Abstract
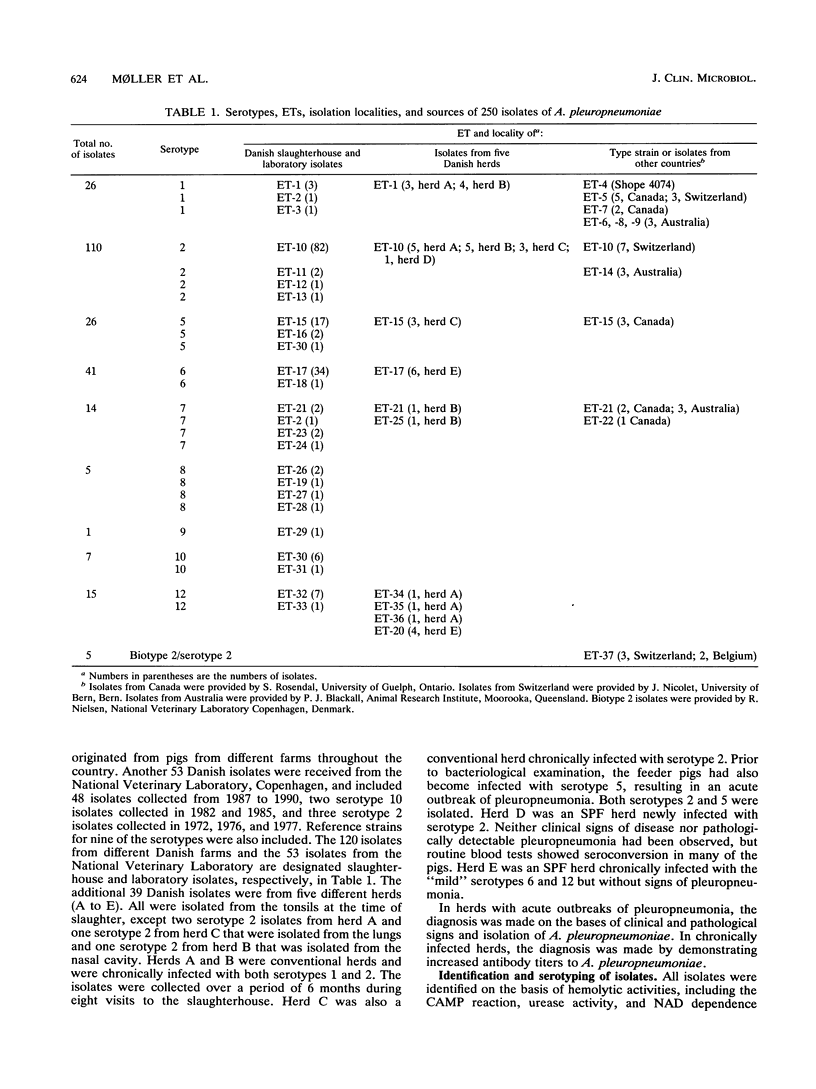
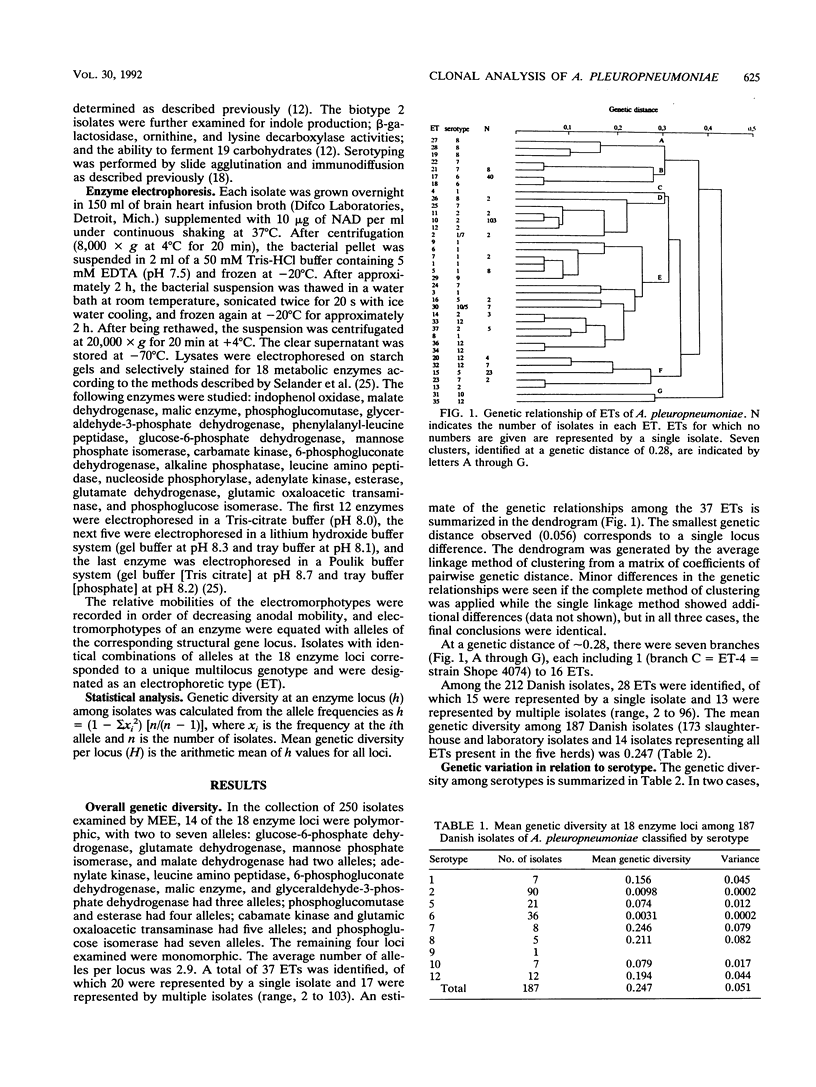
The genetic diversity among 250 isolates of Actinobacillus pleuropneumoniae from lungs of pigs with pleuropneumonia and from tonsils of apparently healthy pigs at slaughter was estimated by multilocus enzyme electrophoresis. The Danish strains were derived from both specific-pathogen-free and conventional herds. Sixty-six percent of the isolates belonged to three electrophoretic types (ETs) of a total of 37 ETs detected. While five biotype 2 isolates constituted a separate ET closely related to biotype 1 isolates, the type strain of the species (Shope 4074) belonged to its own ET, with a genetic distance of 0.30 from its nearest neighbor. Isolates of serotypes traditionally considered to have less pathogenic potential (serotypes 6, 10, and 12) from herds with acute outbreaks of pleuropneumonia belonged to the same ETs as isolates from apparently healthy pigs, suggesting that factors such as cross immunity and management may lead to divergent clinical results. Isolates from four herds harboring more than one serotype showed distinct profiles between the serotypes, indicating no or only limited chromosomal recombination among clones. Isolates from tonsils belonged to the same ET as isolates from lungs. The same ET was isolated from widely different parts of the world. Evidence from this study indicates that multilocus enzyme electrophoresis may be a valuable tool for the epidemiological analysis of A. pleuropneumoniae.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beutin L., Orskov I., Orskov F., Zimmermann S., Prada J., Gelderblom H., Stephan R., Whittam T. S. Clonal diversity and virulence factors in strains of Escherichia coli of the classic enteropathogenic serogroup O114. J Infect Dis. 1990 Dec;162(6):1329–1334. doi: 10.1093/infdis/162.6.1329. [DOI] [PubMed] [Google Scholar]
- Borr J. D., Ryan D. A., MacInnes J. I. Analysis of Actinobacillus pleuropneumoniae and related organisms by DNA-DNA hybridization and restriction endonuclease fingerprinting. Int J Syst Bacteriol. 1991 Jan;41(1):121–129. doi: 10.1099/00207713-41-1-121. [DOI] [PubMed] [Google Scholar]
- Bossé J. T., Johnson R. P., Rosendal S. Serodiagnosis of pleuropneumonia using enzyme-linked immunosorbent assay with capsular polysaccharide antigens of Actinobacillus pleuropneumoniae serotypes 1, 2, 5 and 7. Can J Vet Res. 1990 Oct;54(4):427–431. [PMC free article] [PubMed] [Google Scholar]
