet Elec J Mol Design. 2004;3:737. [Google Scholar]; (b) Stierle AA, Stierle DB, Goldstein E, Parker K, Bugni T, Baarson C, Gress J, Blake D. J Nat Prod. 2003;66:1097. doi: 10.1021/np030044w. [DOI] [PubMed] [Google Scholar]; (c) Peng GP, Tian G, Huang XF, Lou FC. Phytochem. 2003;63:877. doi: 10.1016/s0031-9422(03)00222-x. [DOI] [PubMed] [Google Scholar]
- 9.(a) Carney EW, Freshour NL, Dittenber DA, Dryzga MD. Toxicol Sci. 1999;50:117. doi: 10.1093/toxsci/50.1.117. [DOI] [PubMed] [Google Scholar]; (b) Bove KE. Amer J Clin Pathol. 1966;45:46. doi: 10.1093/ajcp/45.1.46. [DOI] [PubMed] [Google Scholar]
- 10.Knauseder F, Brandl E. J Antibiot. 1976;29:125. doi: 10.7164/antibiotics.29.125. [DOI] [PubMed] [Google Scholar]
- 11.Saito N, Kameyama N, Kubo A. Tetrahedron. 2000;56:9937. [Google Scholar]
- 12.Costantino V, Dell'Aversano C, Fattorusso E, Mangoni A. Steroids. 2000;65:138. doi: 10.1016/s0039-128x(99)00096-3. [DOI] [PubMed] [Google Scholar]
- 13.http://dtp.nci.nih.gov/dtpstandard/cancerscreeningdata/index.jsp
- 14.Paull KD, Shoemaker RH, Hodes L, Monks A, Scudiero DA, Rubinstein L, Plowman J, Boyd MR. J Natl Cancer Inst. 1989;81:1088. doi: 10.1093/jnci/81.14.1088. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Full details on the subpanel sensitivity selection of P. engleri, plant collection, isolation and spectral data, 2 cell assay methodology and NCI60 cell data for 1 and 2. This material is available free of charge via the Internet at http://pubs.acs.org.