Abstract
Summary
Background
Excess body mass index (BMI) has been associated with adverse outcomes in prostate cancer, and hyperinsulinemia is a candidate mediator, but prospective data are sparse. We assessed the influence of prediagnostic BMI and plasma C-peptide (reflecting insulin secretion) on prostate cancer-specific mortality after diagnosis.
Methods
BMI was available at baseline (1982) and in 1990 among 2,546 men who developed prostate cancer (281 prostate cancer deaths). Baseline C-peptide concentration were available in 827 men (117 prostate cancer deaths). We used Cox proportional hazards regression models controlling for age, smoking, time between BMI measurement and prostate cancer diagnosis, and competing causes of death.
Findings
Compared with men of normal weight (BMI<25 kg/m2) at baseline, overweight men (BMI 25–29.9 kg/m2) and obese men (BMI≥30 kg/m2) had significantly higher risk of prostate cancer mortality; the proportional hazard ratio (HR)s (95% confidence interval, CI) were 1.47 (1.16–1.88) for overweight and 2.66 (1.62–4.39; Ptrend<0.0001) for obesity. The trend remained significant after controlling for clinical stage and Gleason grade and was stronger for prostate cancer diagnosed during the PSA screening era (1991–2007) or using BMI obtained in 1990. Men with C-peptide concentrations in the highest quartile (high), versus the lowest quartile (low), also had higher risk (HR=2.38; 1.31–4.30). Compared with men with BMI<25 kg/m2 and low C-peptide concentrations, those with BMI≥25 kg/m2 and high C-peptide concentration had a four times higher risk (HR=4.12; 1.97–8.61; Pinteraction=0.001) independent of clinical predictors.
Interpretation
Excess body weight and high plasma concentration of C-peptide each predispose men with a subsequent diagnosis of prostate cancer to increased likelihood of dying of this disease; those with both factors have the worst outcome.
Introduction
Prostate cancer and obesity are major public health concerns for middle aged and older men. Excess body weight, as measured by body mass index (BMI, kg/m2), has been associated with increased prostate cancer progression, although it appears to be unrelated to risk of incident prostate cancer in most prospective studies.(1–5) Some studies found that higher BMI, measured before disease onset, was associated with a lower risk of localized prostate cancer but a higher risk of lethal cancer.(5) Most,(6–13) but not all (14, 15) studies suggest that obesity at the time of prostate cancer diagnosis is associated with higher risk of biochemical failure. In one study, retrospectively assessed obesity (BMI≥30 kg/m2) at ages 25 and 40 were stronger predictors for risk of biochemical failure than obesity assessed at diagnosis.(10) To date, among five studies that have examined the risk of prostate cancer-specific mortality (11, 16–18) three reported a positive association with BMI at time of treatment or recall of BMI in the year before diagnosis.(11, 17, 19) Taken together, these data suggest that obesity prior to a prostate cancer diagnosis predisposes men to increased risk of dying of the disease.(20) However, no long-term prospective study of prostate cancer-specific mortality has been conducted, and a concomitant assessment of biological mechanism(s) is lacking.
Obesity causes many metabolic changes that may mediate the association with increased prostate cancer mortality. Hyperinsulinemia is a candidate mediator. In a recent laboratory study, mice fed a high energy diet had increased weight gain, hyperinsulinemia, accelerated growth of prostate cancer xenografts, and increased signaling downstream of the insulin receptor in neoplastic prostate tissue.(21) In addition, we recently observed abundant expression of the insulin receptor in human prostate cancer tissue.(22) To our knowledge, no studies have reported the association of plasma concentrations of insulin or C-peptide, a marker of insulin secretion, (23) prior to prostate cancer diagnosis with risk of prostate cancer mortality.
We examined the role of prediagnostic BMI and plasma C-peptide concentration in prostate cancer-specific mortality in a well-defined cohort of US male physicians diagnosed with prostate cancer during the 24 years of follow-up. We also evaluated the potential influences of known clinical predictors of prostate cancer progression, including age at diagnosis, Gleason grade, and clinical stage, on these associations.
Methods
Study population and baseline questionnaire
This study is based on 2,546 men diagnosed with prostate cancer during the 24-years of follow-up (median follow-up between diagnosis and death or end of follow-up was 7 years, range from 1 day to 24 years) in the Physicians’ Health Study, a randomized trial of aspirin and beta carotene among 22,071 U.S. male physicians, aged 40–84 in 1982, without a history of heart disease, cancer, or major chronic diseases.(24) At baseline, participants reported height and body weight and, at the 8th year of follow-up, the participants reported body weight again, from which baseline and the 8th year body mass index (BMI, kg/m2) were calculated and categorized as normal (BMI<25 kg/m2), overweight (BMI 25 to 29.9 kg/m2) or obese (BMI≥30 kg/m2). Cigarette smoking (never, past, current) and history of diabetes were also ascertained at baseline. Between the fall of 1982 and the end of 1984, 14,916 men provided blood samples. In a subgroup selected for nested case-control biomarker studies, we assayed plasma C-peptide concentrations for 827 men and PSA concentration for 718, using baseline blood samples. All patients provided written informed consent for inclusion in this study. This study was approved by the Human Subjects Committee of the Brigham and Women’s Hospital and Harvard School of Public Health.
Follow-up and confirmation of prostate cancer death
Follow-up questionnaires to ascertain disease outcomes were mailed at 6 and 12 months after randomization and yearly thereafter. Of 2,751 reported prostate cancer diagnoses, 2,549 were confirmed by medical records and pathology reports; all except 3 (who had BMI < 18.5 kg/m2) of the confirmed cases were included in the analysis. Prostate cancer stage is recorded according to the TNM staging system or converted from a modified Whitmore-Jewett classification scheme (for prostate cancer diagnosed during the early years of follow-up). We used clinical stage and Gleason grade whenever the information were available. PSA concentrations at diagnosis were also extracted from medical records. Deaths are ascertained through repeated mailings, telephone calls to non-respondents, and searches of the National Death Index. We seek medical records to assess cause of death, and assignment of prostate cancer specific death is blinded to questionnaire and laboratory data and is based on consensus of the three physicians (Drs. Meir J Stampfer, Samuel Goldhaber and James Taylor) of the End Point Committee using medical records and all available information. Follow-up for morbidity and mortality to March 30, 2007 is 97% complete.