- Brandreth S. R., Smith I. M. Comparative virulence of some English strains of Haemophilus pleuropneumoniae serotypes 2 and 3 in the pig. Res Vet Sci. 1987 Mar;42(2):187–193. [PubMed] [Google Scholar]
- Brandreth S. R., Smith I. M. Prevalence of pig herds affected by pleuropneumonia associated with Haemophilus pleuropneumoniae in eastern England. Vet Rec. 1985 Aug 17;117(7):143–147. doi: 10.1136/vr.117.7.143. [DOI] [PubMed] [Google Scholar]
- Caugant D. A., Bol P., Høiby E. A., Zanen H. C., Frøholm L. O. Clones of serogroup B Neisseria meningitidis causing systemic disease in The Netherlands, 1958-1986. J Infect Dis. 1990 Oct;162(4):867–874. doi: 10.1093/infdis/162.4.867. [DOI] [PubMed] [Google Scholar]
- Caugant D. A., Levin B. R., Orskov I., Orskov F., Svanborg Eden C., Selander R. K. Genetic diversity in relation to serotype in Escherichia coli. Infect Immun. 1985 Aug;49(2):407–413. doi: 10.1128/iai.49.2.407-413.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komal J. P., Mittal K. R. Studies on the interactions of two different serotypes of Actinobacillus pleuropneumoniae. Comp Immunol Microbiol Infect Dis. 1990;13(1):25–34. doi: 10.1016/0147-9571(90)90005-e. [DOI] [PubMed] [Google Scholar]
- Lymbery A. J., Hampson D. J., Hopkins R. M., Combs B., Mhoma J. R. Multilocus enzyme electrophoresis for identification and typing of Treponema hyodysenteriae and related spirochaetes. Vet Microbiol. 1990 Mar;22(1):89–99. doi: 10.1016/0378-1135(90)90127-h. [DOI] [PubMed] [Google Scholar]
- MacInnes J. I., Borr J. D., Massoudi M., Rosendal S. Analysis of southern Ontario Actinobacillus (Haemophilus) pleuropneumoniae isolates by restriction endonuclease fingerprinting. Can J Vet Res. 1990 Apr;54(2):244–250. [PMC free article] [PubMed] [Google Scholar]
- Mittal K. R. Cross-reactions between Actinobacillus (Haemophilus) pleuropneumoniae strains of serotypes 1 and 9. J Clin Microbiol. 1990 Mar;28(3):535–539. doi: 10.1128/jcm.28.3.535-539.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morozumi T., Nicolet J. Morphological variations of Haemophilus parasuis strains. J Clin Microbiol. 1986 Jan;23(1):138–142. doi: 10.1128/jcm.23.1.138-142.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morozumi T., Nicolet J. Some antigenic properties of Haemophilus parasuis and a proposal for serological classification. J Clin Microbiol. 