Abstract
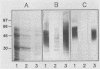
A preparation of Brucella abortus cytoplasmic proteins was depleted of lipopolysaccharide (LPS) by immunoadsorption with a monoclonal antibody (MAb), BC68, specific for the O antigen of B. abortus smooth LPS. Two enzyme-linked immunosorbent assay (ELISA) systems were developed and used in this study. The first system includes an LPS-free cytoplasmic protein preparation; the second one was based on antigen capture on MAb BC68. By using these systems, we have demonstrated that 94% (33 of 35) of the brucellosis patients studied showed immunoglobulin G antiprotein response and also that all of the patients showed a strong anti-LPS reactivity. Thirty-six serologically positive individuals with no active infection at the time of examination (SPI) were also included. No immunoglobulin G antibodies against proteins were detected in 34 of them (92%), whereas 31 SPI (86%) showed various degrees of anti-LPS reactivity. The use of the LPS-free protein extract in ELISAs made it possible to establish differential reactivity patterns between active and inactive brucellosis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bricker B. J., Tabatabai L. B., Deyoe B. L., Mayfield J. E. Conservation of antigenicity in a 31-kDa Brucella protein. Vet Microbiol. 1988 Dec;18(3-4):313–325. doi: 10.1016/0378-1135(88)90096-x. [DOI] [PubMed] [Google Scholar]
- Bricker B. J., Tabatabai L. B., Judge B. A., Deyoe B. L., Mayfield J. E. Cloning, expression, and occurrence of the Brucella Cu-Zn superoxide dismutase. Infect Immun. 1990 Sep;58(9):2935–2939. doi: 10.1128/iai.58.9.2935-2939.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galfrè G., Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 1981;73(Pt B):3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Moreno E., Berman D. T., Boettcher L. A. Biological activities of Brucella abortus lipopolysaccharides. Infect Immun. 1981 Jan;31(1):362–370. doi: 10.1128/iai.31.1.362-370.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyon I., Berman D. T. Isolation, purification, and partial characterization of Brucella abortus matrix protein. Infect Immun. 1983 Jan;39(1):394–402. doi: 10.1128/iai.39.1.394-402.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyón I., Gamazo C., Díaz R. Properties of the outer membrane of Brucella. Ann Inst Pasteur Microbiol. 1987 Jan-Feb;138(1):89–91. doi: 10.1016/0769-2609(87)90082-2. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicer T., Ariza J., Foz A., Pallares R., Gudiol F. Specific antibodies detected during relapse of human brucellosis. J Infect Dis. 1988 May;157(5):918–924. doi: 10.1093/infdis/157.5.918. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Riezu-Boj J. I., Moriyón I., Blasco J. M., Gamazo C., Díaz R. Antibody response to Brucella ovis outer membrane proteins in ovine brucellosis. Infect Immun. 1990 Feb;58(2):489–494. doi: 10.1128/iai.58.2.489-494.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan J. D., Jr, Watson S. W. Factors affecting the sensitivity of Limulus lysate. Appl Microbiol. 1974 Dec;28(6):1023–1026. doi: 10.1128/am.28.6.1023-1026.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang V. C., Peralta J. M., Simons A. R. Enzyme-linked immunoelectrotransfer blot techniques (EITB) for studying the specificities of antigens and antibodies separated by gel electrophoresis. Methods Enzymol. 1983;92:377–391. doi: 10.1016/0076-6879(83)92032-3. [DOI] [PubMed] [Google Scholar]
- Verstreate D. R., Creasy M. T., Caveney N. T., Baldwin C. L., Blab M. W., Winter A. J. Outer membrane proteins of Brucella abortus: isolation and characterization. Infect Immun. 1982 Mar;35(3):979–989. doi: 10.1128/iai.35.3.979-989.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voller A., Bidwell D., Huldt G., Engvall E. A microplate method of enzyme-linked immunosorbent assay and its application to malaria. Bull World Health Organ. 1974;51(2):209–211. [PMC free article] [PubMed] [Google Scholar]