C-peptide assay
Plasma C-peptide concentrations were measured in blood that had been frozen at −82 °C, using standard ELISA methodology and a single production lot of reagents (Diagnostic Systems Limited, Webster, TX) at Dr. M Pollak’s laboratory. Blinded embedded quality control samples showed within assay CV of 5% and a between assay variability of 9%.
Statistical Analysis
We characterized the clinical predictors of lethal prostate cancer and other potential confounding variables according to the three BMI categories using Chi-square tests and analysis of covariance. A competing risk analysis using Cox proportional hazards regression (25) was used to evaluate associations of baseline and the 8th year follow-up BMI (three categories as the major exposure) with risk of prostate cancer-specific mortality (the major outcome) using proportional hazard ratios (HR) and 95% confidence intervals (95% CI). This competing risk model is a semi-parametric multiplication hazard model assuming that the log relative hazard is linearly related to covariates. The implementation of the model is based on a stacked data set technique that allows some covariates have the identical effects for several causes. In our analysis, we assume that no covariates have identical effects on the two failure types (prostate cancer death and death due to other causes).(26) Person-years were counted from the date of prostate cancer diagnosis until the date of prostate cancer death (event), death due to other causes, or the end of follow-up (March 31, 2007) (censored), whichever came first. We also estimated the HR in association with a one unit incremental increase in BMI and present the P-values of the tests for trend.
We controlled in the basic model for age at diagnosis, baseline cigarette smoking status, and time between BMI measurement and prostate cancer diagnosis in all analyses. Controlling for the randomized trial components, aspirin and beta-carotene, had no influence so these were not included in the analyses. To assess the independent effect of BMI, we further controlled for clinical stage and Gleason grade in some analyses. To further assess the impact of PSA screening, we stratified the analysis by year of diagnosis (before or after 1990, when PSA screening became widespread). In subgroup analyses, we also controlled for baseline PSA (<4, 4–9, 10+ ng/mL) (n=718) or PSA at diagnosis (n=1869). To further reduce the potential influence of obesity/overweight on PSA screening or treatment options, we conducted sensitivity analyses by excluding stage T1 or stage N1/M1 cancer. We also evaluated models excluding current smokers, men with history of diabetes, non-Caucasians (less than 6% of the cohort), or men who died of any cause within the first five years of follow-up.
All analyses of plasma C-peptide concentrations (in quartiles) were controlled for baseline age and time since last meal, and subsequent analyses controlled for baseline BMI, or clinical stage and Gleason grade to assess the independent association of C-peptide. Tests for trend were conducted by treating median concentration of quartiles as a continuous variable. We also examined the joint association between BMI (<25 kg/m2 vs. ≥25 kg/m2) and quartile of C-peptide concentration and tested the significance of the interaction by including a product term of the two variables with the main exposures. Because excluding 11 men with history of diabetes at baseline did not change the results materially, we presented data including all men with plasma C-peptide levels. We used Cox proportional hazards regression models adjusting for age at diagnosis and smoking categories to produce plots of prostate cancer-specific survival curves for the three BMI categories or for the quartiles of C-peptide concentration. In addition, we conducted log rank tests controlling for age at diagnosis and smoking status to test if the survival curves estimated via Kaplan-Meier method for the three BMI categories or for the quartiles of C-peptide concentration are equal. All statistics were calculated using SAS (version 9.1.3; SAS Institute Inc, Cary, NC), with a two-sided significance level of 0.05.
Role of the funding source
The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Of the 2,546 men diagnosed with prostate cancer during the follow-up, 989 (39%) were overweight (BMI 25–29.9 kg/m2) and 87 (3.4%) were obese (BMI≥30 kg/m2) at baseline (Table 1). Overweight men had characteristics similar to men of normal weight. Greater proportions of obese men were past smokers, and were more likely to have extraprostatic, metastatic, or high Gleason grade (8–10) cancer at diagnosis. BMI was unrelated to PSA concentration, with spearman correlation coefficients between BMI and baseline PSA concentrations of 0.06 (P=0.09, n=718) and −0.03 between BMI and PSA concentration at diagnosis (P=0.25, n=1869). As expected, baseline plasma C-peptide concentrations were weakly positively correlated with age (spearman partial correlation r=0.12, P = 0.001, controlling for fasting status and assay batch) and BMI (r=0.25, P <0.0001, controlling for fasting, batch, and age).
Table 1.