1986 Jun;23(6):1022–1025. doi: 10.1128/jcm.23.6.1022-1025.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser J. M., Granoff D. M., Pattison P. E., Selander R. K. A population genetic framework for the study of invasive diseases caused by serotype b strains of Haemophilus influenzae. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5078–5082. doi: 10.1073/pnas.82.15.5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser J. M., Rapp V. J., Selander R. K. Clonal diversity in Haemophilus pleuropneumoniae. Infect Immun. 1987 May;55(5):1207–1215. doi: 10.1128/iai.55.5.1207-1215.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller K., Kilian M. V factor-dependent members of the family Pasteurellaceae in the porcine upper respiratory tract. J Clin Microbiol. 1990 Dec;28(12):2711–2716. doi: 10.1128/jcm.28.12.2711-2716.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen R., Plambeck T., Foged N. T. Blocking enzyme-linked immunosorbent assay for detection of antibodies to Actinobacillus pleuropneumoniae serotype 2. J Clin Microbiol. 1991 Apr;29(4):794–797. doi: 10.1128/jcm.29.4.794-797.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp V. J., Munson R. S., Jr, Ross R. F. Outer membrane protein profiles of Haemophilus pleuropneumoniae. Infect Immun. 1986 May;52(2):414–420. doi: 10.1128/iai.52.2.414-420.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers R. J., Eaves L. E., Blackall P. J., Truman K. F. The comparative pathogenicity of four serovars of Actinobacillus (Haemophilus) pleuropneumoniae. Aust Vet J. 1990 Jan;67(1):9–12. doi: 10.1111/j.1751-0813.1990.tb07382.x. [DOI] [PubMed] [Google Scholar]
- Rosendal S., Boyd D. A., Gilbride K. A. Comparative virulence of porcine Haemophilus bacteria. Can J Comp Med. 1985 Jan;49(1):68–74. [PMC free article] [PubMed] [Google Scholar]
- Rosendal S., Mittal K. R. Serological cross-reactivity between a porcine Actinobacillus strain and Haemophilus pleuropneumoniae. Can J Comp Med. 1985 Apr;49(2):164–170. [PMC free article] [PubMed] [Google Scholar]
- Selander R. K., Caugant D. A., Ochman H., Musser J. M., Gilmour M. N., Whittam T. S. Methods of multilocus enzyme electrophoresis for bacterial population genetics and systematics. Appl Environ Microbiol. 1986 May;51(5):873–884. doi: 10.1128/aem.51.5.873-884.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]