Characteristics among 2,546 prostate cancer cases (1982–2007), by baseline BMI
| 18.6 – 24.9 | BMI (kg/m2) 25.0 – 29.9 | 30.0–36.1 | P value | |
|---|---|---|---|---|
| All prostate cancer (%) | 1470 (57.7) | 989 (38.8) | 87 (3.4) | |
| Pre-PSA era (1982–1990, %) | 237 (57.1) | 165 (39.8) | 13 (3.1) | |
| PSA era (1991–2007, %) | 1233 (57.9) | 824 (38.7) | 74 (3.5) | 0.88 |
| Deaths due to prostate cancer (%) | 134 (9.1) | 129 (13.0) | 18 (20.7) | 0.0001 |
| Overall follow-up, median (min, max, yr) | ||||
| Baseline to diagnosis | 14.3 (0.0, 23.9) | 14.0 (0.2, 23.8) | 13.1 (0.5, 23.0) | |
| Diagnosis to end of follow-up | 7.5 (0.0, 22.7) | 7.3 (0.1, 24.3) | 6.2 (0.5, 17.4) | |
| Age at baseline (yr) | 56.7± 9.3 | 57.2 ± 8.4 | 55.9 ± 7.5 | 0.31 |
| Age at diagnosis (yr) | 70.6 ± 7.7 | 70.8 ± 7.2 | 69.2 ± 6.7 | 0.13 |
| Smoking status at baseline (%) | ||||
| Non-smoker | 774 (52.7) | 464 (46.9) | 35 (40.2) | 0.02 |
| Past smoker | 572 (38.9) | 421 (42.6) | 42 (48.3) | |
| Current smoker | 124 (8.4) | 104 (10.5) | 10 (11.5) | |
| Baseline diabetes (%) | 23 (1.6) | 15 (1.5) | 0 (0.0) | 0.50 |
| Clinical Stage, N (%)a | ||||
| T1/T2 | 1141 (89.8) | 767 (90.0) | 58 (78.4) | 0.0010 |
| T3/T4 | 87 (6.9) | 48 (5.6) | 7 (9.5) | |
| N1/M1 | 42 (3.3) | 37 (4.3) | 9 (12.2) | |
| Clinical Stage, N (%) Unknown | 200 | 137 | 13 | |
| Gleason score, N (%) a | ||||
| 2–6 | 897 (63.1) | 569 (59.6) | 49 (60.5) | |
| 7 | 343 (24.1) | 269 (28.2) | 16 (19.8) | 0.06 |
| 8–10 | 182 (12.8) | 117 (12.3) | 16 (19.8) | |
| Gleason score, N (%) Unknown | 48 | 34 | 6 | |
| Baseline PSA, ng/mL, N, (%) b | ||||
| 4 – 9.9 | 87 (20.8) | 62 (22.4) | 6 (27.3) | 0.71 |
| ≥ 10 | 63 (15) | 46 (16.6) | 5 (22.7) | 0.58 |
| PSA at diagnosis, ng/mL, N (%) b | ||||
| 4 – 9.9 | 580 (53.2) | 421 (58.3) | 30 (52.6) | 0.09 |
| ≥ 10 | 375 (34.4) | 224 (31.0) | 22 (38.6) | 0.22 |
| Plasma C-peptide concentrations (ng/mL), median (10th–90th percentile) b | 1.5 (0.7–3.9) | 1.9 (0.8–4.5) | 2.8 (1.4–4.7) | <0.0001 |
Among the 2,546 men, 350 (14%) had unknown stage and 88 (3%) had unknown Gleason grade information.
Baseline plasma PSA concentration were available for 718 men; data for PSA at diagnosis were available for 1869 men; and baseline plasma C-peptide concentrations were available for 827 men.
During the 24 years of follow-up, 281 (11.0%) men subsequently died of prostate cancer and 485 (19%) men died of other causes. Higher baseline BMI was significantly associated with higher risk of prostate cancer-specific mortality, independent of age at diagnosis and baseline smoking status (Table 2, Figure 1A). Compared with men of normal weight, the HRs were 1.47 (1.16–1.88) for overweight men and 2.66 (1.62–4.39) for obese men (Ptrend < 0.0001). Controlling for the two trial components, aspirin and beta-carotene, did not change the results (Table 3).
Table 2.
Cox proportional hazard ratio (HR) a and 95% confidence interval (95% CI) of prostate cancer-specific mortality according to BMI
| <25 | BMI (kg/m2) 25–29.9 | ≥ 30 | Per unit increase in BMI | Ptrend | |
|---|---|---|---|---|---|
| Normal | Overweight | Obese | |||
| BMI measured in 1982 (n=2546) | |||||
| All prostate cancer, 1982–2007 | |||||
| No. | 134/1,470 a | 129/989 | 18/87 | ||
| HR (95% CI) b | 1.00 (ref.) | 1.47 (1.16–1.88) | 2.66 (1.62–4.39) | 1.09 (1.05–1.14) | <0.0001 |
| HR (95% CI) c | 1.00 (ref.) | 1.26 (0.98–1.62) | 1.95 (1.17–3.23) | 1.07 (1.02–1.12) | 0.0042 |
| Pre-PSA era prostate cancer, 1982–1990 | |||||
| No. | 68/237 | 63/165 | 9/13 | ||
| HR (95% CI) b | 1.00 (ref.) | 1.44 (1.02–2.04) | 3.15 (1.52–6.51) | 1.10 (1.03–1.18) | 0.0045 |
| HR (95% CI) c | 1.00 (ref.) | 1.06 (0.73–1.53) | 1.55 (0.74–3.24) | 1.06 (0.99–1.13) | 0.1113 |
| PSA era prostate cancer, 1991–2007 | |||||
| No. | 66/1,233 | 66/824 | 9/74 | ||
| HR (95% CI) b | 1.00 (ref.) | 1.45(1.03–2.05) | 2.41(1.19–4.89) | 1.09 (1.02–1.16) | 0.0064 |
| HR (95% CI) c | 1.00 (ref.) | 1.57 (1.11–2.24) | 2.50 (1.22–5.13) | 1.08 (1.02–1.15) | 0.0146 |
| BMI measured in 1990 (n=2078) | |||||
| PSA era Prostate cancer, 1991–2007 | |||||
| No. | 59/1094 | 66/877 | 9/107 | ||
| HR (95% CI) b | 1.00 (ref.) | 1.49(1.05–2.13) | 2.24(1.09–4.57) | 1.09 (1.03–1.15) | 0.0033 |
| HR (95% CI) c | 1.00 (ref.) | 1.61 (1.12–2.32) | 2.23 (1.07–4.64) | 1.10 (1.04–1.16) | 0.0015 |
Number of prostate cancer deaths/men diagnosed with prostate cancer.
Adjusted for age at diagnosis (age <65, 65<= age<70, 70<= age<75, 75<= age<80, >=80 years) and baseline smoking status (never, past, and current smoker) and time interval from BMI measurement to prostate cancer diagnosis.
Adjusted for age at diagnosis (age <65, 65<= age<70, 70<= age<75, 75<= age<80, >=80 years), baseline smoking status (never, past, and current smoker), time interval from BMI measurement to prostate cancer diagnosis, clinical stage and Gleason grade.
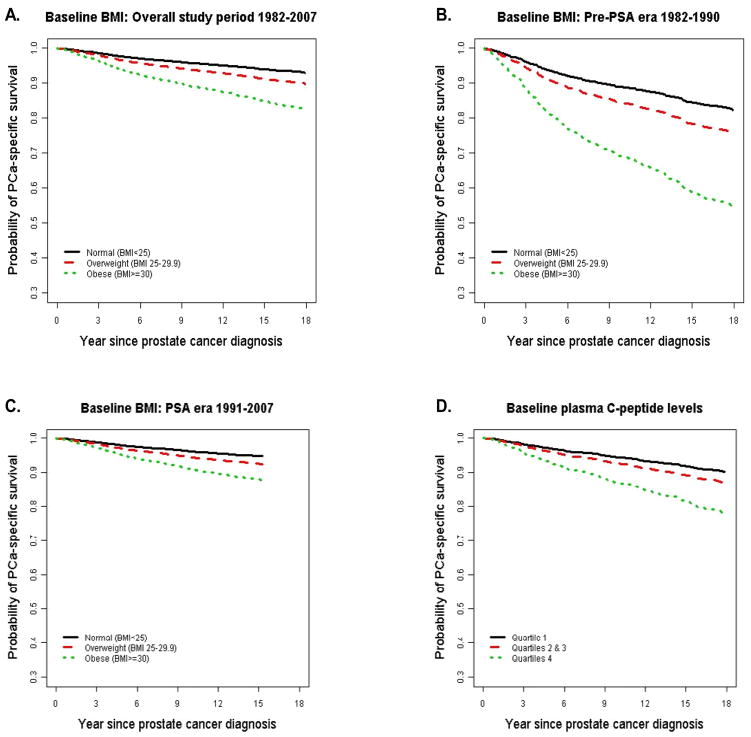
Figure 1.
Survival curves show the probability of prostate cancer-specific survival after diagnosis according to baseline BMI measured in 1982 controlling for age at diagnosis, smoking status, and time between BMI measurement and cancer diagnosis (1A, 2,546 prostate cancer diagnosed during the overall study period 1982–2007, 281 prostate cancer deaths; 1B, 415 prostate cancer diagnosed during the pre-PSA era 1982–1990, 140 prostate cancer deaths; 1C, 2,131 prostate cancer diagnosed during the PSA era 1991–2007, 141 prostate cancer deaths) and according to baseline plasma C-peptide concentration (quartile, 1D, 827 prostate cancer diagnosed during 1982–2007, 117 prostate cancer deaths). The p-values for log rank tests were all less than 0.023.
Table 3.
Cox proportional hazard ratio (HR) a and 95% confidence interval (95% CI) of prostate cancer-specific mortality according to one unit increase in baseline BMI
| HR (95% CI) | P-value | |
|---|---|---|
| All prostate cancer | 1.09 (1.05–1.14) | <0.0001 |
| Controlling for aspirin and beta-carotene | 1.09 (1.05–1.14) | <0.0001 |
| Excluding any deaths occurred first 5 years | 1.10 (1.05–1.15) | <0.0001 |
| Excluding current smokers | 1.11 (1.06–1.16) | <0.0001 |
| Excluding diabetes | 1.09 (1.05–1.14) | <0.0001 |
| Excluding non-Caucasians | 1.11 (1.05–1.16) | <0.0001 |
| Excluding stage T1 | 1.10 (1.05–1.15) | <0.0001 |
| Excluding metastasis (N1/M1) | 1.08 (1.02–1.13) | 0.0039 |
All adjusted for age at diagnosis, smoking status at baseline (past smoking for the analysis excluding current smokers) and time interval from baseline to prostate cancer diagnosis.
We further included clinical stage and Gleason grade in the multivariate model to assess the independent association between baseline BMI and fatal prostate cancer. Controlling for these clinical predictors somewhat attenuated the magnitude of the association; the HRs were 1.26 (0.98–1.62) for overweight men and 1.95 (1.17–3.23) for obese men. However, the positive trend of increase in risk for each unit increase in BMI remained statistically significant (HR= 1.07, 1.02–1.12; Ptrend = 0.004, Table 2). As expected, high Gleason score (7 or 8–10) and regional (clinical stageT3/T4/N0/M0) and metastatic disease (N1/M1) at diagnosis were strong predictors of lethal prostate cancer. The HRs were 2.25 (1.62–3.12) for Gleason grade 7 tumors (80 prostate cancer deaths) and 4.70 (3.37–6.56) for Gleason 8–10 tumors (93 prostate cancer deaths), compared with Gleason 2–6 (73 prostate cancer deaths). The HRs were 3.62 (2.61–5.02) for stage T3/T4/N0/M0 (78 prostate cancer deaths) and 10.62 (7.45–15.14) for stage N1/M1 disease (67 prostate cancer deaths) compared with localized (stage T1/T2/N0/M0) disease (105 prostate cancer deaths). Further controlling for PSA at diagnosis (<4, 4–9,≥10 ng/mL) in a subgroup of 1869 men (diagnosed in PSA era) strengthened the association for overweight (HR=1.80, 1.15–2.83) but attenuated the association for obesity (HR=1.61, 0.56–4.58). Controlling for baseline PSA (<4, 4–9,≥10 ng/mL, n=718 cases, most of whom were diagnosed during the pre-PSA era) in the multivariate model with clinical predictors did not materially change the results (HR=1.61, 1.11–2.34 for overweight and HR=2.83, 1.31–6.11 for obese).
Widespread PSA screening since early 1990s has significantly changed the clinical presentation of prostate cancer. Because information on screening was not uniformly available, we used the period of 1982–1990 and 1991–2007 as a surrogate of the pre-PSA and PSA screening era. Among the 415 men diagnosed with prostate cancer during 1982–1990 (pre-PSA era), 140 (33.7%) died of prostate cancer. Among the 2,131 men diagnosed with prostate cancer during 1991–2007 (PSA screening era), 141 (6.6%) died of the disease. Although the overall prostate cancer-specific mortality was dramatically different between the two periods (Figure 1B and 1C), the relative risk of prostate cancer-specific mortality in association with baseline BMI remained similar in age- and smoking-adjusted model (Table 2). Further controlling for clinical stage and Gleason grade significantly attenuated the association for prostate cancer diagnosed during 1982–1990. However, for prostate cancer diagnosed during the PSA screening era, excess body weight many years before diagnosis was a strong and significant predictor of poor survival.
The median time between baseline BMI and prostate cancer diagnosis was 13 to 14 years (Table 1), we therefore controlled for time between BMI measurements to prostate cancer diagnosis in all the analyses. In addition, BMI obtained in the 8th year of follow-up (in 1990) was highly correlated with baseline BMI in 1982 (correlation coefficient = 0.8), suggesting strong tracking over time. The prospective association between prediagnostic BMI and prostate cancer-specific mortality in the PSA screening era (1991–2007) was quite similar using BMI obtained in 1982 or in 1990 (Table 2), with or without controlling for clinical stage and Gleason grade, further demonstrating the robust relationship.
To evaluate potential confounding factors, we conducted a series of subgroup sensitivity analyses with baseline BMI as a continuous variable, which gives more statistical power (Table 3), and controlling for age and smoking status. Compared with the overall risk of prostate cancer-specific mortality with a one unit increase in BMI (HR = 1.09, 95% CI: 1.05–1.14), the association remained virtually unchanged after each of the following exclusions: men who died of any cause during the first five years of follow-up, current smokers, men with history of diabetes, or non-Caucasians. This suggests that these factors cannot explain the strong positive association between baseline BMI and prostate cancer mortality. In addition, excluding men with stage T1 or stage N1/M1 prostate cancer at diagnosis did not materially change the results suggesting that early cancer detection by PSA (stage T1) or delayed diagnosis (metastasis) had little impact on the association.
We have baseline blood available in a subgroup of 827 men; 634 of these blood samples were collected less than 8 hours since last meal (nonfasting). We therefore measured plasma C-peptide as a surrogate for insulin secretion, and assessed the link between C-peptide concentration and prostate cancer-specific mortality, adjusting for time between last meal and blood draw. Baseline characteristics and clinical features in this subgroup of men were similar to those in the overall study population (data shown in Supplemental Webtable 1). Among the 117 prostate cancer deaths, a significantly higher proportion (44, 21%) had baseline C-peptide concentrations in the highest quartile compared to those in the lowest quartile (21, 10%). After controlling for age, fasting status, and time interval from baseline to prostate cancer diagnosis, men with baseline C-peptide concentration in the highest quartile had an HR of 2.38 (1.31–4.30) for prostate cancer mortality compared with the lowest quartile, Ptrend=0.008 (Figure 1D, Table 4). The increased risk was mainly among men in the highest quartile suggesting a threshold effect (Table 4). Including BMI in the model slightly attenuated the association for C-peptide (inter-quartile HR = 2.01, 95% CI: 1.11–3.66; Ptrend=0.03) but BMI remained a strong predictor (HR = 1.61, 95% CI: 1.10–2.35, for overweight, HR = 2.37, 95% CI: 1.04–5.37, for obesity; Ptrend=0.023). The HR for the highest quartile of C-peptide remained statistically significant (HR = 1.93; 95% CI: 1.03–3.63; Ptrend=0.09) after controlling for clinical stage and Gleason grade. However, including both BMI and clinical predictors in the same model attenuated the associations for both BMI (HR=1.76, 95%CI: 1.19–2.61, for overweight and HR=1.87, 95%CI: 0.80–4.37, for obesity) and C-peptide (inter-quartile HR = 1.72, 95% CI: 0.92–3.24, Ptrend=0.11). This finding suggests that part of the impact of BMI on prostate cancer prognosis is mediated through insulin (Table 4).
Table 4.
Cox proportional hazard ratio (HR) and 95% confidence interval (95% CI) of prostate cancer-specific mortality according baseline plasma C-peptide concentration
| Plasma C-peptide Quartile | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | Pa | |
| Median C-peptide concentrations (ng/mL) | 0.77 | 1.34 | 2.06 | 3.89 | |
| No. | 21/207 b | 32/206 | 20/208 | 44/206 | |
| Simple model c | 1.00 | 1.54 (0.87–2.71) | 1.10 (0.58–2.09) | 2.38 (1.31–4.30) | 0.008 |
| Simple model + BMI | 1.00 | 1.42 (0.80–2.51) | 0.95 (0.50–1.82) | 2.01 (1.11–3.66) | 0.03 |
| Simple model + clinical stage, grade | 1.00 | 1.15 (0.63–2.10) | 1.08 (0.56–2.08) | 1.93 (1.03–3.63) | 0.09 |
| Simple model + BMI, clinical stage, grade | 1.00 | 1.01 (0.54–1.86) | 0.97 (0.50–1.87) | 1.72 (0.92–3.24) | 0.11 |
Test for trend using median concentration of C-peptide in each of the quartiles.
Number of prostate cancer deaths/men diagnosed with prostate cancer;
Controlling for age, fasting status and time interval from baseline to prostate cancer diagnosis.
When assessing the joint association between BMI and C-peptide, we found that the increased risk of prostate cancer mortality associated with higher concentrations of C-peptide was statistically significant among men with BMI≥25 kg/m2 (Ptrend=0.007) but not among men with BMI<25 kg/m2 (Ptrend=0.38) (Table 5). Overweight men with C-peptide in the highest quartile were over four times (the multivariate-adjusted HR=4.22, 95% CI: 2.10–8.48, Pinteraction=0.017) more likely to die of prostate cancer compared to men of normal weight and with C-peptide in the lowest quartile. Further controlling for clinical stage and Gleason grade did not change the result (the multivariate-adjusted HR=4.12, 95% CI: 1.97–8.61, Pinteraction=0.001).
Table 5.
Cox proportional hazard ratio (HR) and 95% confidence interval (95% CI) of prostate cancer-specific mortality according baseline BMI and plasma C-peptide concentrations
| BMI < 25 kg/m2 | BMI >= 25 kg/m2 | |||
|---|---|---|---|---|
| No. | HR (95% CI) | No. | HR (95% CI) | |
| Plasma C-peptide concentration | ||||
| Simple model a | ||||
| Q1 | 13/148 b | 1.00 (ref) | 8/59 | 1.71 (0.71–.4.15) |
| Q2 | 19/123 | 1.83 (0.89–3.76) | 13/83 | 1.79 (0.81–3.94) |
| Q3 | 10/119 | 1.22 (0.52–2.88) | 10/89 | 1.31 (0.56–3.03) |
| Q4 | 12/99 | 1.33 (0.57–3.13) | 32/107 | 4.22 (2.10–8.48) |
| Ptrend | 0.38 | 0.007 | ||
| Pinteraction | 0. 017 | |||
| Simple model + clinical stage, grade | ||||
| Q1 | 13/148 | 1.00 (ref) | 8/59 | 2.07 (0.84–5.13) |
| Q2 | 19/123 | 1.64 (0.78–3.47) | 13/83 | 1.04 (0.44–2.44) |
| Q3 | 10/119 | 1.15 (0.48–2.74) | 10/89 | 1.56 (0.65–3.73) |
| Q4 | 12/99 | 0.97 (0.39–2.38) | 32/107 | 4.12 (1.97–8.61) |
| Ptrend | 0.48 | 0.006 | ||
| Pinteraction | 0.001 | |||
Controlling for age, fasting status and time interval from baseline to prostate cancer diagnosis;
Number of prostate cancer deaths/men diagnosed with prostate cancer.
Discussion
In this large cohort with long-term follow-up, men who are overweight or obese and who have a subsequent diagnosis of prostate cancer are at increased risk of prostate cancer-specific death. Compared to those of normal weight at baseline in 1982, overweight men and obese men had significant higher risk of dying of prostate cancer after initial cancer diagnosis. The magnitude of the association increased monotonically; the HR was 1.09 (95% CI: 1.05–1.14) for each unit increase in BMI (Ptrend <0.0001); the results remained largely unchanged after further excluding current smokers at baseline, men with history of diabetes, non-Caucasians, or those who died of any cause within five years of follow-up. Moreover, we found that men with baseline C-peptide concentration in the top quartile had a 2.4-time higher risk of dying of prostate cancer than those in the lowest quartile.
In the PSA screening era, obesity may delay prostate cancer diagnosis because higher BMI has been associated with lower serum PSA concentrations.(27) A less sensitive PSA test in obese men could delay diagnosis and treatment, perhaps leading to worse prognosis. However, we observed no correlation between baseline BMI and PSA concentrations measured at baseline or PSA concentration recorded at diagnosis. We further evaluated the association separately by pre-PSA and PSA screening eras, controlling for clinical predictors (stage, Gleason grade, and PSA concentration at diagnosis), or excluding stage T1 or stage N1/M1 prostate cancer from the analysis, and found that the significant association between BMI and prostate cancer mortality remained largely unchanged. Thus, the positive associations between high BMI and poor prostate cancer outcomes are unlikely to be attributable to differences in cancer detection through PSA screening.
Another concern is whether the association between obesity and high prostate cancer mortality could be due to different choice of treatment among obese men that affected the outcome. Although we cannot fully address this issue given limited treatment information, our findings are in line with many previous clinical studies showing that, among patients either receiving prostatectomy or radiotherapy, obesity at diagnosis predicts subsequent PSA failure.(6–13) In addition to the relation with obesity, which accounts for only 3.4% of our study population, we found that overweight men (38.9% of the study population) also had a significant 47% higher risk of prostate cancer specific mortality. Although one may argue that obesity leads to treatment differences, this seems less plausible for overweight men with BMI under 30.
In our study, higher prediagnostic BMI and plasma C-peptide concentrations were both independent positive predictors of prostate cancer-specific mortality and men with both factors had the worst outcome. High insulin concentration may promote tumor progression via insulin receptor, and/or the insulin-like growth factor type I receptor and downstream pathways.(28) Significantly elevated insulin concentrations were observed in prostate cancer cases versus healthy controls in a Chinese case-control study,(29) among men with high risk prostate cancer versus those with low risk cancer,(30) and among men who died of prostate cancer (n=20 patients) versus survivors.(31) As these retrospective studies measured insulin concentration after the cancer diagnosis, it is unclear whether insulin concentrations were influenced by disease severity or hormonal therapy, which affects hyperinsulinemia or insulin resistance.(32, 33) Two recent prospective studies reported a null association between fasting insulin or plasma C-peptide concentration and risk of incident prostate cancer,(34, 35) but neither specifically addressed the association with prostate cancer progression or survival.
Major strengths of this study are the prospective design which minimizes possible recall bias of BMI or influences of disease severity and treatment on blood biomarkers. The long follow-up allows us to examine independently the influence of both baseline BMI and BMI at 8th year of follow-up on prostate cancer mortality. Additionally, we conducted a series of sensitivity analyses to evaluate potential biases and confounding factors. One limitation is that we had no detailed information about PSA screening and cancer treatment. However, given the prospective design and the homogenous study population of US physicians, confounding by PSA screening and treatment is unlikely to explain our findings Another limitation is that, although plasma C-peptide is a more reliable measurement of insulin secretion than insulin itself,(23) especially using nonfasting samples, we have only one C-peptide measure at baseline, taken years before prostate cancer diagnosis. C-peptide is relatively stable; the within-person correlation coefficient for C-peptide measured 4 years apart in a similar cohort of men was 0.57, a correlation similar to blood cholesterol measurement.(36) Participants are not a representative sample of prostate cancer patients in general population. All are physicians in good health at baseline, and further selected by being trial participants. However, we believe that studying this more homogenous population can avoid many unknown confounding factors such as socioeconomic status that may influence obesity, access to medical care, and cancer treatment options. Moreover, the biological relations of overweight and prostate cancer prognosis observed in this population are broadly generalizable.
Our findings, taken together with other evidence, are consistent with the hypothesis that insulin and obesity-related metabolic factors influence prostate cancer prognosis. The observations further suggest that the “seed/soil” hypothesis proposed by Stephen Paget more than 100 years ago(37) may apply to metabolic aspects of host-tumor interactions, and imply that the overweight/obese, hyperinsulinemic host may provide a host environment that favors aggressive neoplastic behavior. The association of high C-peptide concentrations with prostate cancer mortality is also of interest in the context of evidence that the androgen ablation leads to hyperinsulinimia, and might increase diabetic and cardiovascular morbidity in long-term prostate cancer survivors.(32, 33) Our findings raise the speculative possibility that hyperinsulinemia may also favor aggressive androgen-independent disease progression.
Findings from this study have several implications for prostate cancer risk prediction, prevention, and treatment. First, men living in affluent societies are facing two epidemics, obesity and prostate cancer. In parallel with the obesity epidemic, the prevalence of hyperinsulinemia has increased remarkably among nondiabetic U.S. adults.(38) The over-treatment of prostate cancer detected by PSA screening is a well recognized issue, and the need to identify prognostic factors that will improve our ability to identify men with life threatening prostate cancer who may benefit from novel and more aggressive treatment is clear. If confirmed, our prospective data provide evidence that overweight/obesity and high C-peptide concentration are adverse prognostic factors, and that they operate independently of clinical predictors. This provides further impetus for men to avoid overweight and to reduce risk of metabolic syndrome through physical activity and diet. Second, our data suggest that the recent progress in prostate cancer control may be attenuated by increased prevalence of obesity and hyperinsuliniemia. It also adds to the rationale for investigation of novel therapeutic and prevention strategies such as using insulin-lowering or antidiabetic drugs,(39) as well as novel agents that target the insulin/IGF-I receptor family as an adjuvant therapy for prostate cancer.
Supplementary Material
Acknowledgments
The authors thank Haiyan Zhang and Manyee To for their assistance in data management and technical assistance. The authors are also indebted to the participants in the Physicians’ Health Study.
Funding: The National Institutes of Health research grants CA 42182, CA90598, CA58684, CA34944, CA40360, HL26490, HL34595 and the National Cancer Institute of Canada.
Footnotes
Conflict of Interest Statement
The authors state no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.MacInnis RJ, English DR. Body size and composition and prostate cancer risk: systematic review and meta-regression analysis. Cancer Causes Control. 2006 Oct;17(8):989–1003. doi: 10.1007/s10552-006-0049-z. [DOI] [PubMed] [Google Scholar]
- 2.Littman AJ, White E, Kristal AR. Anthropometrics and prostate cancer risk. Am J Epidemiol. 2007 Jun 1;165(11):1271–9. doi: 10.1093/aje/kwm013. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez C, Freedland SJ, Deka A, et al. Body mass index, weight change, and risk of prostate cancer in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol Biomarkers Prev. 2007 Jan;16(1):63–9. doi: 10.1158/1055-9965.EPI-06-0754. [DOI] [PubMed] [Google Scholar]
- 4.Wright ME, Chang SC, Schatzkin A, et al. Prospective study of adiposity and weight change in relation to prostate cancer incidence and mortality. Cancer. 2007 Feb 15;109(4):675–84. doi: 10.1002/cncr.22443. [DOI] [PubMed] [Google Scholar]
- 5.Giovannucci E, Liu Y, Platz EA, Stampfer MJ, Willett WC. Risk factors for prostate cancer incidence and progression in the health professionals follow-up study. Int J Cancer. 2007 Oct 1;121(7):1571–8. doi: 10.1002/ijc.22788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amling CL, Riffenburgh RH, Sun L, et al. Pathologic variables and recurrence rates as related to obesity and race in men with prostate cancer undergoing radical prostatectomy. J Clin Oncol. 2004 Feb 1;22(3):439–45. doi: 10.1200/JCO.2004.03.132. [DOI] [PubMed] [Google Scholar]
- 7.Bassett WW, Cooperberg MR, Sadetsky N, et al. Impact of obesity on prostate cancer recurrence after radical prostatectomy: data from CaPSURE. Urology. 2005 Nov;66(5):1060–5. doi: 10.1016/j.urology.2005.05.040. [DOI] [PubMed] [Google Scholar]
- 8.Freedland SJ, Aronson WJ, Kane CJ, et al. Impact of obesity on biochemical control after radical prostatectomy for clinically localized prostate cancer: a report by the Shared Equal Access Regional Cancer Hospital database study group. J Clin Oncol. 2004 Feb 1;22(3):446–53. doi: 10.1200/JCO.2004.04.181. [DOI] [PubMed] [Google Scholar]
- 9.Mallah KN, DiBlasio CJ, Rhee AC, Scardino PT, Kattan MW. Body mass index is weakly associated with, and not a helpful predictor of, disease progression in men with clinically localized prostate carcinoma treated with radical prostatectomy. Cancer. 2005 May 15;103(10):2030–4. doi: 10.1002/cncr.20991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strom SS, Wang X, Pettaway CA, et al. Obesity, weight gain, and risk of biochemical failure among prostate cancer patients following prostatectomy. Clin Cancer Res. 2005 Oct 1;11(19 Pt 1):6889–94. doi: 10.1158/1078-0432.CCR-04-1977. [DOI] [PubMed] [Google Scholar]
- 11.Efstathiou JA, Bae K, Shipley WU, et al. Obesity and mortality in men with locally advanced prostate cancer: analysis of RTOG 85-31. Cancer. 2007 Dec 15;110(12):2691–9. doi: 10.1002/cncr.23093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strom SS, Kamat AM, Gruschkus SK, et al. Influence of obesity on biochemical and clinical failure after external-beam radiotherapy for localized prostate cancer. Cancer. 2006 Aug 1;107(3):631–9. doi: 10.1002/cncr.22025. [DOI] [PubMed] [Google Scholar]
- 13.Stroup SP, Cullen J, Auge BK, L’Esperance JO, Kang SK. Effect of obesity on prostate-specific antigen recurrence after radiation therapy for localized prostate cancer as measured by the 2006 Radiation Therapy Oncology Group-American Society for Therapeutic Radiation and Oncology (RTOG-ASTRO) Phoenix consensus definition. Cancer. 2007 Sep 1;110(5):1003–9. doi: 10.1002/cncr.22873. [DOI] [PubMed] [Google Scholar]
- 14.Chun FK, Briganti A, Graefen M, et al. Body mass index does not improve the ability to predict biochemical recurrence after radical prostatectomy. Eur J Cancer. 2007 Jan;43(2):375–82. doi: 10.1016/j.ejca.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 15.Gallina A, Karakiewicz PI, Hutterer GC, et al. Obesity does not predispose to more aggressive prostate cancer either at biopsy or radical prostatectomy in European men. Int J Cancer. 2007 Aug 15;121(4):791–5. doi: 10.1002/ijc.22730. [DOI] [PubMed] [Google Scholar]
- 16.Merrick GS, Galbreath RW, Butler WM, Wallner KE, Allen ZA, Adamovich E. Obesity is not predictive of overall survival following permanent prostate brachytherapy. Am J Clin Oncol. 2007 Dec;30(6):588–96. doi: 10.1097/COC.0b013e318068b506. [DOI] [PubMed] [Google Scholar]
- 17.Palma D, Pickles T, Tyldesley S. Obesity as a predictor of biochemical recurrence and survival after radiation therapy for prostate cancer. BJU Int. 2007 Aug;100(2):315–9. doi: 10.1111/j.1464-410X.2007.06897.x. [DOI] [PubMed] [Google Scholar]
- 18.Siddiqui SA, Inman BA, Sengupta S, et al. Obesity and survival after radical prostatectomy: A 10-year prospective cohort study. Cancer. 2006 Aug 1;107(3):521–9. doi: 10.1002/cncr.22030. [DOI] [PubMed] [Google Scholar]
- 19.Gong Z, Agalliu I, Lin DW, Stanford JL, Kristal AR. Obesity is associated with increased risks of prostate cancer metastasis and death after initial cancer diagnosis in middle-aged men. Cancer. 2007 Mar 15;109(6):1192–202. doi: 10.1002/cncr.22534. [DOI] [PubMed] [Google Scholar]
- 20.Freedland SJ, Giovannucci E, Platz EA. Are findings from studies of obesity and prostate cancer really in conflict? Cancer Causes Control. 2006 Feb;17(1):5–9. doi: 10.1007/s10552-005-0378-3. [DOI] [PubMed] [Google Scholar]
- 21.Venkateswaran V, Haddad AQ, Fleshner NE, et al. Association of diet-induced hyperinsulinemia with accelerated growth of prostate cancer (LNCaP) xenografts. J Natl Cancer Inst. 2007 Dec 5;99(23):1793–800. doi: 10.1093/jnci/djm231. [DOI] [PubMed] [Google Scholar]
- 22.Cox M, Gleave M, Zakikhani M, et al. Insulin receptor expression by human prostate cancers. The Prostate. 2008 doi: 10.1002/pros.20852. in press. [DOI] [PubMed] [Google Scholar]
- 23.Little RR, Rohlfing CL, Tennill AL, et al. Standardization of C-Peptide Measurements. Clin Chem. 2008 Apr 17; doi: 10.1373/clinchem.2007.101287. [DOI] [PubMed] [Google Scholar]
- 24.Final report on the aspirin component of the ongoing Physicians’ Health Study. Steering Committee of the Physicians’ Health Study Research Group. N Engl J Med. 1989 Jul 20;321(3):129–35. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed] [Google Scholar]
- 25.Andersen PK, Borgan O, Gill RD, Keidling N. Statistical Models Based on Counting Processes. New York: Springer-Verlag; 1993. [Google Scholar]
- 26.Cheng SC, Fine JP, Wei LJ. Prediction of cumulative incidence function under the proportional hazards model. Biometrics. 1998 Mar;54(1):219–28. [PubMed] [Google Scholar]
- 27.Banez LL, Hamilton RJ, Partin AW, et al. Obesity-related plasma hemodilution and PSA concentration among men with prostate cancer. JAMA. 2007 Nov 21;298(19):2275–80. doi: 10.1001/jama.298.19.2275. [DOI] [PubMed] [Google Scholar]
- 28.Frasca F, Pandini G, Sciacca L, et al. The role of insulin receptors and IGF-I receptors in cancer and other diseases. Arch Physiol Biochem. 2008 Feb;114(1):23–37. doi: 10.1080/13813450801969715. [DOI] [PubMed] [Google Scholar]
- 29.Hsing AW, Chua S, Jr, Gao YT, et al. Prostate cancer risk and serum levels of insulin and leptin: a population-based study. J Natl Cancer Inst. 2001 May 16;93(10):783–9. doi: 10.1093/jnci/93.10.783. [DOI] [PubMed] [Google Scholar]
- 30.Lehrer S, Diamond EJ, Stagger S, Stone NN, Stock RG. Serum insulin level, disease stage, prostate specific antigen (PSA) and Gleason score in prostate cancer. Br J Cancer. 2002 Sep 23;87(7):726–8. doi: 10.1038/sj.bjc.6600526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hammarsten J, Hogstedt B. Hyperinsulinaemia: a prospective risk factor for lethal clinical prostate cancer. Eur J Cancer. 2005 Dec;41(18):2887–95. doi: 10.1016/j.ejca.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Keating NL, O’Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006 Sep 20;24(27):4448–56. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 33.Basaria S, Muller DC, Carducci MA, Egan J, Dobs AS. Hyperglycemia and insulin resistance in men with prostate carcinoma who receive androgen-deprivation therapy. Cancer. 2006 Feb 1;106(3):581–8. doi: 10.1002/cncr.21642. [DOI] [PubMed] [Google Scholar]
- 34.Chen C, Lewis SK, Voigt L, Fitzpatrick A, Plymate SR, Weiss NS. Prostate carcinoma incidence in relation to prediagnostic circulating levels of insulin-like growth factor I, insulin-like growth factor binding protein 3, and insulin. Cancer. 2005 Jan 1;103(1):76–84. doi: 10.1002/cncr.20727. [DOI] [PubMed] [Google Scholar]
- 35.Stocks T, Lukanova A, Rinaldi S, et al. Insulin resistance is inversely related to prostate cancer: a prospective study in Northern Sweden. Int J Cancer. 2007 Jun 15;120(12):2678–86. doi: 10.1002/ijc.22587. [DOI] [PubMed] [Google Scholar]
- 36.Ma J, Giovannucci E, Pollak M, et al. A prospective study of plasma C-peptide and colorectal cancer risk in men. J Natl Cancer Inst. 2004 Apr 7;96(7):546–53. doi: 10.1093/jnci/djh082. [DOI] [PubMed] [Google Scholar]
- 37.Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006 Aug;12(8):895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- 38.Li C, Ford ES, McGuire LC, Mokdad AH, Little RR, Reaven GM. Trends in hyperinsulinemia among nondiabetic adults in the U.S. Diabetes Care. 2006 Nov;29(11):2396–402. doi: 10.2337/dc06-0289. [DOI] [PubMed] [Google Scholar]
- 39.Shaw RJ, Lamia KA, Vasquez D, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005 Dec 9;310(5754):1642–6. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.